- 1School of Biomedical Sciences, Faculty of Health, University of Plymouth, Plymouth, United Kingdom
- 2School of Dentistry, Faculty of Health, University of Plymouth, Plymouth, United Kingdom
Background: Oral squamous cell carcinoma (OSCC) is a common malignant cancer in humans. An abundance of tumour associated macrophages (TAMs) create an immunosuppressive tumour microenvironment (TME). TAM markers (CD163 and CD68) are seen to serve as prognostic factors in OSCC. PD-L1 has seen to widely modulate the TME but its prognostic significance remains controversial. The aim of this meta-analysis is to evaluate the prognostic role of CD163+, CD68+ TAMs and PD-L1 in OSCC patients.
Methods: Searches in PubMed, Scopus and Web of Science were performed; 12 studies were included in this meta-analysis. Quality assessment of included studies was performed according to REMARK guidelines. Risk of bias across studies was investigated according to the rate of heterogeneity. Meta-analysis was performed to investigate the association of all three biomarkers with overall survival (OS).
Results: High expression of CD163+ TAMs were associated with poor overall survival (HR = 2.64; 95% Cl: [1.65, 4.23]; p < 0.0001). Additionally, high stromal expression of CD163+ TAMs correlated with poor overall survival (HR = 3.56; 95% Cl: [2.33, 5.44]; p < 0.00001). Conversely, high CD68 and PD-L1 expression was not associated with overall survival (HR = 1.26; 95% Cl: [0.76, 2.07]; p = 0.37) (HR = 0.64; 95% Cl: [0.35, 1.18]; p = 0.15).
Conclusion: In conclusion, our findings indicate CD163+ can provide prognostic utility in OSCC. However, our data suggests CD68+ TAMs were not associated with any prognostic relevance in OSCC patients, whereas PD-L1 expression may prove to be a differential prognostic marker dependent on tumour location and stage of progression.
Introduction
Oral squamous cell carcinoma (OSCC) is a common malignant neoplasm (80%–90%) of the oral cavity, derived from the head and neck region of the body. It is associated with the common risk factors of smoking and alcohol consumption (1, 2). Contributing the highest incidence and mortality rate in both males and females, there were 354,864 new cases and 177,384 deaths worldwide in 2018 and was the leading cause of mortality in Central Asia (3). Whilst there is an improvement in advancing therapies such as surgery and chemotherapy, the 5-year survival rate remains 50% in various countries over the past 3 decades (4). This insufficient improvement in prognosis could be explained by the lack of consideration of immunological parameters in prognostic classification and treatment of OSCC (5). Moreover, poor prognosis in OSCC may be a result of its aggressive local invasion and metastasis, leading to an uncontrollable recurrence (6).
Metastasis is achieved through the interaction of tumour cells and the surrounding tumour microenvironment (TME) (7). This TME plays a critical role in tumourigenesis, tumour progression, invasion and tumour tissue infiltration by tumour-associated macrophages (TAMs) (8). TAMs are abundant in both the tumour and tumour stroma, playing a significant role in cancer progression (9, 10). MCP-1 (Monocyte chemoattractant protein-1 or CCL2) plays a role in recruiting and attracting TAMs to tumour sites (11, 12). These TAMs may exhibit either of two functional phenotypes, M1 or M2, dependent on cytokine, chemokine, chemokine receptor and other regulator expression (13). M1 TAMs exhibit pro-inflammatory and anti-tumoural properties, mediated by IL-12, TNF-α, IFN-γ, and stimulate strong Th1 IFNγ-driven cell mediated responses resulting in tumouricidal function. M2 TAMs are generally anti-inflammatory and pro-tumoural, expressing IL-10, IL-13, MR (mannose receptor) and are capable of inducing humoral Th2-driven cytokine responses, secreting IL-4, IL-13 and high levels of chemokines and growth factors such as VEGF, TGF-β, FGF and uPA, promoting angiogenesis, immunosuppression, tumour invasion and metastasis (14, 15).
TAM polarisation to distinct M1 and M2 subsets however, remains unclear, as recent evidence suggests functional plasticity and the ability to repolarise from one phenotype to the other (16). Human ovarian cancer TAMs have been observed to repolarise from M2 to M1-like phenotype suppressing levels of CCL18, MMP9 and VEGF when exposed to IFN-γ (17). TME TAMs have been shown to favour tumourigenesis, tumour survival and angiogenesis (18). This role in the TME however, is controversial; in colorectal cancer, for example, TAMs exhibit pro-inflammatory anti-tumour effects, leading to a favourable prognosis (19, 20). This may be explained by these M1 TAMs inducing the secretion of galectin-3 in human colon cells which further induces TAM infiltration and release of the pro-inflammatory cytokines, IL-1β and TNF-α, causing a strong anti-tumour response (20). Nevertheless, whilst TAMs may exhibit either phenotype, studies recognise TAMs to be predominantly of the M2 phenotype and correlate with a poor prognosis (15, 21).
TAMs may thus serve as potential biomarkers for the prognosis and therapeutic targeting of several cancers, particularly OSCC. Interestingly, over 80% of studies reveal a high number of TAMs correlates with poor patient prognosis (21). Investigations have shown CD163 (M2 macrophage class B scavenger receptor) (22), as a biomarker for macrophage activation in lung, breast and hepatocellular carcinoma (23-25). Recently, overexpression of M2-like CD163+ TAMs in head and neck squamous cell carcinoma (HNSCC) patients, revealed a poor clinical prognosis in both overall survival (OS) and progression-free survival (PFS) (26). CD163 functionality involves eradicating and endocytosing the haemoglobin/haptoglobin complex, thereby protecting tissues from oxidative damage (27). Used as a biomarker for M2c deactivated macrophages, it presents both anti-inflammatory and pro-tumoural functions (28).
Monocyte/macrophages, specifically M2 macrophages, abundantly express CD68, a glycosylated type I transmembrane glycoprotein belonging to the LAMP (lysosomal-associated membrane proteins) family (29). Its primary function is poorly understood, but as a class D scavenger receptor, it plays a role in promoting phagocytosis, clearing cellular debris and mediates recruitment/activation of macrophages (30). In contrast, CD68 is considered to be a pan-macrophage marker expressed by both M1 and M2 subsets, derived from anti-CD14-purified peripheral blood monocytes (31). This may explain observations where overexpression of CD68+ TAMs was associated with poor overall survival and disease-free survival (DFS) in breast cancer patients (32), whereas conversely, high CD68+ TAM expression conferred a longer overall survival and disease-free survival in hepatocellular carcinoma patients (33). Therefore, its prognostic relevance to OSCC needs clarification.
PD-L1 (programmed death ligand-1 also known as CD274, B7-H1) is a cell surface type I glycoprotein expressed on antigen-presenting cells (APCs) and located in dendritic cells and macrophages. Belonging to the B7 family, it is a co-inhibitory ligand which binds PD-1 (programmed death receptor-1). PD-1 functions as a T-cell checkpoint protein, regulating T-cell suppression (34). PD-1 is a member of CTLA-4 (cytotoxic T lymphocyte-associated protein 4) family, primarily expressed by cytotoxic T cells (Tc), which predominate anti-tumour responses. PD-1 ligation suppresses T-cell function via an inhibitory signal involving SHP-2 which inhibits CD28-mediated PI3K and Akt activity (35). In various cancers, PD-1 can be expressed on tumour infiltrating lymphocytes (TILs), where CD4+ and CD8+ TILs exhibit an increased PD-1 expression on Treg and Tc, effectively resulting in Treg-mediated immunosuppression and Tc anergy/loss of CTL function (36). In order to maintain homeostasis, PD-1/PD-L1 induces immune tolerance and effectively suppresses excessive tissue inflammation and autoimmune disease. In tumours, however, binding of PD-L1 to its PD-1 receptor on activated T cells results in T-cell suppression and immune escape by inhibiting perforin/granzyme production, suppressing IL-2 and IFN-γ production and promoting apoptosis, effectively inducing tumour growth (37). Several studies investigated the relationship between TAM PD-L1 expression and cancer patient prognosis. High PD-L1 expression revealed a poor clinical prognosis in malignant pleural mesothelioma (MPM) and renal cell carcinoma patients (38, 39), whereas other studies reached controversial and inconsistent conclusions. Conversely, high PD-L1 expression was associated with longer overall and disease-free survival (40, 41). Therefore, its prognostic relevance needs further clarification.
This study aims to investigate the prognostic role of CD68+, CD163+ TAMs and PD-L1 expression in OSCC, through a meta-analysis of the current literature. Furthermore, the prognostic role of these biomarkers was investigated in different sub-locations in OSCC (tumour versus stroma). This study hypothesised a high expression of CD68+, CD163+ TAMs and PD-L1 would lead to worse survival in OSCC patients, whereas concluded that CD163+ TAMs located in both tumour and stroma were predictive of a poor prognosis in OSCC and that PD-L1 may prove to be indicative of a positive outcome in these patients.
Materials and Methods
Search Strategy
In order to identify potential studies, a systematic search was conducted on the following online databases: PubMed, Scopus and Web of Science. Two Boolean operators (AND, OR) were used to select specific keywords. The following terms include: (macrophage OR TAM OR “tumour-associated macrophage” OR CD68 OR CD163) AND (“oral cancer” OR “oral squamous cell carcinoma” OR OSCC) AND (survival OR prognosis OR mortality OR death) AND (PD-L1 OR programmed death ligand 1 OR PDL1 OR B7-H1). Title and abstracts were screened based on inclusion and exclusion criteria (Refer to eligibility criteria section below). After inspecting full texts, the final predetermined articles were selected.
Eligibility Criteria—Included and Excluded Studies
Studies that had met the following inclusion criteria were included in the meta-analysis: 1) English language publication. 2) Studies that reported the prognostic significance and role of CD163, CD68 and PD-L1 in OSCC 3) Studies analysing the protein level expression of CD163, CD68 and PD-L1 in clinical analysis such as immunohistochemistry (IHC) sections in OSCC. 4) Evaluate the association of CD163, CD68 and PD-L1 and patient prognosis according to the following parameters: overall survival (OS). 5) Provided sufficient survival data which included only hazard ratio (HR) with 95% confidence interval (Cl), p-value P) alongside Kaplan Meier survival graphs. Studies that had less than 30 patients and did not meet the parameters were excluded from the meta-analysis.
Data Extraction (Outcomes)
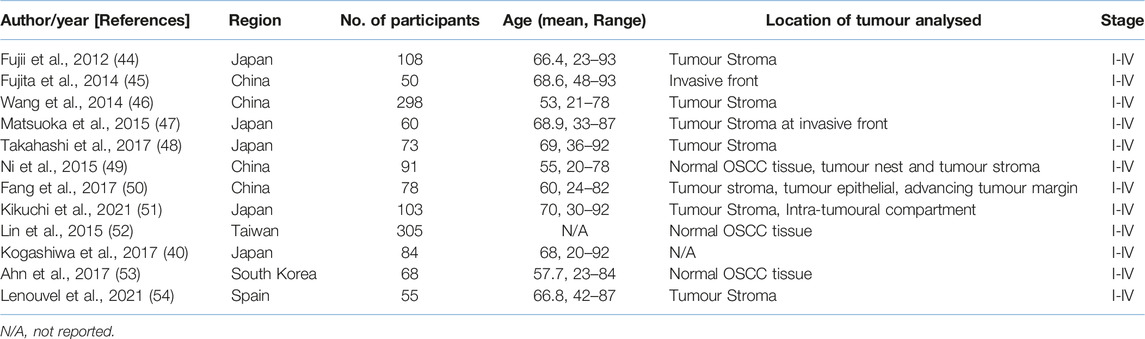
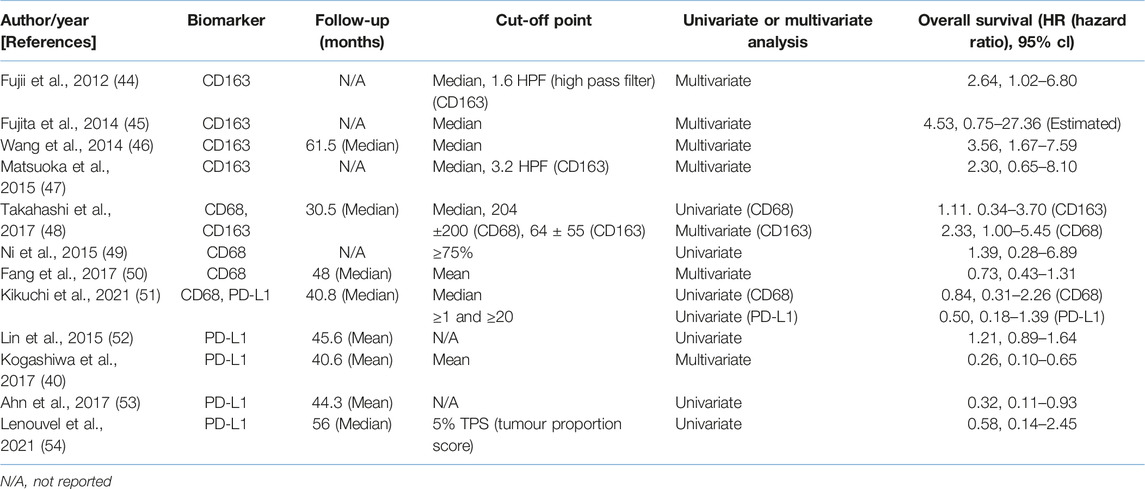
Included studies that met the criteria had their extracted data in accordance to: name of first author, year of publication, region of study, sample size, age, type of biomarkers used (CD163, CD68 or both and PD-L1), stage of cancer (TNM stage), location of tumour analysed, follow-up, cut-off values (threshold for prognostic factor and corresponding outcome based on high-risk and low-risk groups) and univariate and/or multivariate analysis outcomes to extract HR and 95% Cl for OS. Articles providing survival data are visualised in Kaplan-Meier curves.
Risk of Bias
To determine the risk of bias for each study, a quality assessment was conducted in accordance with the REMARK (Reporting Recommendations for Tumour Marker Prognostic Studies) (42). The risk of bias consists of six components: 1) samples, 2) clinical data of the group, 3) immunohistochemistry, 4) prognostication, 5) statistics and 6) prognostic factors. Each component was considered as: sufficient, insufficient or N/A (no description). The assessment scores for each study are shown in Table 1 according to REMARK assessment criteria guidelines presented in Supplementary Table S2.
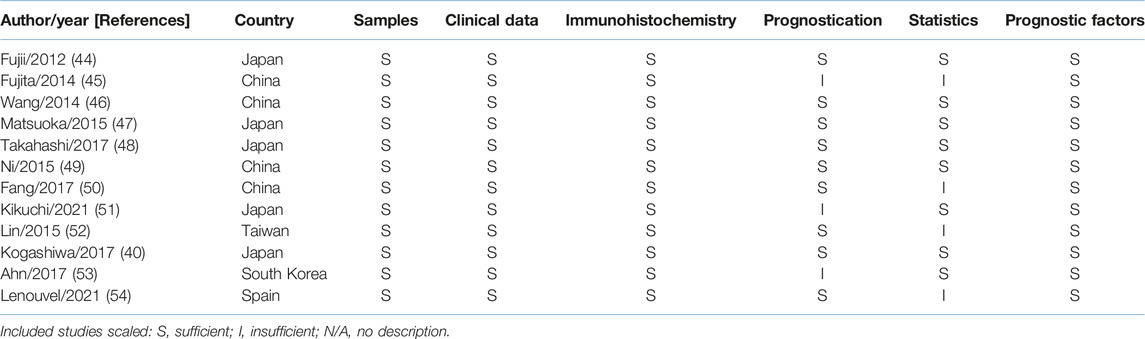
TABLE 1. Quality assessment of studies included in the meta-analysis according to REMARK guidelines.
Quantitative Data Analysis
RevMan (Review Manager) 5.4.1 was used to extract and construct quantitative data for the meta-analysis. Hazard ratios (either univariate or multivariate estimates), 95% confidence intervals (95% Cl) and p-value P) were extracted from the included studies. This data was constructed in forest plots with all in a random effect model. HRs for all immune biomarkers were sorted in a high vs. low direction. If HR estimates are reported in the opposite direction, HR and 95% Cl values were inverted. An HR >1 corresponds to worse survival in the group with high CD68+, CD163+ TAMs or PD-L1 expression. Estimated values of CD163, CD68 and PD-L1 expression were performed based on survival variables such as overall survival (OS). Other survival rates including disease-free survival (DFS) and disease-specific survival (DSS) was not included due to insufficient data. p-value lower than 0.05 was considered statistically significant. Heterogeneity (I2) is assessed and classified by Higgins index with: low heterogeneity (25%), medium heterogeneity (50%) and high heterogeneity (70%) (43).
Results
Study Selection and Study Results
Searches revealed 1881 records from commonly used databases (PubMed, Scopus and Web of Science) which justified the best array of literature. A total of 207 records were screened by title and abstract. Of these 207 articles, 177 were excluded due to providing insufficient data. 30 articles met the initial assessment of the inclusion criteria. Eventually, 12 studies were considered for inclusion in the meta-analysis (Figure 1) analysing data from 1373 patients (see also Supplementary Table S1). Three studies analysed CD68+ in the stroma and intra-tumour location of OSCC. Three studies analysed CD163+ TAM in the stroma. 12 of the included studies were predominately performed in Asia and one in Europe. The main characteristics of eligible studies and data extraction are shown in Tables 2, 3.
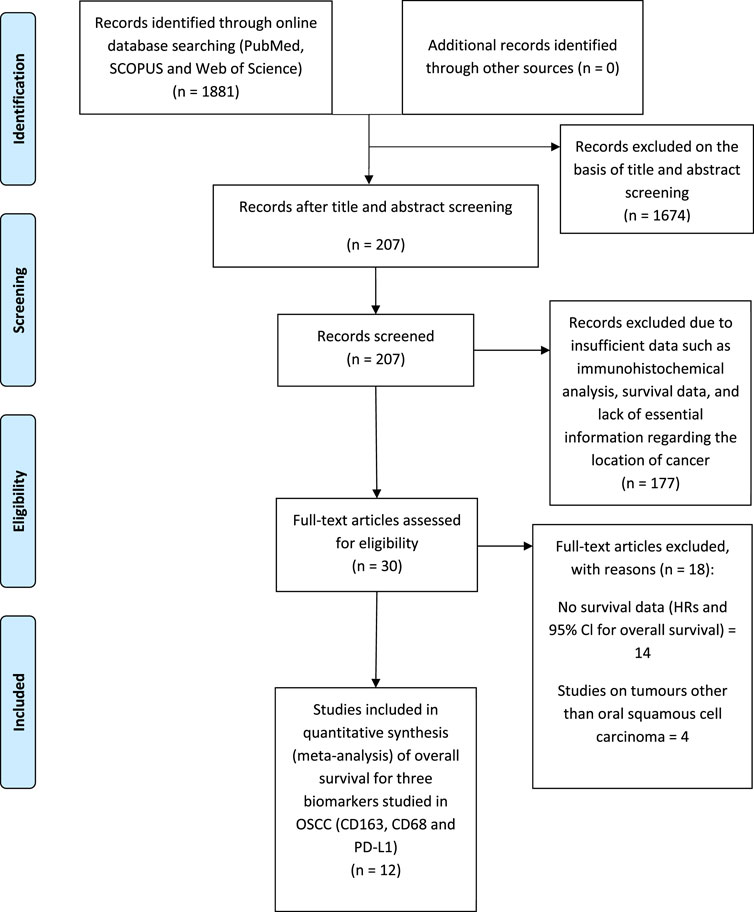
FIGURE 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) flow diagram of the study selection process for meta-analysis summarising literature searching, screening and assessment of eligibility of identified studies.
CD163+ TAMs are Associated With Poor Prognosis in OSCC
Due to observations that M2-like CD163+ TAMs were associated with poor prognosis in HNSCC, breast, gastric, colorectal and hepatocellular cancers, this study investigated whether CD163+ TAMs could also be adopted as a prognostic indicator in OSCC. The meta-analysis was executed at a random-effect model as a result of its low rate of heterogeneity (I2 = 0%). Five eligible studies reported the prognostic value of CD163+ TAM in OSCC. The pooled analysis revealed a high expression of CD163+ TAM and overall survival (OS) corresponded to a worse survival in OSCC patients (HR = 2.64; 95% Cl: [1.65, 4.23]; p < 0.0001) (Figure 2). Furthermore, in accordance to the stromal localisation of CD163+ TAM, it revealed similar results with the association being significant in stromal expression in OSCC patients (HR = 3.56; 95% Cl: [2.33, 5.44]; p < 0.00001) (Figure 3).
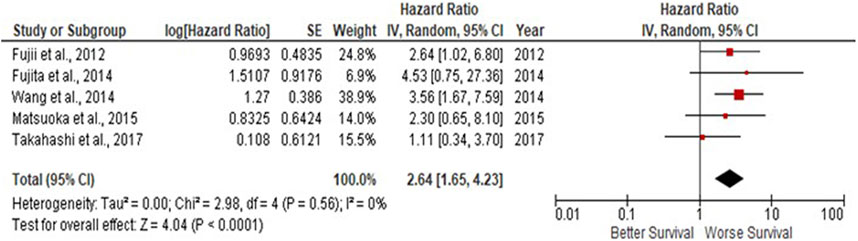
FIGURE 2. CD163+ TAMs are associated with poor overall survival in OSCC Forest plot reveals Hazard ratios (HRs) and 95% Cl for the association of CD163+ TAMs and overall survival (OS) in OSCC patients. Red square represents hazard ratio for each study, horizontal lines represent 95% confidence intervals and vertical line represents line of no effect. Black diamond represents the mean weighted overall hazard ratio among all studies (pooled estimate). An HR >1 illustrates a higher risk of death or progression associated with high CD163+ TAM expression. Forest plot reveals statistical significance between high CD163+ TAMs expression and OS in OSCC patients (p < 0.0001). Heterogeneity equates to 0% and results are conducted in a random-effect model.
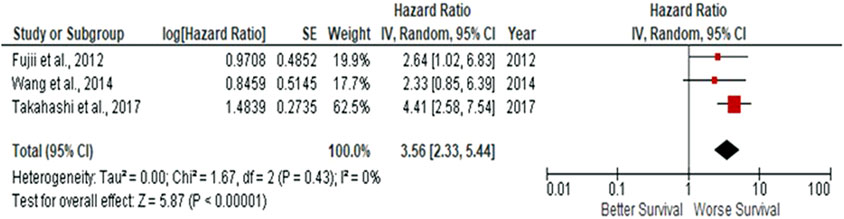
FIGURE 3. Stromal-located CD163+ TAMs are also associated with poor overall survival in OSCC Forest-plot reveals hazard ratios (HRs) and 95% Cl in accordance to stromal localisation of CD163+ TAMs in OSCC samples. Red square represents hazard ratio for each study, horizontal lines represent 95% confidence intervals and vertical line represents line of no effect. Black diamond represents the mean weighted overall hazard ratio among all studies (pooled estimate). An HR >1 illustrates a higher risk of death or progression associated with high stromal CD163+ TAMs. Forest plot reveals statistical significance between high CD163+ TAMs expression in accordance to stromal localisation in OSCC samples (p < 0.00001). Heterogeneity equates to 0% and results are conducted in a random-effect model.
Tumour and Stromal CD68+ TAMs Fail to Predict Prognosis in OSCC
Similar to findings of M2-like CD163+ TAMs, the presence of CD68+ TAMs is associated with poor prognosis in nasopharyngeal carcinoma (NPC), gastric and hepatocellular cancers, this study investigated whether CD68+ TAMs could also be adopted as a prognostic indicator in OSCC. The meta-analysis was executed at a random-effect model as a result of its low rate of heterogeneity (I2 = 41%). Four eligible studies reported the prognostic value of CD68+ TAM in OSCC. It should be noted in this analysis, included studies evaluate the expression of CD68+ TAM in more than one area (stroma and tumour (intra-tumoural) area). The pooled analysis revealed the association between high CD68+ TAMs and OS showed no statistically significant difference in OSCC patients (HR = 1.26; 95% Cl: [0.76, 2.07]; p = 0.37) (Figure 4A). In addition, CD68+ TAM expression was evaluated in different sample locations (stroma vs. tumour). The subgroup analysis revealed no association between stromal (HR = 1.30; 95% Cl: [0.55, 3.04]; p = 0.55) or tumour (intra-tumoural) (HR = 1.40; 95% Cl: [0.40, 4.90]; p = 0.60) expression of CD68+ TAMs and OS in OSCC patients (Figure 4B).
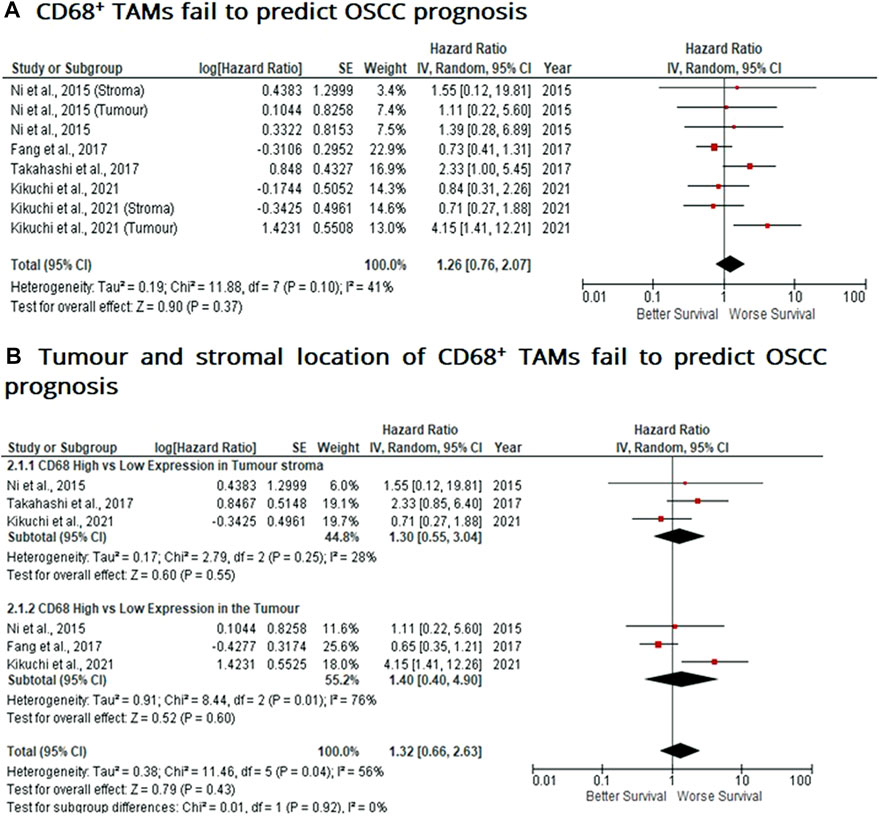
FIGURE 4. Tumour and stromal CD68+ TAMs fail to predict prognosis in OSCC Forest-plot reveals hazard ratios (HR) and 95% Cl for the association of CD68+ TAMs and OS in OSCC patients. (A) Studies were released evaluating the expression of CD68+ TAMs in several areas of the samples from the same group, whereas (B) is a Forest-plot presenting hazard ratios (HRs) and 95% Cl in a subgroup analysis related to survival in accordance to stromal or intra-tumour localisation of CD68+ TAMs in OSCC samples. Red square represents hazard ratio for each study, horizontal lines represent 95% confidence intervals and vertical line represents line of no effect. Black diamond represents the mean weighted overall hazard ratio among all studies (pooled estimate). An HR >1 illustrates a higher risk of death or progression associated with high levels of CD68+ TAMs. Tests for overall effect reveals statistical significance between CD68+ TAMs and OS in OSCC patients (p value) according to tumour as a whole (A) or specific tumour location (B).
PD-L1 Expression May be Associated With a Positive Prognosis in OSCC
When considering the immune-suppressive nature of PD-L1, it was not surprising to note that this marker was associated with a poor prognosis in breast, bladder and non-small cell lung cancer as well as in malignant pleural mesothelioma and renal cell carcinoma, whereas the opposite prognosis was observed in breast cancer and HKSCC. The present study wished to investigate whether PD-L1 acted as either a positive or negative prognostic indicator. Five eligible studies reported the prognostic value of PD-L1 expression in OSCC. A high rate of heterogeneity was found (I2 = 70%), therefore a random effect model was performed. In this analysis, one study evaluated PD-L1 expression twice (two areas of the sample from the same cohort) was included. Pooled analysis revealed the association of high PD-L1 expression and OS showed no statistically significant difference in OSCC patients (HR = 0.64; 95% Cl: [0.35, 1.18]; p = 0.15) (Figure 5A). In addition, PD-L1 expression was evaluated in different sample locations (stroma vs. tumour). The subgroup analysis revealed no association between stromal (HR = 0.53; 95% Cl: [0.23, 1.21]; p = 0.13) or tumour (intra-tumour) (HR = 2.24; 95% Cl: [0.83, 6.02]; p = 0.11) expression of PD-L1 and OS in OSCC patients (Figure 5B).
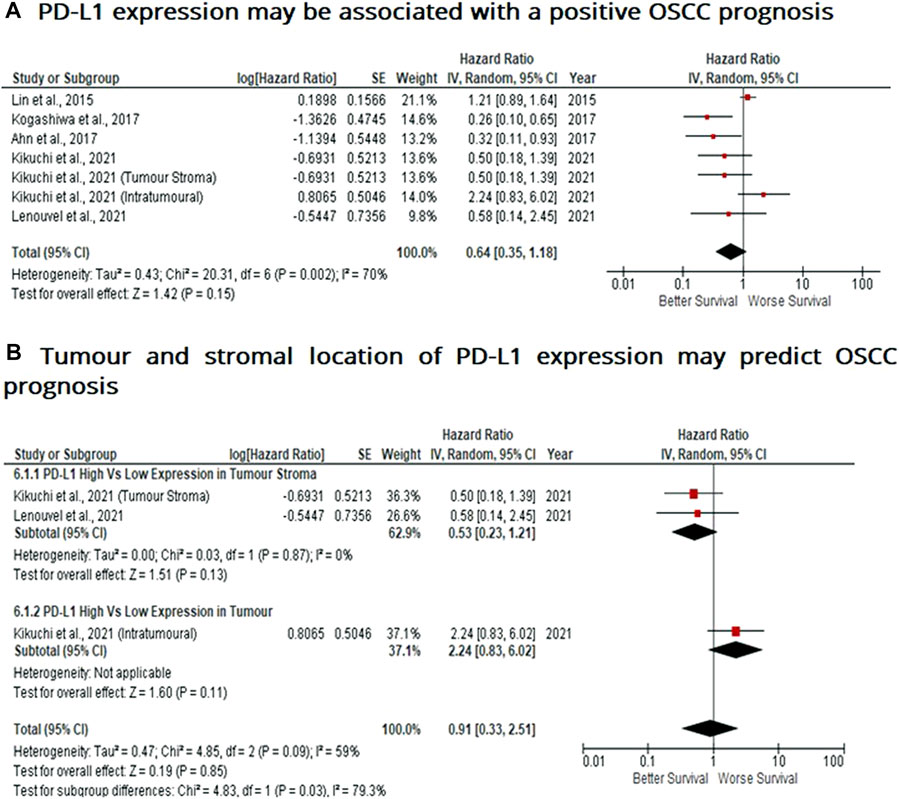
FIGURE 5. PD-L1 expression may be associated with a positive prognosis in OSCC Forest-plot reveals hazard ratios (HRs) and 95% Cl for the association of PD-L1 expression and OS in OSCC patients. (A) Studies evaluating the expression of PD-L1 in several areas of tumour samples from the same group, whereas (B) Forest-plot reveals hazard ratios (HRs) and 95% Cl in a subgroup analysis related to survival in accordance to stromal or intra-tumour localisation of PD-L1 in OSCC samples. Red square represents hazard ratio for each study, horizontal lines represent 95% confidence intervals and vertical line represents line of no effect. Black diamond represents the mean weighted overall hazard ratio among all studies (pooled estimate). An HR <1 illustrates a better overall survival associated with PD-L1 expression. Tests for overall effect reveals statistical significance between PD-L1 expression and OS in OSCC patients (p value) according to tumour as a whole (A) or specific tumour location (B). Percentage heterogeneity (I2) is indicated and as such, results are conducted in a random-effect model.
Discussion
This meta-analysis has reviewed the current literature on the prognostic potential of tissue biopsy tumour-associated macrophages; CD163+ TAMs and CD68+ TAMs as well as PD-L1 expression in OSCC. This meta-analysis demonstrated a significant association between high CD163+ TAMs with poor survival/prognosis in OSCC patients. Additional results revealed an insignificant association of CD68+ TAMs with OS, whereas PD-L1 expression approaches significance, indicating a potential positive prognosis associated with OSCC patients. Therefore, it reveals CD163+ TAMs to exhibit the best prognostic potential of macrophage subsets in both intra-tumour and stromal OSCC biopsies.
The present pooled analysis revealed a high density of CD163+ TAMs was associated with worse overall survival in OSCC (HR = 2.64; 95% Cl: [1.65, 4.23]; p < 0.0001). These findings are consistent in several other studies, reporting the correlation of CD163+ macrophages and worse survival in breast, gastric, colorectal and hepatocellular cancers (24, 55–57). Also, pooled analysis found CD163+ located within the tumour stroma to be associated with poor survival in OSCC patients (HR = 3.56; 95% Cl: [2.33, 5.44]; p < 0.00001). These findings were consistent with high CD163+ TAM densities in the tumour stroma and associated with poor survival in SCCHN (squamous cell carcinoma of the head and neck) (26). Similarly, high levels of tumour stroma CD163+ TAMs were associated with lymph node metastasis in OSCC (58). These high levels of OSCC CD163+ TAMs could be explained by the ability of TAMs to directly stimulate EGF (epidermal-growth factor) as well as anti-inflammatory cytokines (such as IL-6, IL-10), pro-inflammatory cytokines such as TNF-α, and chemokines such as CXCL12, CCL16, CCL18. Collectively, these factors induce tumour cell growth and survival factors which enhance tumour cell proliferation, migration and metastasis (59, 60).
Interestingly, the expression of CD163 is not only restricted to TAMs but may also be associated with cell fusion where the fusion of cancer cells and TAMs can increase metastatic potential with migratory leukocytes in cancer patients and plays a role in cancer progression. This can lead to a more aggressive and metastatic phenotype causing an unfavourable prognosis as demonstrated in OSCC (61, 62). Therefore, it is imperative to discriminate CD163+ malignant cells and macrophages when examining the influence of CD163+ on prognosis. Nevertheless, many studies reveal consistent findings with the present results demonstrating CD163 may serve as a significant prognostic biomarker in OSCC. Interestingly, the results presented about CD163+ TAMs may provide clinical implications. More specifically, they may serve as therapeutic targets for anticancer therapeutic regimens which may include the repolarisation of TAMs from M2-like TAMs to an M1-like phenotype, to restrain tumour progression (63). Therefore, a greater understanding of TAM function and OSCC progression is critical for future research in TAM-targeted therapies.
Compared to CD163+ TAMs, pooled results demonstrated high CD68+ TAMs were not statistically significant in revealing poor overall survival in OSCC patients (HR = 1.26; 95% Cl: [0.76, 2.07]; p = 0.37). However, other meta-analysis, has revealed high CD68+ TAM densities were associated with worse overall survival and disease-free survival in nasopharyngeal carcinoma (NPC) patients (64). Similar studies revealed CD68+ TAMs were associated with poor survival in gastric cancer and hepatocellular carcinoma, respectively (65, 66). These findings may be indicative of CD68+ TAMs possessing immunosuppressive and pro-tumour responses, favouring cancer progression. Interestingly, CD68+ TAMs have been shown to suppress cytotoxic activity of CD8+ T-cells and increase tumour growth (67). On the other hand, in the case of oesophageal squamous cell carcinoma, CD68+ TAMs were correlated with a favourable prognosis (68), suggestive that CD68+ TAMs may also function as M1 macrophages, revealing pro-inflammatory and anti-tumour effects. Whilst these molecular mechanisms are not fully understood, studies reveal TAMs may exert tumoricidal activity in vitro; more specifically, to polarise into M1 TAMs orchestrated by the production of IFN-γ which also activates cytotoxic CD8+ T and NK cell responses to initiate tumour cell killing (12, 19).
Similar to findings in this study, CD68+ TAMs did not significantly correlate with overall survival and recurrence-free survival (RFS) in multivariate analysis in basal-like breast cancer (BLBC) and triple-negative cancer of the breast (24, 69). In addition, no prognostic utility was found between CD68+ TAMs and OS in SCCHN patients (26). These findings reveal CD68+ TAMs may serve as a poor prognostic biomarker as demonstrated in this study focussed on OSCC, but more importantly, may indicate CD68 as a pan-macrophage marker expressed by both M1-like and M2-like TAMs, capable of exhibiting opposing effects on the tumour microenvironment (70). Therefore, this study’s findings and the mounting conflicting evidence between different cancers, indicates that CD68+ TAMs may be a poor prognostic biomarker in OSCC or at least requires further investigation across a variety of cancers/tumours as well as their TMEs.
Contrary to expectation, pooled results also revealed high PD-L1 expression had a non-significant positive impact on overall survival in OSCC patients (HR = 0.64; 95% Cl: [0.35, 1.18]; p = 0.15). A high level of heterogeneity (I2 = 70%) among the included studies were revealed, demonstrating conflicting results with each other. In contrast to these data however, numerous studies reveal PD-L1 expression was associated with poor prognosis and overall survival (OS) in solid cancers, such as head and neck squamous cell carcinoma (HNSCC) (71), breast cancer (72), non-small cell lung cancer (NSCLC) (73) and bladder cancer (74). This association with poor prognosis may be suggested by PD-L1/PD-1 binding to suppress CD8+ cytotoxic T-lymphocyte activation, leading to the evasion of the host immune anti-tumour response, thereby decreasing the survival rate in many cancers (37, 75). Additionally, whilst PD-L1 expression may protect macrophages from cell death, OSCC tumour cells induce TAM PD-L1 expression via IL-10 and induce T-cell apoptosis, further reinforcing an unfavourable prognosis (76).
Contradictory to these studies, yet consistent with findings in this investigation, PD-L1 expression in primary tumour cells was associated with prolonged DFS (Disease-free survival) in HNcSCC (head and neck cutaneous squamous cell carcinoma) (77). Similarly, high PD-L1 expression correlated better OS and DFS in breast cancer patients (78). This observation in prolonging survival in patients with PD-L1 expression may be due to the induction of an anti-tumour immune response. More specifically, IFN-γ, released by tumour-infiltrating lymphocytes as an adaptive immune-resistance mechanism to inhibit local effector T-cell function, can upregulate PD-L1 expression in tumour cells (36). Interestingly, IFN-γ also induces protein kinase D isoform 2 (PKD2), an important negative regulator of PD-L1 expression in OSCC. Thus PDK2 inhibits PD-L1 expression and promotes anti-tumour effects (blocking PD-1/PD-L1 dependent tumour antigen-specific CD8+ T cell apoptosis) (79). In addition, a high expression of PD-L1 was not statistically associated with OS in oesophageal squamous cell carcinoma (80), and more recently, pooled analysis of high PD-L1 expression did not have a statistically significant association with OS, DFS, DSS (Disease-specific survival) in OSCC patients (81). The findings of this investigation (Figure 5) are consistent with these studies focussed on OSCC and oesophageal SCC. In addition, further subgroup analysis suggested that stromal expression of PD-L1 may be associated with improved survival, whereas intra-tumour PD-L1 expression may be associated with poor prognosis and overall survival. This may be indicative of PD-L1+ cell location is predictive of survival and may reflect stage of cancer, or, when contrasted with the poor survival observed for CD163+ TAMs, is suggestive that stromal PD-L1 and CD163 are expressed on different TAM subsets or that PD-L1 may not be expressed on TAMs at all. Thus, this current investigation goes some way to indicating PD-L1 as a prognostic marker of survival, or indeed stage of cancer progression in OSCC which may reach statistical significance with the inclusion of more clinical studies. Further investigation may also potentially validate PD-1/PD-L1 interaction as a future therapeutic target for OSCC.
Conclusion
In conclusion, this meta-analysis confirmed the prognostic role of CD163+ TAMs, where a high cell number was associated with poor overall survival in OSCC. This indicates CD163+ TAMs may be a useful novel prognostic biomarker for OSCC and may suggest TAMs as a potential therapeutic target. Both CD68+ TAMs and PD-L1 revealed an insignificant correlation with overall survival in OSCC patients and limits the prognostic value of both biomarkers in OSCC, however the fact that the OS approached significance for PD-L1 is potentially indicative of PD-L1 being revealed as a positive prognostic indicator in the future.
Summary Table
What is Known About Subject
• Presence of TAMs are associated with poor prognosis of tumours including OSCC.
What This Paper Adds
• In contrast to other cancers, CD68+ TAMs fail to indicate OSCC prognosis.
• CD163+ TAMs and expression of PD-L1 could serve as both prognostic indicators of survival and stage of tumour progression: counter-intuitive, as these markers are normally associated with M2 subset, which is described as pro-tumoral.
• TAM subset analysis and location (tumour or stroma) is indicative of OSCC stage and prognosis.
Summary Sentence
• TAMs, and their location, are indeed, indicative of OSCC survival; where both tumour and stromal located CD163+ TAMs are indicative of poor prognosis whereas stromal PD-L1 expression may be indicative of a better prognosis when compared to tumour expressed PD-L1.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MC conceived, designed experiments undertook experimental data capture, analysed data and drafted the manuscript. MP contributed to experimental design, data analysis and drafting of manuscript. PL-Y involved in experimental design, data interpretation and analysis, and edited manuscript. VS supervised the design of experimental approach, data interpretation and analysis, and edited this manuscript. AF supervised experimental design, data capture and analysis and edited this manuscript. All authors read and approved the final manuscript.
Funding
This study was internally funded by the School of Biomedical Sciences, Faculty of Health, University of Plymouth.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge the expert input regarding meta-analysis methodology by Dr Nicola King, School of Biomedical Sciences, Faculty of Health, University of Plymouth, Plymouth PL4 8AA, United Kingdom and Dr Vito Carlo Alberto Caponio, DDS, Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/bjbs.2023.11065/full#supplementary-material
References
1. Pires, FR, Ramos, AB, Oliveira, JBCD, Tavares, AS, Luz, PSRD, and Santos, TCRBD. Oral Squamous Cell Carcinoma: Clinicopathological Features from 346 Cases from a Single Oral Pathology Service during an 8-year Period. J Appl Oral Sci (2013) 21(5):460–7. doi:10.1590/1679-775720130317
2. Chi, AC, Day, TA, and Neville, BW. Oral Cavity and Oropharyngeal Squamous Cell Carcinoma—An Update. CA: a Cancer J clinicians (2015) 65(5):401–21. doi:10.3322//caac.21293
3. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer J clinicians (2018) 68(6):394–424. doi:10.3322/caac.21492
4. Suárez-Sánchez, FJ, Lequerica-Fernández, P, Suárez-Canto, J, Rodrigo, JP, Rodriguez-Santamarta, T, Domínguez-Iglesias, F, et al. Macrophages in Oral Carcinomas: Relationship with Cancer Stem Cell Markers and PD-L1 Expression. Cancers (2020) 12(7):1764. doi:10.3390/cancers12071764
5. Weber, M, Iliopoulos, C, Moebius, P, Büttner-Herold, M, Amann, K, Ries, J, et al. Prognostic Significance of Macrophage Polarization in Early Stage Oral Squamous Cell Carcinomas. Oral Oncol (2016) 52:75–84. doi:10.1016/j.oraloncology.2015.11.001
6. Wang, B, Zhang, S, Yue, K, and Wang, XD. The Recurrence and Survival of Oral Squamous Cell Carcinoma: a Report of 275 Cases. Chin J. Cancer (2013) 32(11):614–8. doi:10.5732/cjc.012/10219
7. McAllister, SS, and Weinberg, RA. The Tumour-Induced Systemic Environment as a Critical Regulator of Cancer Progression and Metastasis. Nat Cel Biol (2014) 16(8):717–27. doi:10.1038/ncb3015
8. Lin, Y, Xu, J, and Lan, H. Tumor-associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J Hematol Oncol (2019) 12(1):76–16. doi:10.1186/s13045-019-0760-3
9. Li, C, Shintani, S, Terakado, N, Nakashiro, K, and Hamakawa, H. Infiltration of Tumor-Associated Macrophages in Human Oral Squamous Cell Carcinoma. Oncol Rep (2002) 9:1219–23. doi:10.3892/or.9.6.1219
10. Merry, R, Belfield, L, McArdle, P, McLennan, A, Crean, S-J, and Foey, A. Oral Health and Pathology: a Macrophage Account. Br J Oral Maxillofacial Surg. (2012) 50:2–7. doi:10.1016/j.bjoms.2010.10.020
11. Ireland, LV, and Mielgo, A. Macrophages and Fibroblasts, Key Players in Cancer Chemoresistance. Front Cel Developmental Biol (2018) 6:131. doi:10.3389/fcell.2018.00131
12. Klimp, AH, De Vries, EGE, Scherphof, GL, and Daemen, T. A Potential Role of Macrophage Activation in the Treatment of Cancer. Crit Revs Oncol./Hematol (2002) 44(2):143–61. doi:10.1016/s1040-8428(01)00203-7
13. Sica, A, and Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J Clin Invest (2012) 122(3):787–95. doi:10.1172/JCI59643
14. Atri, C, Guerfali, FZ, and Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci (2018) 19(6):1801. doi:10.3390/ijms19061801
15. Evrard, D, Szturz, P, Tijeras-Raballand, A, Astorgues-Xerri, L, Abitbol, C, Paradis, V, et al. Macrophages in the Microenvironment of Head and Neck Cancer: Potential Targets for Cancer Therapy. Oral Oncol (2019) 88:29–38. doi:10.1016/j.oraloncology.2018.10.040
16. Zheng, X, Turkowski, K, Mora, J, Brüne, B, Seeger, W, Weigert, A, et al. Redirecting Tumor-Associated Macrophages to Become Tumoricidal Effectors as a Novel Strategy for Cancer Therapy. Oncotarget (2017) 8(29):48436–52. doi:10.18632/oncotarget.17061
17. Duluc, D, Corvaisier, M, Blanchard, S, Catala, L, Descamps, P, Gamelin, E, et al. Interferon-γ Reverses the Immunosuppressive and Protumoral Properties and Prevents the Generation of Human Tumor-associated Macrophages. Int J Cancer (2009) 125(2):367–73. doi:10.1002/ijc.24401
18. Chanmee, T, Ontong, P, Konno, K, and Itano, N. Tumor-associated Macrophages as Major Players in the Tumor Microenvironment. Cancers (Basel) (2014) 6(3):1670–90. doi:10.3390/cancers6031670
19. Ong, SM, Tan, YC, Beretta, O, Jiang, D, Yeap, WH, Tai, JJ, et al. Macrophages in Human Colorectal Cancer Are Pro-inflammatory and Prime T Cells towards an Anti-tumour Type-1 Inflammatory Response. Eur J Immunol (2012) 42(1):89–100. doi:10.1002/eji.201141825
20. Dumont, P, Berton, A, Nagy, N, Sandras, F, Tinton, S, Demetter, P, et al. Expression of Galectin-3 in the Tumor Immune Response in colon Cancer. Lab Invest (2008) 88(8):896–906. doi:10.1038/labinvest.2008.54
21. Yang, L, and Zhang, Y. Tumor-associated Macrophages: from Basic Research to Clinical Application. J Hematol. Oncol. (2017) 10(1):58–12. doi:10.1186/s13045-017-0430-2
22. Fabriek, BO, Dijkstra, CD, and van den Berg, TK. The Macrophage Scavenger Receptor CD163. Immunobiol (2005) 210(2-4):153–60. doi:10.1016/j.imbio.2005.05.010
23. Sumitomo, R, Hirai, T, Fujita, M, Murakami, H, Otake, Y, and Huang, CL. M2 Tumor-Associated Macrophages Promote Tumor Progression in Non-small-cell Lung Cancer. Exp Ther Med (2019) 18(6):4490–8. doi:10.3892/etm.2019.8068
24. Jamiyan, T, Kuroda, H, Yamaguchi, R, Abe, A, and Hayashi, M. CD68-and CD163-Positive Tumor-Associated Macrophages in Triple Negative Cancer of the Breast. Virchows Archiv (2020) 477(6):767–75. doi:10.1007/s00428-020-02855-z
25. Kong, LQ, Zhu, XD, Xu, HX, Zhang, JB, Lu, L, Wang, WQ, et al. The Clinical Significance of the CD163+ and CD68+ Macrophages in Patients with Hepatocellular Carcinoma. PloS ONE (2013) 8(3):e59771. doi:10.1371/journal.pone.0059771
26. Troiano, G, Caponio, VCA, Adipietro, I, Tepedino, M, Santoro, R, Laino, L, et al. Prognostic Significance of CD68+ and CD163+ Tumor Associated Macrophages in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol (2019) 93:66–75. doi:10.1016/j.oraloncology.2019.04.019
27. Etzerodt, A, and Moestrup, SK. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid Redox Signal (2013) 18(17):2352–63. doi:10.1089/ars.2012.4834
28. Foey, AD. Macrophage Polarisation: a Collaboration of Differentiation, Activation and Pre-programming. J Clin Cel Immunol. (2015) 6(1):293. doi:10.4172/2155-9899.1000293
29. Chistiakov, DA, Killingsworth, MC, Myasoedova, VA, Orekhov, AN, and Bobryshev, YV. CD68/macrosialin: Not Just a Histochemical Marker. Lab Invest (2017) 97(1):4–13. doi:10.1038/labinvest.2016.116
30. Yu, X, Guo, C, Fisher, PB, Subjeck, JR, and Wang, XY. Scavenger Receptors: Emerging Roles in Cancer Biology and Immunology. Adv Cancer Res (2015) 128:309–64. doi:10.1016/bs.acr.2015.04.004
31. Raggi, F, Pelassa, S, Pierobon, D, Penco, F, Gattorno, M, Novelli, F, et al. Regulation of Human Macrophage M1–M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front Immunol (2017) 8:1097. doi:10.3389/fimmu.2017.01097
32. Yuan, J, He, H, Chen, C, Wu, J, Rao, J, and Yan, H. Combined High Expression of CD47 and CD68 Is a Novel Prognostic Factor for Breast Cancer Patients. Cancer Cel Int (2019) 19(1):238–12. doi:10.1186/s12935-019-0957-0
33. Ren, CX, Leng, RX, Fan, YG, Pan, HF, Li, BZ, Wu, CH, et al. Intratumoral and Peritumoral Expression of CD68 and CD206 in Hepatocellular Carcinoma and Their Prognostic Value. Oncol Rep (2017) 38(2):886–98. doi:10.3892/or.2017.5738
34. Kythreotou, A, Siddique, A, Mauri, FA, Bower, M, and Pinato, DJ. PD-L1. J Clin Pathol (2018) 71(3):189–94. doi:10.1136/jclinpath-2017-204853
35. Riella, LV, Paterson, AM, Sharpe, AH, and Chandraker, A. Role of the PD-1 Pathway in the Immune Response. Am J Transplant (2012) 12(10):2575–87. doi:10.1111/j.1600-6143.2012.04224.x
36. Qin, W, Hu, L, Zhang, X, Jiang, S, Li, J, Zhang, Z, et al. The Diverse Function of PD-1/PD-L Pathway beyond Cancer. Front Immunol (2019) 10:2298. doi:10.3389/fimmu.2019.02298
37. Jiang, Y, Chen, M, Nie, H, and Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum Vaccin Immunother (2019) 15(5):1111–22. doi:10.1080/21645515.2019.1571892
38. Nguyen, BH, Montgomery, R, Fadia, M, Wang, J, and Ali, S. PD-L1 Expression Associated with Worse Survival Outcome in Malignant Pleural Mesothelioma. Asia-Pacific J Clin Oncol (2018) 14(1):69–73. doi:10.1111/ajco.12788
39. Xu, F, Xu, L, Wang, Q, An, G, Feng, G, and Liu, F. Clinicopathological and Prognostic Value of Programmed Death Ligand-1 (PD-L1) in Renal Cell Carcinoma: a Meta-Analysis. Int J Clin Exp Med (2015) 8(9):14595–603.
40. Kogashiwa, Y, Yasuda, M, Sakurai, H, Nakahira, M, Sano, Y, Gonda, K, et al. PD-L1 Expression Confers Better Prognosis in Locally Advanced Oral Squamous Cell Carcinoma. Anticancer Res (2017) 37(3):1417–24. doi:10.21873/anticanres.11465
41. Thierauf, J, Veit, JA, Affolter, A, Bergmann, C, Grünow, J, Laban, S, et al. Identification and Clinical Relevance of PD-L1 Expression in Primary Mucosal Malignant Melanoma of the Head and Neck. Melanoma Res (2015) 25(6):503–9. doi:10.1097/CMR.0000000000000197
42. Altman, DG, McShane, LM, Sauerbrei, W, and Taube, SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med (2012) 9(5):e1001216. doi:10.1371/journal.pmed.1001216
43. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi:10.1136/bmj.327.7414.557
44. Fujii, N, Shomori, K, Shiomi, T, Nakabayashi, M, Takeda, C, Ryoke, K, et al. Cancer-associated Fibroblasts and CD163-positive Macrophages in Oral Squamous Cell Carcinoma: Their Clinicopathological and Prognostic Significance. J Oral Pathol Med (2012) 41(6):444–51. doi:10.1111/j.1600-0714.2012.01127.x
45. Fujita, Y, Okamoto, M, Goda, H, Tano, T, Nakashiro, KI, Sugita, A, et al. Prognostic Significance of Interleukin-8 and CD163-Positive Cell-Infiltration in Tumor Tissues in Patients with Oral Squamous Cell Carcinoma. PloS ONE (2014) 9(12):e110378. doi:10.1371/journal.pone.0110378
46. Wang, S, Sun, M, Gu, C, Wang, X, Chen, D, Zhao, E, et al. Expression of CD163, Interleukin-10, and Interferon-Gamma in Oral Squamous Cell Carcinoma: Mutual Relationships and Prognostic Implications. Eur J Oral Sci (2014) 122(3):202–9. doi:10.1111/eos.12131
47. Matsuoka, Y, Yoshida, R, Nakayama, H, Nagata, M, Hirosue, A, Tanaka, T, et al. The Tumour Stromal Features Are Associated with Resistance to 5-FU-based Chemoradiotherapy and a Poor Prognosis in Patients with Oral Squamous Cell Carcinoma. APMIS (2015) 123(3):205–14. doi:10.1111/apm.12344
48. Takahashi, H, Sakakura, K, Kudo, T, Toyoda, M, Kaira, K, Oyama, T, et al. Cancer-associated Fibroblasts Promote an Immunosuppressive Microenvironment through the Induction and Accumulation of Protumoral Macrophages. Oncotarget (2017) 8(5):8633–47. doi:10.18632/oncotarget.14374
49. Ni, YH, Ding, L, Huang, XF, Dong, YC, Hu, QG, and Hou, YY. Microlocalization of CD68+ Tumor-Associated Macrophages in Tumor Stroma Correlated with Poor Clinical Outcomes in Oral Squamous Cell Carcinoma Patients. Tumor Biol (2015) 36(7):5291–8. doi:10.1007/s13277-015-3189-5
50. Fang, J, Li, X, Ma, D, Liu, X, Chen, Y, Wang, Y, et al. Prognostic Significance of Tumor Infiltrating Immune Cells in Oral Squamous Cell Carcinoma. BMC Cancer (2017) 17(1):375. doi:10.1186/s12885-017-3317-2
51. Kikuchi, M, Yamashita, D, Hara, S, Takebayashi, S, Hamaguchi, K, Mizuno, K, et al. Clinical Significance of Tumor-associated Immune Cells in Patients with Oral Squamous Cell Carcinoma. Head & Neck (2021) 43(2):534–43. doi:10.1002/hed.26498
52. Lin, YM, Sung, WW, Hsieh, MJ, Tsai, SC, Lai, HW, Yang, SM, et al. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PloS ONE (2015) 10(11):e0142656. doi:10.1371/journal.pone.0142656
53. Ahn, H, Yang, JM, Kim, H, Chung, JH, Ahn, SH, Jeong, WJ, et al. Clinicopathologic Implications of the miR-197/pd-L1 axis in Oral Squamous Cell Carcinoma. Oncotarget (2017) 8(39):66178–94. doi:10.18632/oncotarget.19842
54. Lenouvel, D, González Moles, MÁ, Ruiz Ávila, I, Chamorro-Santos, C, González Ruiz, L, González Ruiz, I, et al. Clinicopathological and Prognostic Significance of PD L1 in Oral Cancer: A Preliminary Retrospective Immunohistochemistry Study. Oral Dis (2021) 27(2):173–82. doi:10.1111/odi.13509
55. Zhu, Q, Wu, X, Tang, M, and Wu, L. Observation of Tumor-Associated Macrophages Expression in Gastric Cancer and its Clinical Pathological Relationship. Medicine (2020) 99(17):e19839. doi:10.1097/MD.0000000000019839
56. Ding, D, Yao, Y, Yang, C, and Zhang, S. Identification of Mannose Receptor and CD163 as Novel Biomarkers for Colorectal Cancer. Cancer Biomarkers (2018) 21(3):689–700. doi:10.3233/CBM-170796
57. Minami, K, Hiwatashi, K, Ueno, S, Sakoda, M, Iino, S, Okumura, H, et al. Prognostic Significance of CD68, CD163 and Folate Receptor-β Positive Macrophages in Hepatocellular Carcinoma. Exp Ther Med (2018) 15(5):4465–76. doi:10.3892/etm.2018.5959
58. Yamagata, Y, Tomioka, H, Sakamoto, K, Sato, K, Harada, H, Ikeda, T, et al. CD163-positive Macrophages within the Tumor Stroma Are Associated with Lymphangiogenesis and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. J Oral Maxillofacs Surg (2017) 75(10):2144–53. doi:10.1016/j.joms.2017.03.009
59. Allavena, P, Sica, A, Solinas, G, Porta, C, and Mantovani, A. The Inflammatory Micro-environment in Tumor Progression: the Role of Tumor-Associated Macrophages. Crit Rev Oncology/Hematology (2008) 66(1):1–9. doi:10.1016/j.critrevonc.2007.07.004
60. Ostuni, R, Kratochvill, F, Murray, PJ, and Natoli, G. Macrophages and Cancer: from Mechanisms to Therapeutic Implications. Trends Immunol (2015) 36(4):229–39. doi:10.1016/j.it.2015.02.004
61. Skytthe, MK, Graversen, JH, and Moestrup, SK. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int J Mol Sci (2020) 21(15):5497. doi:10.3390/ijms21155497
62. Manjunath, Y, Porciani, D, Mitchem, JB, Suvilesh, KN, Avella, DM, Kimchi, ET, et al. Tumor-Cell–Macrophage Fusion Cells as Liquid Biomarkers and Tumor Enhancers in Cancer. Int J Mol Sci (2020) 21(5):1872. doi:10.3390/ijms21051872
63. Poh, AR, and Ernst, M. Targeting Macrophages in Cancer: from Bench to Bedside. Front Oncol (2018) 8:49. doi:10.3389/fonc.2018.00049
64. Chen, YL. Prognostic Significance of Tumor-Associated Macrophages in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. Medicine (2020) 99(39):e21999. doi:10.1097/MD.0000000000021999
65. Zhang, J, Yan, Y, Yang, Y, Wang, L, Li, M, Wang, J, et al. High Infiltration of Tumor-Associated Macrophages Influences Poor Prognosis in Human Gastric Cancer Patients, Associates with the Phenomenon of EMT. Medicine (2016) 95(6):e2636. doi:10.1097/MD.0000000000002636
66. Ding, W, Tan, Y, Qian, Y, Xue, W, Wang, Y, Jiang, P, et al. Clinicopathologic and Prognostic Significance of Tumor-Associated Macrophages in Patients with Hepatocellular Carcinoma: A Meta-Analysis. PloS ONE (2019) 14(10):e0223971. doi:10.1371/journal.pone.0223971
67. Kitamura, T, Qian, BZ, and Pollard, JW. Immune Cell Promotion of Metastasis. Nat Rev Immunol (2015) 15(2):73–86. doi:10.1038/nri3789
68. Li, J, Zhang, BZ, Qin, YR, Bi, J, Liu, HB, Li, Y, et al. CD68 and Interleukin 13, Prospective Immune Markers for Esophageal Squamous Cell Carcinoma Prognosis Prediction. Oncotarget (2016) 7(13):15525–38. doi:10.18632/oncotarget.6900
69. Yang, M, Li, Z, Ren, M, Li, S, Zhang, L, Zhang, X, et al. Stromal Infiltration of Tumor-Associated Macrophages Conferring Poor Prognosis of Patients with Basal-like Breast Carcinoma. J Cancer (2018) 9(13):2308–16. doi:10.7150/jca.25155
70. Wu, K, Lin, K, Li, X, Yuan, X, Xu, P, Ni, P, et al. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol (2020) 11:1731. doi:10.3389/fimmu.2020.01731
71. Müller, T, Braun, M, Dietrich, D, Aktekin, S, Höft, S, Kristiansen, G, et al. PD-L1: a Novel Prognostic Biomarker in Head and Neck Squamous Cell Carcinoma. Oncotarget (2017) 8(32):52889–900. doi:10.18632/oncotarget.17547
72. Huang, W, Ran, R, Shao, B, and Li, H. Prognostic and Clinicopathological Value of PD-L1 Expression in Primary Breast Cancer: a Meta-Analysis. Breast Cancer Res Treat (2019) 178(1):17–33. doi:10.1007/s10549-019-05371-0
73. Zhou, ZJ, Zhan, P, and Song, Y. PD-L1 Over-expression and Survival in Patients with Non-small Cell Lung Cancer: a Meta-Analysis. Translational Lung Cancer Res (2015) 4(2):203–8. doi:10.3978/j.issn.2218-6751.2015.03.02
74. Zhu, L, Sun, J, Wang, L, Li, Z, Wang, L, and Li, Z. Prognostic and Clinicopathological Significance of PD-L1 in Patients with Bladder Cancer: a Meta-Analysis. Front Pharmacol (2019) 10:962. doi:10.3389/fphar.2019.00962
75. Kuol, N, Stojanovska, L, Nurgali, K, and Apostolopoulos, V. PD-1/PD-L1 in Disease. Immunotherapy (2018) 10(2):149–60. doi:10.2217/imt-2017-0120
76. Kubota, K, Moriyama, M, Furukawa, S, Rafiul, HA, Maruse, Y, Jinno, T, et al. CD163+ CD204+ Tumor-Associated Macrophages Contribute to T Cell Regulation via Interleukin-10 and PD-L1 Production in Oral Squamous Cell Carcinoma. Scientific Rep (2017) 7(1):1755–12. doi:10.1038/s41598-017-01661-z
77. Roper, E, Lum, T, Palme, CE, Ashford, B, Ch'ng, S, Ranson, M, et al. PD-L1 Expression Predicts Longer Disease Free Survival in High Risk Head and Neck Cutaneous Squamous Cell Carcinoma. Pathology (2017) 49(5):499–505. doi:10.1016/j.pathol.2017.04.004
78. Matikas, A, Zerdes, I, Lövrot, J, Richard, F, Sotiriou, C, Bergh, J, et al. Prognostic Implications of PD-L1 Expression in Breast Cancer: Systematic Review and Meta-Analysis of Immunohistochemistry and Pooled Analysis of Transcriptomic Data. Clin Cancer Res (2019) 25(18):5717–26. doi:10.1158/1078-0432.CCR-19-1131
79. Chen, J, Feng, Y, Lu, L, Wang, H, Dai, L, Li, Y, et al. Interferon-γ-induced PD-L1 Surface Expression on Human Oral Squamous Carcinoma via PKD2 Signal Pathway. Immunobiology (2012) 217(4):385–93. doi:10.1016/j.imbio.2011.10.016
80. Qu, HX, Zhao, LP, Zhan, SH, Geng, CX, Xu, L, Xin, YN, et al. Clinicopathological and Prognostic Significance of Programmed Cell Death Ligand 1 (PD-L1) Expression in Patients with Esophageal Squamous Cell Carcinoma: a Meta-Analysis. J Thorac Dis (2016) 11:3197–204. doi:10.21037/jtd.2016.11.01
81. Troiano, G, Caponio, VC, Zhurakivska, K, Arena, C, Pannone, G, Mascitti, M, et al. High PD L1 Expression in the Tumour Cells Did Not Correlate with Poor Prognosis of Patients Suffering for Oral Squamous Cells Carcinoma: A Meta Analysis of the Literature. Cel Prolif (2019) 52(2):e12537. doi:10.1111/cpr.12537
Keywords: macrophages, oral cancer, CD68, PD-L1, CD163
Citation: Chohan MH, Perry M, Laurance-Young P, Salih VM and Foey AD (2023) Prognostic Role of CD68+ and CD163+ Tumour-Associated Macrophages and PD-L1 Expression in Oral Squamous Cell Carcinoma: A Meta-Analysis. Br J Biomed Sci 80:11065. doi: 10.3389/bjbs.2023.11065
Received: 18 November 2022; Accepted: 10 February 2023;
Published: 16 June 2023.
Copyright © 2023 Chohan, Perry, Laurance-Young, Salih and Foey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew D. Foey, YW5kcmV3LmZvZXlAcGx5bW91dGguYWMudWs=
†These authors share senior authorship
 Mohammed Haseeb Chohan1,2
Mohammed Haseeb Chohan1,2 Paul Laurance-Young
Paul Laurance-Young Vehid M. Salih
Vehid M. Salih Andrew D. Foey
Andrew D. Foey