- Jean and Paul Amos Parkinson’s disease and Movement Disorder Program, Emory University School of Medicine, Atlanta, GA, United States
Tardive dystonia (TD), the second most common but most disabling form of tardive syndrome, was initially described in 1982. It is caused by exposure to dopamine receptor blocking agents including antipsychotics and antiemetics. It most commonly presents as cranial or cervical dystonia. Characteristics suggestive of a TD diagnosis include a young age of onset, male predominance, and the higher prevalence of phasic cervical dystonia and retrocollis. Treatment of TD is limited. In this paper we review the literature on treatment options for TD as well as discussing a strategic approach. Options include use of clozapine which appears to have anti-dystonia properties. Other medications reported on with limited evidence include VMAT2 inhibitors, anticholinergics, clonazepam, and baclofen. Botulinum toxin has been shown to provide relief in TD in a manner similar to primary dystonia. The largest literature is on the use of deep brain stimulation (DBS) of the globus pallidus pars interna which includes blinded studies. We finish with providing an algorithm based on current knowledge.
Introduction
Tardive dystonia (TD) is the second most common variant of tardive syndrome (TS) after orofacial tardive dyskinesia and the most disabling (1). It is the result of chronic treatment with dopamine receptor blocking agents (DRBAs) including first and second generation antipsychotics and certain promotility and antiemetic agents such as metoclopramide which are also used for the treatment of migraine. TD is defined by its persistence for at least 1 month following discontinuation of the offending agent as opposed to acute dystonic reactions (2). While TD is usually included under the encompassing term of TS (also referred to generally as tardive dyskinesia in older literature), which also includes the more common orofacial dyskinetic phenotype among others, the first large series that focused specifically on tardive dystonia was described in 1982 and included 42 patients (3). While the classical tardive dyskinesia usually indicates chorea predominantly affecting the orofacial region, the term tardive dystonia (TD) is used for sustained and involuntary movements, as is typical of idiopathic dystonia. In its initial description, TD was defined as persistent dystonia with onset during or following the use of antipsychotic drugs, in a patient with no family history of dystonia and a negative work-up for other secondary dystonias. A metanalysis of 41 studies, including a total of 11,493 patients on antipsychotics at the time of TS assessment, showed an average prevalence of TS of 25.3% (4). Lower (7.2%) figures were seen in patients treated with second generation antipsychotics (SGA) and naïve to first generation antipsychotics (FGA), compared to patients on SGA but previously exposed to FGA (23.4%) (4). Similar data regarding the overall prevalence of TS (23.4%) was reported in 1988, with an incidence of 5% per year (5). TD prevalence ranges in the literature from 0.5% to 21.6% (6–10), with a more recent series including 30 patients with history of DRBAs use reporting a 36.6% prevalence of TD (11). The phenomenology of TS includes choreoathetoid and stereotypical movements, dystonia, akathisia, and less commonly myoclonus, tics, and tremor (12). Due to its relatively higher prevalence, most of the available research of TS pathophysiology is performed in classical orofacial tardive dyskinesia (13). The main hypothesis attributes TS to upregulation of dopamine D2 receptors following chronic receptor blockade, resulting in a hyperdopaminergic state; however, this is not fully supported by animal models and human studies (1). The D2 receptor hypersensitivity theory is supported by the fact that DRBAs such as clozapine and quetiapine, which quickly dissociate from D2 receptors (14), have a lower incidence of such neurological side effects. However, there appears to be some difference in pathophysiology between TD and the classical choreiform syndrome. One important difference is the role of the cholinergic system, as anticholinergic medications may worsen and even increase the risk of classical tardive dyskinesia, while they are often used in TD treatment (13). The literature reporting on therapeutic interventions also focuses on TS with few studies specifically examining TD. Due to the relative rarity of TD, most studies include it within a larger sample of TS patients, limiting the conclusions that can be inferred regarding TD treatment specifically. We aim to summarize the available literature of TD treatment and provide a practical workflow to address this disabling condition.
Clinical features
In the original TD cohort reported by Burke et al. (3), mean age of onset was 34 years, and male predominance was reported. The majority of patients (64%) had segmental dystonia, followed by focal (21%), and generalized (14%). Two expanded series of 67 patients (including 16 of those previously reported by Burke et al. (15) and 107 patients (including 17 of those previously reported by Burke et al. (9) confirmed the initially described characteristics, including a significantly lower age of onset in men compared to women (34 vs. 44 years). The clinical evolution of TD was described by Kiriakakis et al. (9), which showed that while most cases had a focal onset (83%), once the dystonia progressed it was more frequently segmental (60%). The cervical (37%) and cranial (33%) regions were the most commonly affected at onset, becoming more frequently involved after dystonia progression (66% and 60%, respectively). This was true for limb dystonia affecting one or both uppers in 15% at onset and 63% when the condition had fully developed. The risk of TS, in general, increases with treatment duration of exposure (1), and although a few months of treatment are required for diagnosis (2, 16), there seems to be no minimum duration of treatment with DRBAs associated with TS (9, 17). In these studies, the duration of exposure to DRBAs before onset of TD was on average of 3.7–7.3 years, with a very wide range of 3 days to 37.5 years reported (3, 9, 15). There is also a tendency to need a shorter duration of neuroleptic exposure in younger patients (3, 9). Dystonia progression may occur over months to years, with a mean of 1.8 years reported in one study; progression was more rapid in patients whose dystonia onset occurred at a younger age and after a shorter interval from DRBAs initiation (9).
While the clinical phenotype of TD may be indistinguishable from that of primary dystonia (17), comparisons of clinical characteristic of idiopathic versus tardive cervical dystonia found that TD presents more frequently with head jerks, extracervical involvement, retrocollis, antecollis, and right compared to left torticollis (9, 18). Additionally, head tremor was not present in the TD group. A study comparing idiopathic and tardive oromandibular (OMD) dystonia (19) found that isolated OMD and the association of cranial stereotypy were higher in tardive than in idiopathic forms, while cervical dystonia more frequently co-existed with idiopathic than with tardive OMD. In a case series of lingual protrusion dystonia, TD was the most common etiology (20). Task specificity and sensory tricks may also characterize certain forms of TD (17). The likelihood of remission of TD is around 10%, but increases by discontinuing the offending agent as soon as dystonia is diagnosed and with a shorter treatment course (9). Kang et al. (15) reported that out of 42 patients in whom DRBAs could be discontinued, only five experienced a remission (after 11 months to 5 years following DRBA discontinuation). The patients who achieved remission had a younger age at dystonia onset, shorter exposure to DRBAs, earlier medication discontinuation after the onset of TD, and earlier treatment. It has been shown that neither dystonia or dyskinesia has impact on occurrence of remission (21) In addition to its persistent nature, TD can be socially and functionally disabling (22), and be associated with complications such as fractures (22), spine disease and myelopathy (23, 24), dysphagia (25–27), dyspnea (27), and rhabdomyolysis (28).
Methods
A literature search was performed on the PubMed database for publication title and/or abstract including “tardive dystonia,” “tardive dyskinesia,” “tardive syndrome” and a combination of these with clozapine, antipsychotic, VMAT inhibitors, tetrabenazine, valbenazine, deutetrabenazine, benztropine, trihexyphenidyl, clonazepam, baclofen, deep brain stimulation, pallidotomy. The search was limited to papers in English. Abstracts were reviewed for pertinence and papers selected and reviewed. References from these publications were also reviewed and included as appropriate.
Treatment
The medical treatment of TD has been described as limited and unsatisfactory starting from the earlier reports (3, 9, 15). As with classical orofacial tardive dyskinesia, the first step in treatment is discontinuation of DRBAs if possible (9, 17). This should be performed with a progressive taper, as TD may worsen following DRBAs discontinuation (15). It should be noted that the evidence for this approach in reversing TS is lacking (29).
Second generation antipsychotics
Second generation antipsychotics (SGA) are currently utilized more often than first generation agents (FGA) but retain a risk of causing TD and TS (1). If discontinuation of DRBAs is not possible due to psychiatric comorbidities, a second strategy is to substitute FGAs with SGAs or substitute SGAs with one that is less potent in blocking D2 receptors (30). Evidence for this approach is also lacking (29). While DRBAs may be effective in suppressing TD (15), their continuation may worsen or decrease the chances of remission of the TS (9). Their use as a treatment for TD may be justified if TD is severe and painful or associated with muscle damage, if a dystonic storm ensues, or if no improvement is noted after being off DRBAs for a sufficient time (3, 17).
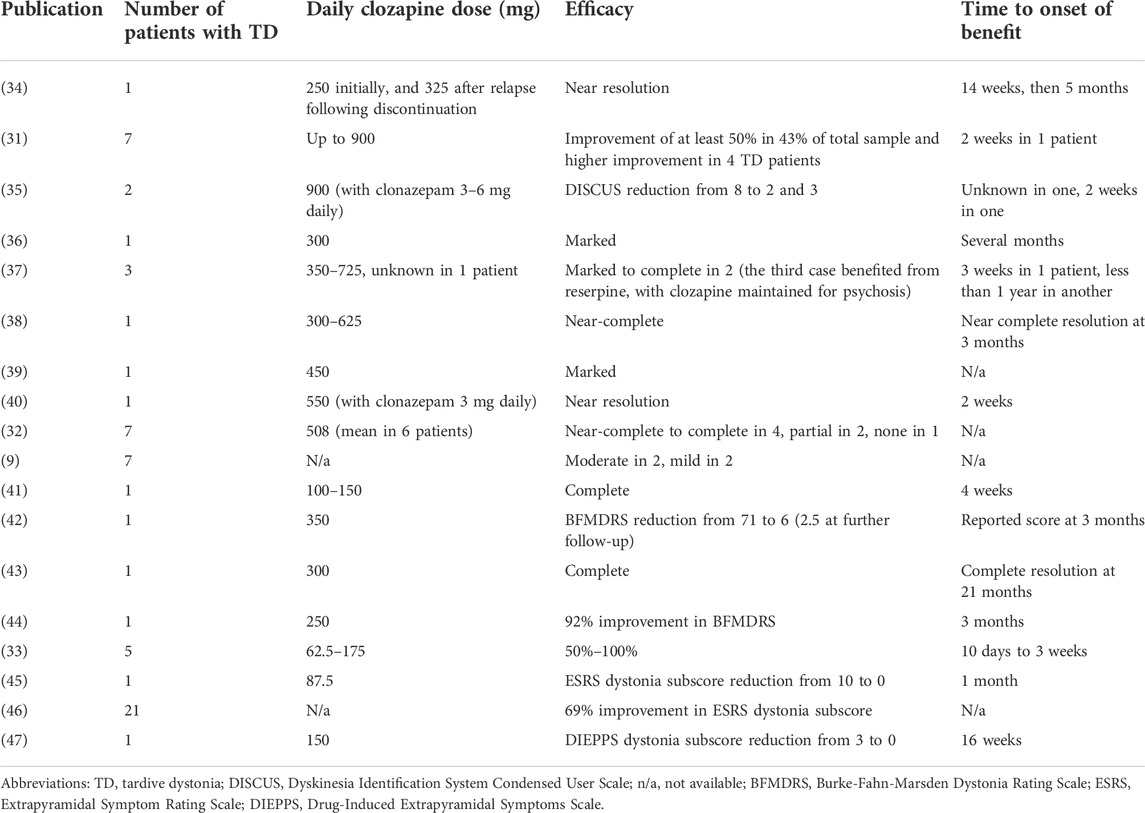
Under these circumstances, clozapine is a particularly interesting agent for treating TD (22). Several studies suggest clozapine may itself improve dystonia through a direct effect, not via masking. Lieberman et al. examined the effect of clozapine on 37 TS patients including seven who had TD (31). Forty-three percent of the total sample had an improvement of at least 50%, and four TD patients experienced a more pronounced benefit. After discontinuation of clozapine in two patients, TD recurred in one. In a case series of seven TD patients treated with clozapine, four achieved total or near total remission while six demonstrated some improvement. One patient had no recurrence after clozapine discontinuation for 2 weeks, while one had worsening of dystonia after stopping the medication (32). Another series of five patients (33) showed positive results of clozapine for TD and tardive dyskinesia within 10 days to 3 weeks. Improvement ranged from 50% to complete resolution. Two patients treated for at least 1 year did not experience a recurrence of TD after clozapine discontinuation. Additional reports of successful treatment of TD with clozapine are listed in Table 1. Three patients responded to combined treatment with clonazepam and clozapine (35, 40). Other reports showed no benefit of clozapine (48, 49), or worsening of TD upon clozapine introduction (50). The data from these experiences would suggest that a randomized controlled trial should be undertaken but this has not developed.
In the treatment of psychosis and TD, some reports have found benefit with olanzapine (51–54), risperidone (9, 55, 56), quetiapine (57–61), and aripiprazole (62–65). It is likely that their effect is mediated through masking of the abnormal movements or by an active therapeutic mechanism.
VMAT2 inhibitors
The VMAT2 inhibitors tetrabenazine, valbenazine, and deutetrabenazine are dopamine depleting medications which act by inhibiting the transport and sequestration of monoamines into presynaptic vesicles, therefore promoting monoamine degradation in the cytosol and reducing dopaminergic transmission. They were approved by the FDA for the treatment of Huntington disease-associated chorea (tetrabenazine and deutetrabenazine) and tardive dyskinesia (deutetrabenazine and valbenazine) following completion of randomized, double-blind, placebo-controlled trials in each disorder (66–71). Both deutetrabenazine (in doses of 24–36 mg daily) and valbenazine (in doses of 80 mg daily) were each shown in two separate randomized, multicenter, double-blind, placebo controlled trials to demonstrate a statistically significant improvement in the Abnormal Involuntary Movement Scale (AIMS) severity scale (∼3 point change, with a 1.9 point change for valbenazine 40 mg daily) for TS with acceptable tolerability, and these results were maintained for 1–3 years (68, 69, 71). These trials included patients with all phenotypes for TS including TD but the phenotype (dystonia, chorea, etc.) is not indicated in the AIMS scale so that the number of patients with dystonia and their response could not be separated from the rest of the TS cases. No large scale trials have been dedicated to studying their efficacy to treat TD, and truncal dystonia was an exclusion criterion in the trials investigating valbenazine in tardive dyskinesia (71). Hence, it remains unclear what the extent of response is for TD.
Tetrabenazine is a much older drug and, while it was not studied in randomized, multicenter controlled trials for TS, there is some data regarding effectiveness in TD in smaller open label cohorts. The first experiences were reported in the initial series by Burke et al. in 1982 (3), with improvement noted in 68% of 19 treated patients. Treatment dosages were not reported. Kang et al. (15) noticed a response in three out of seven patients treated with tetrabenazine alone and ten out of 16 treated with a combination of tetrabenazine and other agents (with a tetrabenazine dosage of 12.5–250 mg, mean of 174 mg, in responders). No details are available regarding the degree of improvement. Successful treatment of tardive oculogyric crises was later reported in a case series of four patients (72). Kiriakakis et al. (9) reported improvement in 16 out of 39 patients (mild in ten, moderate in five, and marked in one; unknown dosage). A larger open label study showed that tetrabenazine may be more effective in TD than in idiopathic dystonia (82% of patients with TD versus 62.9% of those with idiopathic dystonia achieving marked improvement) (73). In this study, patients with TD were treated with a mean maximal daily dose of 125 mg (ranging from 37.5 to 400 mg). In one patient, tetrabenazine doses up to 75 mg daily led to remission of tardive cervical and truncal dystonia after 4 weeks of treatment (74). Benefit of tetrabenazine in TD ranges from transient (75) to complete resolution after its discontinuation (76).
Anticholinergics
Anticholinergics have been utilized to treat TD because of their effectiveness in idiopathic dystonia (77), but experience is limited to case series and case reports, and dosages needed for therapeutic efficacy are often not reported. In general, this class of drugs worsens the classical orofacial syndrome, and increases the risk of its development (3, 78). In the largest TD cohort examined, out of 54 patients treated with anticholinergics, Kiriakakis et al. (9) obtained mild benefit in 16, moderate in six, and marked in two. In addition, Burke et al. (3) achieved some benefit in seven out of 18 patients treated with anticholinergics (benztropine, trihexyphenidyl, procyclidine, and ethopropazine). Subsequent smaller experiences included response in three out of eight patients and in four out of nine treated with 10–32 mg daily of trihexyphenidyl alone or in combination with other agents, respectively (15). A similar response rate was seen with ethopropazine (100–450 mg) alone or in combination (in three out of 11 and five out of 12 patients). Trihexyphenidyl (6–36 mg daily) in the setting of DRBAs discontinuation or reduction also led to amelioration of TD in case reports (79–81).
Benzodiazepines
The benefit of clonazepam in TD is occasionally reported in combination treatment with clozapine (Table 1) (35, 40) or less commonly with clonazepam alone (5.5 mg daily) (82). Five of 25 patients showed response in Kang et al.‘s series (15); two out of six responded to diazepam (dose of 15–30 mg in responders) in combination treatment, one out of four and one out of six to clonazepam (dose of 10–12 mg in responders) alone or in combination respectively, and one out of nine to lorazepam (dose of 12 mg in responders) in combination treatment. A double-blind randomized placebo-controlled trial (83) of clonazepam for TS included six patients with predominant TD who exhibited an improvement of 41.5% in dyskinesia scores with a mean dose of 3.83 mg per day. Scores during treatment with clonazepam were significantly decreased compared to baseline and placebo administration.
Baclofen
While there are several trials of baclofen reported for TS with mixed results (1), few reports of the use of baclofen specifically for TD are available. Benefit (mild in four and moderate in one) was obtained in five out of nine patients of one series (9), while lower success rates were noted in another series in which one patient out of eight responded to a baclofen dosage of 80 mg (15). Case reports showed a range of results from modest benefit with 60 mg of baclofen per day (34) up to near-complete symptom resolution when baclofen (80 mg per day) was used in combination with reserpine (36). This combination therapy led to worsening psychosis and needed to be discontinued. Intrathecal baclofen has also been used in one patient to improve axial dystonia following failure of oral medications (including oral baclofen) or of botulinum toxin, and transient response to neurotomy (84). The patient benefited from a continuous intrathecal baclofen infusion of 100 μg/day with marked improvement of her dystonia.
Botulinum toxin
Botulinum toxin (BoNT) is an established and efficacious treatment for idiopathic dystonia which received FDA approval in 1989 (85, 86). Over the years, botulinum toxin use has been expanded to the phenomenologically similar TD using the same techniques, but there are no double-blind, controlled trials. Most of the results are open label experiences and use onabotulinumtoxinA. Kaufman reported the use of botulinum toxin in three patients affected by tardive cervical dystonia, with results including complete symptom resolution in one patient (87). Kiriakakis et al. (9) reported improvement in 83% of patients with tardive cervical dystonia and in 86% of patients with tardive blepharospasm. An open label study including 34 patients with TD treated with botulinum toxin injections achieved moderate to marked improvement in the majority of patients (29 out of 38 treated body regions) (88). A comparison study of botulinum toxin type A in idiopathic cervical dystonia versus tardive cervical dystonia included 149 patients with idiopathic cervical dystonia (ICD) and 7 with TD, and showed a similar improvement in severity in the two groups, but with a higher average dose used in the TD group compared to ICD (mean of 287 vs. 220 units) (89). A subsequent study comparing 92 patients with idiopathic and 24 with tardive oromandibular dystonia (19) confirmed a moderate benefit of BoNT and found no differences in dose and efficacy of botulinum toxin between the two groups, when injecting only the masseters, geniohyoid, digastric, and mylohyoid muscles. Molho et al. (18) compared 78 patients with idiopathic and eight with tardive cervical dystonia and found no differences in efficacy or dosage used. Other reports with variable results include cases of oromandibular TD (90) and tardive lingual protrusion dystonia (20, 91). The addition of electromyographic or ultrasound guidance can be considered (92). Partial efficacy has also been reported in tardive truncal dystonia, with five patients experiencing a 32% mean subjective improvement in symptoms after treatment with abobotulinumtoxin A injections in the paraspinal or rectus abdominis muscles (93).
Surgical treatment
Deep brain stimulation of the globus pallidus pars interna
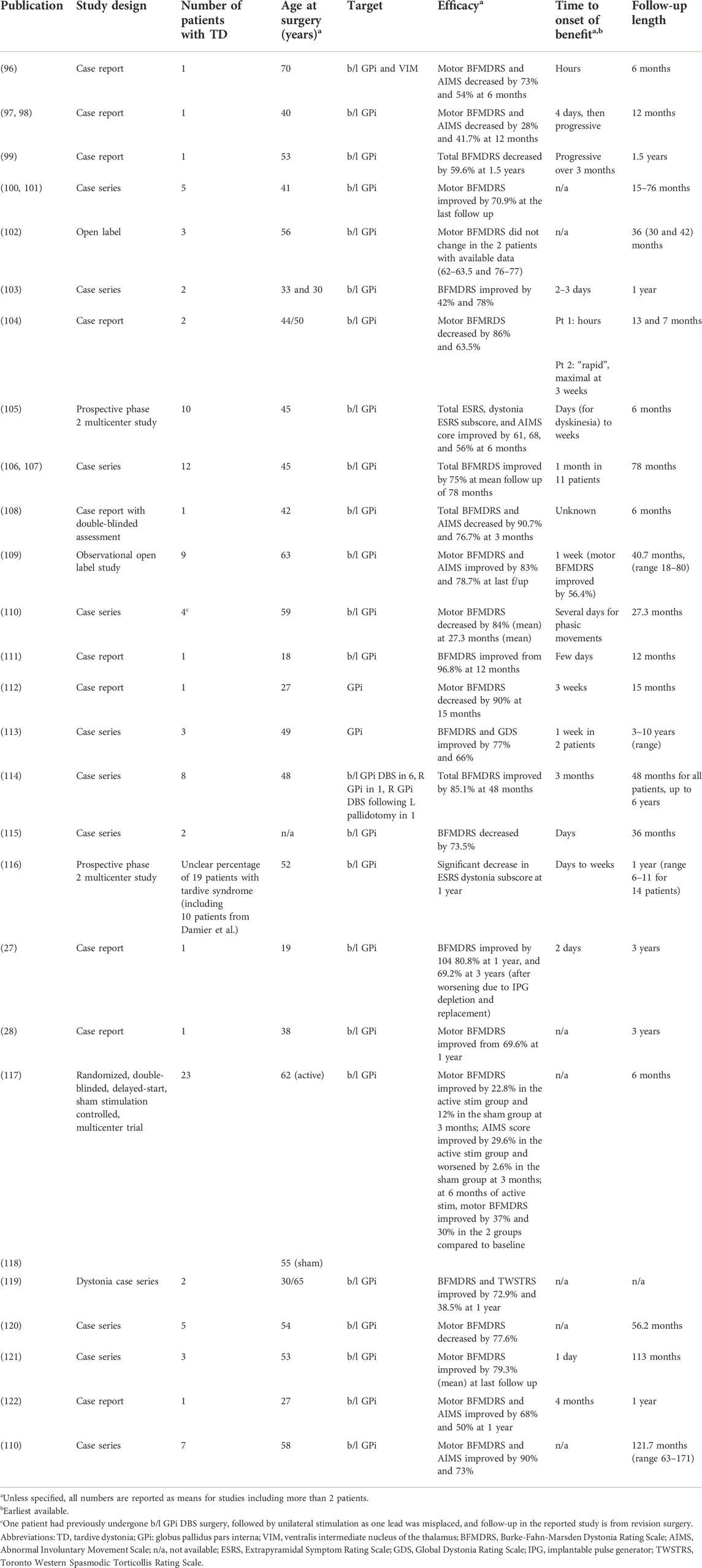
Deep brain stimulation (DBS) of the globus pallidus pars interna (GPi) is an established treatment for isolated dystonia (94, 95), leading to investigations of its use for TD refractory to medications or botulinum toxin injections (Table 2). An initial case report (96) described combined bilateral GPi and ventralis intermediate nucleus of the thalamus (VIM) DBS to treat a 70-year-old woman with tardive dyskinesia and dystonia with blepharospasm and neck and arm involvement. While VIM stimulation was not beneficial, an improvement was noted hours after GPi stimulation was started, and at 6 months the motor Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) and AIMS were decreased by 73% and 54%, respectively. Symptoms worsened after stimulation was discontinued for one to 2 hours. Additional early single cases included a patient with severe camptocormia, who experienced improvement starting 4 days after stimulation initiation, and whose latest reported motor BFMDRS improved by 28% compared to presurgically (97, 98). After 1 month of stimulation, the patient was able to eat in an upright position and at 6 months he could stand upright and walk for 50 feet. The patient’s symptom would reappear after stimulation discontinuation for two to 12 h. Despite the report of better outcomes of GPi DBS or pallidotomy for primary versus secondary dystonia (99), multiple successful open label reports of DBS for TD followed (Table 2).
The STARDYS study group (105) included ten patients with TD with or without associated orofacial tardive dyskinesia, assessed preoperatively and at three and 6 months after bilateral GPi DBS. Each of the postoperative assessments included an evaluation on stimulation and off stimulation on two consecutive days. At the six-month follow-up, these were performed in a double-blinded fashion. Results included improvement of 61% at 6 months in the Extrapyramidal Symptom Rating Scale (ESRS) score (and by a mean of 68% in the dystonia subscore), 56% in the AIMS score compared to baseline. Improvement was noted days or weeks after the beginning of stimulation. An extension of this study including 19 patients with TS showed a 64% improvement of the ESRS dystonia subscore at 12 months (116).
The largest study, and the only randomized double-blind sham-stimulation controlled trial for GPi DBS for TD/TS (117), included 25 patients, of which 23 completed the trial. The subjects were randomized to active stimulation for 6 months or to sham stimulation for 3 months followed by 6 months of active stimulation. The outcome was evaluated by two independent blinded raters of videos obtained at baseline and after three and 6 months of active stimulation. There was no significant difference in improvement of BFMDRS at 3 months (primary outcome) between the groups, except for a significant difference in improvement in the limb dystonia subscore, favoring the active stimulation group. The AIMS score improvement was also significantly better in the active group compared to the sham. Only the active stimulation group exhibited a significant improvement in motor BFMDRS compared to baseline, with a mean improvement of 22.8% at three and of 41.5% at 6 months. Of note, the target sample size was not reached, possibly affecting results. Skin erosion in the sham group, confusion for 3 months with active stimulation, worsening of dystonia during sham stimulation, and extension of hospitalization for dystonia and pulmonary embolism in another patient were the serious adverse events reported. Non-serious events included gait disorder and dysarthria.
The most recent long-term results were reported in seven patients whose mean BFMDRS motor score improved by 90%, while their BFMDRS disability score improved by 79% at a mean follow-up of 121.7 months (122). Mouth and neck dystonia showed the best improvement. Of note, three patients turned their stimulation off with no worsening of dystonia at the last follow-up, after 6–59 months off stimulation.
A study analyzing the different response of various dystonia distributions included six patients undergoing bilateral GPi DBS, one undergoing right GPi DBS, and one patient undergoing right GPI DBS following left pallidotomy (114). Three patients had an improvement above 80% after 1 month, while the mean total BFMDRS improved by 43.6% in that time frame. The mean improvement in BFMDRS at 48 months was 85.1%. One patient had late worsening in dystonia correlated with a depressive episode. The earliest clinically significant improvement, within 3 months, was noted in speech, swallowing, and mouth dystonia, while neck and trunk dystonia had an early but slowly progressive improvement, requiring stimulation adjustments over 48 months in some cases. Upper and lower limb dystonia exhibited results in 18 and 48 months, respectively.
In comparison to the onset of stimulation benefits in isolated dystonia (99, 103), the effects of GPi DBS in TD seem to be evident very rapidly after stimulation is started, with intervals as short as a day (115, 120). Consistently, the effects of stimulation may quickly dissipate, as shortly as 1 week following battery depletion (113, 123). This may lead to severe adverse events including status dystonicus as reported in one patient who experienced abrupt symptom recurrence 3 years after GPi DBS implantation (28).
A negative result for GPi DBS in TD was reported by Krause et al. (102) within a larger case series including primary and secondary dystonia. No benefit was noticed up to 30–42 months of follow-up in two out of three TD patients. One of the patients had her IPG removed in the early postoperative course due to infection and no data was available. A recent case series by Koyama et al. (106) expanded on their previous results (107) and included 12 patients treated with GPi DBS. While the mean improvement in the total BFMDRS score was 77.8% at 1 month and 75% at the last available follow-up, poor efficacy was noted in two patients who were older and had a longer duration of disease prior to surgery.
Regarding psychiatric effects, Gruber et al. (117) reported improvement in patient-rated Hospital Anxiety and Depression total and anxiety sub-scores but worsening in physician-rated scores. The Mattis Dementia Rating Score did not change. Damier et al. (105) reported mood worsening in three out of ten patients, with spontaneous resolution. A case of worsening mania improved with stimulation changes was reported (106). Although not all studies evaluated the psychiatric comorbidities following surgery, the overall lack of severe psychiatric adverse events or worsening of psychiatric comorbidities is reassuring (100, 105, 107, 113, 117, 124). The importance of a stable psychiatric condition and postoperative psychiatric monitoring is emphasized (100).
Deep brain stimulation of the subthalamic nucleus
The subthalamic nucleus (STN) has been reported as a DBS target for TD in a smaller number of patients (125–129). The first reports showed an improvement of more than 90% in motor BFMDRS at 3 months following bilateral STN DBS in two patients with TD (127, 128). One continued to have persistent benefit at 12 years, with ability to turn off the stimulation for several days (130). Deng et al. later reported a series of ten patients treated with bilateral STN DBS achieving a mean improvement of 88.3% in the motor BFMDRS score at the last follow-up time (mean of 65.6 months) (126). There was a microlesion effect in five patients, and a mean improvement of 55.9% in the motor BFMDRS score was obtained after just 1 week of stimulation. One patient experienced a decline in efficacy at the last follow-up, and this was attributed to ventricular dilatation. In one patient, the stimulation could be turned off with no impairment in light exercise or discomfort.
Ablative surgeries
The experience of pallidotomy in patients with TD is quite limited, but may have benefits for psychiatric patients including the limited follow-up required (131). The eligibility to undergo electroconvulsive therapy (ECT), as no hardware is implanted, is also a mentioned advantage (132). However, no clear evidence is available regarding the safety of ECT following DBS lead implantation (133). The first reported bilateral pallidotomy in severe TD (134) resulted in an improvement in BFMDRS from 76 to 21 in motor score and 22 to 4 in disability score. Following case reports showed a 95% improvement in motor BFMDRS in one patient who underwent bilateral pallidotomy following DBS hardware rejection (135), complete resolution after staged bilateral pallidotomy in a case of tardive camptocormia (132), resolution of life-threatening tardive jaw opening dystonia sustained at a follow-up over 2 years (131), and long-term (5 years) improvement of Unified Dystonia Rating Scale from 24 to 2 after left pallidotomy in a patient with tardive dyskinesia and dystonia (136).
Even more rare is the use of thalamotomy, and the few reported cases include two patients out of a larger series, one with moderate improvement after bilateral thalamotomy and one with mild improvement after unilateral thalamotomy (9), and a successful right thalamotomy (137) for tardive dyskinesia and left arm, neck, and truncal dystonia with an improvement to minimal neck dystonia sustained at 12 months.
Discussion
TD is the most debilitating of tardive syndromes, severely impacts quality of life, and is largely irreversible. First described in the 1980s, investigations of its pathophysiology and treatment have been quite limited because it is less common than classical orofacial tardive dyskinesia. The outcome has been frequently delayed diagnosis and lack of effective therapies and little in the way of reasonable research activity and therapeutic evidence. More recently, DBS (targeting the GPi, and secondarily the STN) has been better studied in TD, providing the best body of literature for beneficial TD treatment. While DBS is nowadays vastly implemented for multiple indications, its use in TD is still limited perhaps because of psychiatric comorbidities.
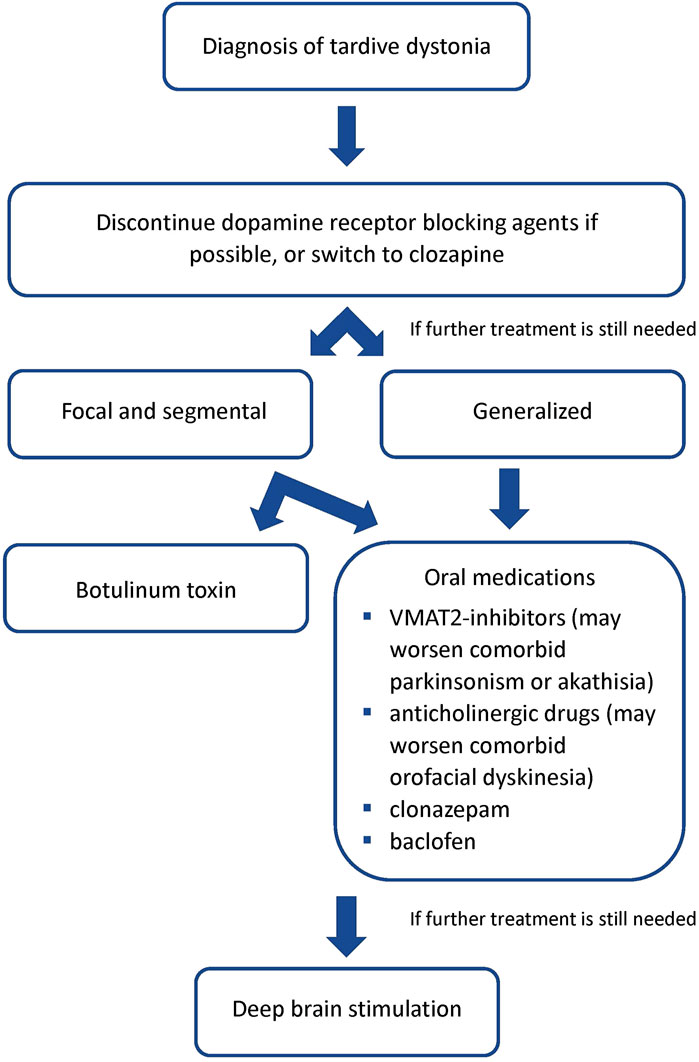
A proposed treatment algorithm is displayed in Figure 1. For treatment of TD patients, we recommend that the first step be the removal of DRBAs. While this is possible in non-psychiatric patients treated with DRBAs for gastrointestinal problems, it is often not feasible in psychiatric patients. In that case efforts should be made, in conjunction with the treating psychiatrist, to change the current antipsychotic regimen in favor of clozapine, which seems to have a unique effect in reversing TD. If after weeks no sufficient improvement is noted, further treatment includes oral medications such as VMAT2-inhibitors, anticholinergic drugs, clonazepam, and baclofen. Among these, the greatest positive experience appears to be with tetrabenazine. However, patients with TD may also present with drug-induced parkinsonism or akathisia, which may worsen with VMAT-2 inhibitors. It should be remembered that classical orofacial tardive dyskinesia may worsen with anticholinergic drugs. For focal dystonia or to address the more incapacitating symptoms in segmental or generalized dystonia, botulinum toxin treatment can be provided using procedures developed for primary dystonia. Finally, we should consider DBS at an earlier stage in severely disabled patients, as current evidence suggests that it is very effective.
Prevention and prompt intervention remain the most important tools to limit this severely detrimental syndrome. We recommend that patients be monitored closely when treated with DRBAs and be prepared to withdraw, switch, or adjust psychiatric medications when symptoms begin to emerge. While this strategy still lacks evidence it certainly is logical. Neurologists should closely question the use of DRBAs when confronted with patients with dystonia in clinic. Additionally, the use of DRBAs for gastrointestinal disorders and migraine should be discouraged.
There are several therapeutic interesting and important research proposals that could bring about useful information in a short period of time to improve treatment of TD. First, clozapine has the potential to be a difference maker in this patient group. It appears to be time to examine its use more closely with a randomized controlled trial. Second, VMAT2 inhibitors have been studied extensively in TS with multicenter trials. The AIMS was the primary efficacy outcome measure. It does not indicate which TS phenotype is present, but videos of all patients were completed. It is possible to review these to find TD cases and examine their response. Finally, DBS is an important approach to treating TD. More studies need to be done to examine the impact of DBS on comorbid psychiatric symptoms, as demonstrating safety in psychiatric populations could increase the opportunity to receive a useful therapy.
Abbreviations
AIMS, Abnormal Involuntary Movement Scale; BFMDRS, Burke-Fahn-Marsden Dystonia Rating Scale; BoNT, botulinum toxin; DBS, Deep brain stimulation; DRBA, dopamine receptor blocking agent; ECT, electroconvulsive therapy; ESRS, Extrapyramidal Symptom Rating Scale; FGA, first generation antipsychotics; GPi, globus pallidus pars interna; ICD, idiopathic cervical dystonia; SGA, second generation antipsychotics; STN, subthalamic nucleus; TD, tardive dystonia; TS, tardive syndromes; VIM, ventralis intermediate nucleus of the thalamus.
Author contributions
PT and SF: Manuscript ideation and writing.
Funding
PT is supported by a Parkinson foundation Fellowship grant. SF is supported by the Sartain Lanier Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AIMS, Abnormal Involuntary Movement Scale; BFMDRS, Burke-Fahn-Marsden Dystonia Rating Scale; BoNT, botulinum toxin; DBS, Deep brain stimulation; DRBA, dopamine receptor blocking agent; ECT, electroconvulsive therapy; ESRS, Extrapyramidal Symptom Rating Scale; FGA, first generation antipsychotics; GPi, globus pallidus pars interna; ICD, idiopathic cervical dystonia; SGA, second generation antipsychotics; STN, subthalamic nucleus; TD, tardive dystonia; TS, tardive syndromes; VIM, ventralis intermediate nucleus of the thalamus.
References
1. Factor, SA. Management of tardive syndrome: Medications and surgical treatments. Neurotherapeutics (2020) 17(4):1694–712. doi:10.1007/s13311-020-00898-3
3. Burke, RE, Fahn, S, Jankovic, J, Marsden, CD, Lang, AE, Gollomp, S, et al. Tardive dystonia: Late-onset and persistent dystonia caused by antipsychotic drugs. Neurology (1982) 32(12):1335–46. doi:10.1212/wnl.32.12.1335
4. Carbon, M, Hsieh, CH, Kane, JM, and Correll, CU. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: A meta-analysis. J Clin Psychiatry (2017) 78(3):e264–e78. doi:10.4088/JCP.16r10832
5. Kane, JM, Woerner, M, and Lieberman, J. Tardive dyskinesia: Prevalence, incidence, and risk factors. J Clin Psychopharmacol (1988) 8(4):57S–6S. doi:10.1097/00004714-198808001-00010
6. Friedman, JH, Kucharski, LT, and Wagner, RL. Tardive dystonia in a psychiatric hospital. J Neurol Neurosurg Psychiatry (1987) 50(6):801–3. doi:10.1136/jnnp.50.6.801
7. Yassa, R, Nair, V, and Iskandar, H. A comparison of severe tardive dystonia and severe tardive dyskinesia. Acta Psychiatr Scand (1989) 80(2):155–9. doi:10.1111/j.1600-0447.1989.tb01319.x
8. van Harten, PN, Matroos, GE, Hoek, HW, and Kahn, RS. The prevalence of tardive dystonia, tardive dyskinesia, parkinsonism and akathisia the curacao extrapyramidal syndromes study: I. Schizophr Res (1996) 19(2-3):195–203. doi:10.1016/0920-9964(95)00096-8
9. Kiriakakis, V, Bhatia, KP, Quinn, NP, and Marsden, CD. The natural history of tardive dystonia. A long-term follow-up study of 107 cases. Brain (1998) 121(11):2053–66. doi:10.1093/brain/121.11.2053
10. Sethi, KD, Hess, DC, and Harp, RJ. Prevalence of dystonia in veterans on chronic antipsychotic therapy. Mov Disord (1990) 5(4):319–21. doi:10.1002/mds.870050411
11. Chouksey, A, and Pandey, S. Clinical spectrum of drug-induced movement disorders: A study of 97 patients. Tremor Other Hyperkinet Mov (N Y) (2020) 10:48. doi:10.5334/tohm.554
12. Friedman, JH. Tardive syndromes. Continuum (2019) 25(4):1081–98. doi:10.1212/CON.0000000000000754
13. Loonen, AJ, and Ivanova, SA. Neurobiological mechanisms associated with antipsychotic drug-induced dystonia. J Psychopharmacol (2021) 35(1):3–14. doi:10.1177/0269881120944156
14. Stahl, SM. Hit-and-Run" actions at dopamine receptors, part 2: Illustrating fast dissociation from dopamine receptors that typifies atypical antipsychotics. J Clin Psychiatry (2001) 62(10):747–8. doi:10.4088/jcp.v62n1001
15. Kang, UJ, Burke, RE, and Fahn, S. Natural history and treatment of tardive dystonia. Mov Disord (1986) 1(3):193–208. doi:10.1002/mds.870010305
16. Jain, R, and Correll, CU. Tardive dyskinesia: Recognition, patient assessment, and differential diagnosis. J Clin Psychiatry (2018) 79(2):nu17034ah1c. doi:10.4088/JCP.nu17034ah1c
17. Skidmore, FWW, and Burke, R. Tardive dyskinesia variants. In: S Factor, A Lang, and W Weiner, editors. Drug induced movement disorders. 2nd ed. Hoboken, NJ: Wiley-Blackwell (2008).
18. Molho, ES, Feustel, PJ, and Factor, SA. Clinical comparison of tardive and idiopathic cervical dystonia. Mov Disord (1998) 13(3):486–9. doi:10.1002/mds.870130319
19. Tan, EK, and Jankovic, J. Tardive and idiopathic oromandibular dystonia: A clinical comparison. J Neurol Neurosurg Psychiatry (2000) 68(2):186–90. doi:10.1136/jnnp.68.2.186
20. Esper, CD, Freeman, A, and Factor, SA. Lingual protrusion dystonia: Frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord (2010) 16(7):438–41. doi:10.1016/j.parkreldis.2010.04.007
21. Zutshi, D, Cloud, LJ, and Factor, SA. Tardive syndromes are rarely reversible after discontinuing dopamine receptor blocking agents: Experience from a university-based movement disorder clinic. Tremor Other Hyperkinet Mov (N Y) (2014) 4:266. doi:10.7916/D8MS3R8C
22. Pinninti, NR, Faden, J, and Adityanjee, A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol (2015) 38(5):183–97. doi:10.1097/WNF.0000000000000106
23. Chen, PL, and Wang, PY. Spondylotic myelopathy in patients with cervical dystonia. J Chin Med Assoc (2012) 75(2):81–3. doi:10.1016/j.jcma.2011.12.003
24. Konrad, C, Vollmer-Haase, J, Anneken, K, and Knecht, S. Orthopedic and neurological complications of cervical dystonia--review of the literature. Acta Neurol Scand (2004) 109(6):369–73. doi:10.1111/j.1600-0404.2004.00281.x
25. Achiron, A, and Melamed, E. Tardive eating dystonia. Mov Disord (1990) 5(4):331–3. doi:10.1002/mds.870050415
26. Ma, CH, Chien, YL, Liu, CC, Chen, IM, and Lin, CH. A case of tardive dystonia associated with long-acting injectable paliperidone palmitate. Eur Neuropsychopharmacol (2016) 26(7):1251–2. doi:10.1016/j.euroneuro.2016.04.006
27. Trezza, A, Antonini, A, Sganzerla, EP, and Landi, A. Globus pallidus internus deep brain stimulation for the treatment of status dystonicus in tardive dystonia. Acta Neurochir (Wien) (2016) 158(9):1789–91. doi:10.1007/s00701-016-2887-0
28. Rohani, M, Munhoz, RP, Shahidi, G, Parvaresh, M, and Miri, S. Fatal status dystonicus in tardive dystonia due to depletion of deep brain stimulation's pulse generator. Brain Stimul (2017) 10(1):160–1. doi:10.1016/j.brs.2016.10.006
29. Bhidayasiri, R, Jitkritsadakul, O, Friedman, JH, and Fahn, S. Updating the recommendations for treatment of tardive syndromes: A systematic review of new evidence and practical treatment algorithm. J Neurol Sci (2018) 389:67–75. doi:10.1016/j.jns.2018.02.010
30. Hasan, A, Falkai, P, Wobrock, T, Lieberman, J, Glenthoj, B, Gattaz, WF, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry (2013) 14(1):2–44. doi:10.3109/15622975.2012.739708
31. Lieberman, JA, Saltz, BL, Johns, CA, Pollack, S, Borenstein, M, and Kane, J. The effects of clozapine on tardive dyskinesia. Br J Psychiatry (1991) 158:503–10. doi:10.1192/bjp.158.4.503
32. Van Harten, PN, Kampuis, DJ, and Matroos, GE. Use of clozapine in tardive dystonia. Prog Neuropsychopharmacol Biol Psychiatry (1996) 20(2):263–74. doi:10.1016/0278-5846(95)00309-6
33. Grover, S, Hazari, N, Kate, N, Chakraborty, K, Sharma, A, Singh, D, et al. Management of tardive syndromes with clozapine: A case series. Asian J Psychiatr (2014) 8:111–4. doi:10.1016/j.ajp.2013.12.016
34. Van Putten, T, Wirshing, WC, and Marder, SR. Tardive Meige syndrome responsive to clozapine. J Clin Psychopharmacol (1990) 10(5):381–2. doi:10.1097/00004714-199010000-00029
35. Blake, LM, Marks, RC, Nierman, P, and Luchins, DJ. Clozapine and clonazepam in tardive dystonia. J Clin Psychopharmacol (1991) 11(4):268–9. doi:10.1097/00004714-199108000-00017
36. Lamberti, JS, and Bellnier, T. Clozapine and tardive dystonia. J Nerv Ment Dis (1993) 181(2):137–8. doi:10.1097/00005053-199302000-00011
37. Friedman, JH. Clozapine treatment of psychosis in patients with tardive dystonia: Report of three cases. Mov Disord (1994) 9(3):321–4. doi:10.1002/mds.870090308
38. Trugman, JM, Leadbetter, R, Zalis, ME, Burgdorf, RO, and Wooten, GF. Treatment of severe axial tardive dystonia with clozapine: Case report and hypothesis. Mov Disord (1994) 9(4):441–6. doi:10.1002/mds.870090411
39. Wolf, ME, and Mosnaim, AD. Improvement of axial dystonia with the administration of clozapine. Int J Clin Pharmacol Ther (1994) 32(6):282–3.
40. Shapleske, J, Mickay, AP, and McKenna, PJ. Successful treatment of tardive dystonia with clozapine and clonazepam. Br J Psychiatry (1996) 168(4):516–8. doi:10.1192/bjp.168.4.516
41. Garcia-Lado, I, Garcia-Caballero, A, Recimil, MJ, Area, R, Ozaita, G, and Lamas, S. Reappearance of tardive dystonia with olanzapine treated with clozapine. Schizophr Res (2005) 76(2-3):357–8. doi:10.1016/j.schres.2004.12.017
42. Aukst-Margetic, B, and Margetic, B. Treatment of generalized tardive dystonia with clozapine. Psychiatr Danub (2008) 20(3):329–31.
43. Kwan, Y, and Sim, K. Resolution of tardive dystonia in a patient with bipolar disorder treated with clozapine: A case report. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34(1):238–9. doi:10.1016/j.pnpbp.2009.09.022
44. Pinninti, NR, Desantis, MJ, and Adityanjee, A. Effectiveness of clozapine in treating severe tardive dystonia and associated pyosis. J Neuropsychiatry Clin Neurosci (2012) 24(1):E5–6. doi:10.1176/appi.neuropsych.11010020
45. Joe, S, Park, J, Lim, J, Park, C, and Ahn, J. Remission of irreversible aripiprazole-induced tardive dystonia with clozapine: A case report. BMC Psychiatry (2015) 15:253. doi:10.1186/s12888-015-0644-1
46. Lee, D, Baek, JH, Bae, M, Choi, Y, and Hong, KS. Long-term response to clozapine and its clinical correlates in the treatment of tardive movement syndromes: A naturalistic observational study in patients with psychotic disorders. J Clin Psychopharmacol (2019) 39(6):591–6. doi:10.1097/JCP.0000000000001114
47. Okamoto, N, Konishi, Y, Tesen, H, Ikenouchi, A, and Yoshimura, R. A low clozapine dose improved refractory tardive dystonia without exacerbating psychiatric symptoms: A case report. Int Med Case Rep J (2021) 14:237–9. doi:10.2147/IMCRJ.S307410
48. Carroll, BJ, Curtis, GC, and Kokmen, E. Paradoxical response to dopamine agonists in tardive dyskinesia. Am J Psychiatry (1977) 134(7):785–9. doi:10.1176/ajp.134.7.785
49. Caine, ED, Polinsky, RJ, Kartzinel, R, and Ebert, MH. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry (1979) 136(3):317–20. doi:10.1176/ajp.136.3.317
50. Mendhekar, DN, and Andrade, C. Prochlorperazine-induced tardive dystonia and its worsening with clozapine in a non-mentally ill patient with migraine. Ann Pharmacother (2011) 45(4):545–6. doi:10.1345/aph.1P194
51. Havaki-Kontaxaki, BJ, Kontaxakis, VP, and Christodoulou, GN. Treatment of tardive pharyngolaryngeal dystonia with olanzapine. Schizophrenia Res (2004) 66(2-3):199–200. doi:10.1016/S0920-9964(03)00093-8
52. Lucetti, C, Bellini, G, Nuti, A, Bernardini, S, Dell'Agnello, G, Piccinni, A, et al. Treatment of patients with tardive dystonia with olanzapine. Clin Neuropharmacol (2002) 25(2):71–4. doi:10.1097/00002826-200203000-00002
53. Jaffe, ME, and Simpson, GM. Reduction of tardive dystonia with olanzapine. Am J Psychiatry (1999) 156(12):2016. doi:10.1176/ajp.156.12.2016
54. Raja, M, Azzoni, A, and Maisto, G. Three cases of improvement of tardive dyskinesia following olanzapine treatment. Int J Neuropsychopharmacol (1999) 2(4):333–4. doi:10.1017/S146114579900156X
55. Chong, SA, Remington, G, and Tan, CH. Risperidone treatment of tardive dyskinesia and dystonia. J Clin Psychiatry (1999) 60(5):340–1. doi:10.4088/jcp.v60n0512i
56. Yoshida, K, Higuchi, H, and Hishikawa, Y. Marked improvement of tardive dystonia after replacing haloperidol with risperidone in a schizophrenic patient. Clin Neuropharmacol (1998) 21(1):68–9.
57. Gourzis, P, Polychronopoulos, P, Papapetropoulos, S, Assimakopoulos, K, Argyriou, AA, and Beratis, S. Quetiapine in the treatment of focal tardive dystonia induced by other atypical antipsychotics: A report of 2 cases. Clin Neuropharmacol (2005) 28(4):195–6. doi:10.1097/01.wnf.0000174933.89758.c9
58. Bouckaert, F, Herman, G, and Peuskens, J. Rapid remission of severe tardive dyskinesia and tardive dystonia with quetiapine. Int J Geriatr Psychiatry (2005) 20(3):287–8. doi:10.1002/gps.1285
59. Sasaki, Y, Kusumi, I, and Koyama, T. A case of tardive dystonia successfully managed with quetiapine. J Clin Psychiatry (2004) 65(4):583–4. doi:10.4088/jcp.v65n0420e
60. Ono, S, Suzuki, Y, Shindo, M, Endo, T, Fukui, N, Sugai, T, et al. Improvement of tardive dyskinesia and dystonia associated with aripiprazole following a switch to quetiapine: Case report and review of the literature. J Clin Pharm Ther (2012) 37(3):370–2. doi:10.1111/j.1365-2710.2011.01290.x
61. De Risio, L, Pettorruso, M, Di Nicola, M, Martinotti, G, Janiri, L, and Fasano, A. Management of aripiprazole-induced tardive pisa syndrome: A case report and literature review. Int Clin Psychopharmacol (2016) 31(1):57–60. doi:10.1097/YIC.0000000000000099
62. Kato, K, Andoh, H, and Matsumoto, H. Case of tardive dystonia improved by aripiprazole. Psychiatry Clin Neurosci (2010) 64(3):337–8. doi:10.1111/j.1440-1819.2010.02092.x
63. Imai, N, and Ikawa, M. Efficacy of aripiprazole in sulpiride-induced tardive oromandibular dystonia. Intern Med (2011) 50(6):635–7. doi:10.2169/internalmedicine.50.4475
64. Takagai, S, Nakasato, K, Suzuki, K, Kasai, E, Isogai, S, Morimoto, S, et al. Improvement in intractable tardive dystonia in bipolar disorder after aripiprazole treatment: A case report. J Clin Psychopharmacol (2012) 32(4):563–4. doi:10.1097/JCP.0b013e31825dde22
65. Huh, L, and Lee, BJ. Efficacy of aripiprazole in antidepressants-induced tardive dystonia and tardive dyskinesia: A case report. Psychiatr Danub (2015) 27(2):195–7.
66. Huntington Study, G. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology (2006) 66(3):366–72. doi:10.1212/01.wnl.0000198586.85250.13
67. Huntington Study, G, Frank, S, Testa, CM, Stamler, D, Kayson, E, Davis, C, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: A randomized clinical trial. JAMA (2016) 316(1):40–50. doi:10.1001/jama.2016.8655
68. Fernandez, HH, Factor, SA, Hauser, RA, Jimenez-Shahed, J, Ondo, WG, Jarskog, LF, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: The ARM-TD study. Neurology (2017) 88(21):2003–10. doi:10.1212/WNL.0000000000003960
69. Anderson, KE, Stamler, D, Davis, MD, Factor, SA, Hauser, RA, Isojarvi, J, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): A double-blind, randomised, placebo-controlled, phase 3 trial. The lancet Psychiatry (2017) 4(8):595–604. doi:10.1016/S2215-0366(17)30236-5
70. O'Brien, CF, Jimenez, R, Hauser, RA, Factor, SA, Burke, J, Mandri, D, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study. Mov Disord (2015) 30(12):1681–7. doi:10.1002/mds.26330
71. Hauser, RA, Factor, SA, Marder, SR, Knesevich, MA, Ramirez, PM, Jimenez, R, et al. Kinect 3: A phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry (2017) 174:476–84. doi:10.1176/appi.ajp.2017.16091037
72. FitzGerald, PM, and Jankovic, J. Tardive oculogyric crises. Neurology (1989) 39(11):1434–7. doi:10.1212/wnl.39.11.1434
73. Jankovic, J, and Beach, J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology (1997) 48(2):358–62. doi:10.1212/wnl.48.2.358
74. Rauchverger, B, Isakov, V, and Jabarin, M. Olanzapine-induced tardive dystonia successfully treated by tetrabenazine. J Neuropsychiatry Clin Neurosci (2007) 19(4):484–5. doi:10.1176/jnp.2007.19.4.484a
75. Friedman, JH. Tardive dystonia due to aripiprazole use in a neuroleptic-naive patient. J Clin Psychiatry (2010) 71(5):652–3. doi:10.4088/JCP.09l05836gre
76. Holla, VV. Speech-induced task-specific cranio-cervical tardive dystonia: An unusual phenomenology. J Mov Disord (2021) 14(1):84–5. doi:10.14802/jmd.20067
77. Thenganatt, MA, and Jankovic, J. Treatment of dystonia. Neurotherapeutics (2014) 11(1):139–52. doi:10.1007/s13311-013-0231-4
78. Citrome, L, and Saklad, SR. Revisiting tardive dyskinesia: Focusing on the basics of identification and treatment. J Clin Psychiatry (2020) 81(2). doi:10.4088/JCP.TV18059AH3C
79. Molho, ES, Factor, SA, Podskalny, GD, and Brown, D. The effect of dancing on dystonia. Mov Disord (1996) 11(2):225–7. doi:10.1002/mds.870110220
80. Lohmann, T, Ferbert, A, and Ebel, H. A unique case of tardive dystonia induced by short-term therapy with perazin. Pharmacopsychiatry (1995) 28(6):263–5. doi:10.1055/s-2007-979614
81. Oommen, E, Chand, PK, and Sharma, PS. Aripiprazole-induced tardive dystonia. Prim Care Companion J Clin Psychiatry (2006) 8(6):378–9. doi:10.4088/pcc.v08n0611c
82. Yamamoto, N, Oda, T, and Inada, T. Methamphetamine psychosis in which tardive dystonia was successfully treated with clonazepam. Psychiatry Clin Neurosci (2007) 61(6):691–4. doi:10.1111/j.1440-1819.2007.01732.x
83. Thaker, GK, Nguyen, JA, Strauss, ME, Jacobson, R, Kaup, BA, and Tamminga, CA. Clonazepam treatment of tardive dyskinesia: A practical GABAmimetic strategy. Am J Psychiatry (1990) 147(4):445–51. doi:10.1176/ajp.147.4.445
84. Dressler, D, Oeljeschlager, RO, and Ruther, E. Severe tardive dystonia: Treatment with continuous intrathecal baclofen administration. Mov Disord (1997) 12(4):585–7. doi:10.1002/mds.870120416
85. Simpson, DM, Hallett, M, Ashman, EJ, Comella, CL, Green, MW, Gronseth, GS, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology (2016) 86(19):1818–26. doi:10.1212/WNL.0000000000002560
86. Spiegel, LL, Ostrem, JL, and Bledsoe, IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). (2020) 12(5):332. doi:10.3390/toxins12050332
87. Kaufman, DM. Use of botulinum toxin injections for spasmodic torticollis of tardive dystonia. J Neuropsychiatry Clin Neurosci (1994) 6(1):50–3. doi:10.1176/jnp.6.1.50
88. Tarsy, D, Kaufman, D, Sethi, KD, Rivner, MH, Molho, E, and Factor, S. An open-label study of botulinum toxin A for treatment of tardive dystonia. Clin Neuropharmacol (1997) 20(1):90–3. doi:10.1097/00002826-199702000-00012
89. Brashear, A, Ambrosius, WT, Eckert, GJ, and Siemers, ER. Comparison of treatment of tardive dystonia and idiopathic cervical dystonia with botulinum toxin type A. Mov Disord (1998) 13(1):158–61. doi:10.1002/mds.870130130
90. Shalash, AS, Abushouk, AI, Elsherbeny, MY, Elrassas, H, and Kamel, T. Refractory open jaw oromandibular tardive dystonia with a sensory trick, treated with botulinum toxin: A case report. Neurol India (2019) 67(4):1110–1. doi:10.4103/0028-3886.266235
91. Hennings, JM, Krause, E, Botzel, K, and Wetter, TC. Successful treatment of tardive lingual dystonia with botulinum toxin: Case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(5):1167–71. doi:10.1016/j.pnpbp.2007.09.010
92. Anandan, C, and Jankovic, J. Botulinum toxin in movement disorders: An update. Toxins (Basel). (2021) 13(1):42. doi:10.3390/toxins13010042
93. Mehta, S, Ray, S, Chakravarty, K, and Lal, V. Spectrum of truncal dystonia and response to treatment: A retrospective analysis. Ann Indian Acad Neurol (2020) 23(5):644–8. doi:10.4103/aian.AIAN_542_20
94. Kupsch, A, Benecke, R, Muller, J, Trottenberg, T, Schneider, GH, Poewe, W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med (2006) 355(19):1978–90. doi:10.1056/NEJMoa063618
95. Bruggemann, N, Kuhn, A, Schneider, SA, Kamm, C, Wolters, A, Krause, P, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology (2015) 84(9):895–903. doi:10.1212/WNL.0000000000001312
96. Trottenberg, T, Paul, G, Meissner, W, Maier-Hauff, K, Taschner, C, and Kupsch, A. Pallidal and thalamic neurostimulation in severe tardive dystonia. J Neurol Neurosurg Psychiatr (2001) 70(4):557–9. doi:10.1136/jnnp.70.4.557
97. Nandi, D, Parkin, S, Scott, R, Winter, JL, Joint, C, Gregory, R, et al. Camptocormia treated with bilateral pallidal stimulation. J Neurosurg (2002) 97(2):461–6. doi:10.3171/jns.2002.97.2.0461
98. Yianni, J, Bain, P, Giladi, N, Auca, M, Gregory, R, Joint, C, et al. Globus pallidus internus deep brain stimulation for dystonic conditions: A prospective audit. Mov Disord (2003) 18(4):436–42. doi:10.1002/mds.10380
99. Eltahawy, HA, Saint-Cyr, J, Giladi, N, Lang, AE, and Lozano, AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: Outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery (2004) 54(3):613–9. discussion 9-21. doi:10.1227/01.neu.0000108643.94730.21
100. Chang, EF, Schrock, LE, Starr, PA, and Ostrem, JL. Long-term benefit sustained after bilateral pallidal deep brain stimulation in patients with refractory tardive dystonia. Stereotact Funct Neurosurg (2010) 88(5):304–10. doi:10.1159/000316763
101. Starr, PA, Turner, RS, Rau, G, Lindsey, N, Heath, S, Volz, M, et al. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: Techniques, electrode locations, and outcomes. Neurosurg Focus (2004) 17(1):E4. doi:10.3171/foc.2004.17.1.4
102. Krause, M, Fogel, W, Kloss, M, Rasche, D, Volkmann, J, and Tronnier, V. Pallidal stimulation for dystonia. Neurosurgery (2004) 55(6):1361–8. discussion 8-70. doi:10.1227/01.neu.0000143331.86101.5e
103. Franzini, A, Marras, C, Ferroli, P, Zorzi, G, Bugiani, O, Romito, L, et al. Long-term high-frequency bilateral pallidal stimulation for neuroleptic-induced tardive dystonia. Report of two cases. J Neurosurg (2005) 102(4):721–5. doi:10.3171/jns.2005.102.4.0721
104. Cohen, OS, Hassin-Baer, S, and Spiegelmann, R. Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Parkinsonism Relat Disord (2007) 13(8):541–4. doi:10.1016/j.parkreldis.2006.11.007
105. Damier, P, Thobois, S, Witjas, T, Cuny, E, Derost, P, Raoul, S, et al. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch Gen Psychiatry (2007) 64(2):170–6. doi:10.1001/archpsyc.64.2.170
106. Koyama, H, Mure, H, Morigaki, R, Miyamoto, R, Miyake, K, Matsuda, T, et al. Long-term follow-up of 12 patients treated with bilateral pallidal stimulation for tardive dystonia. Life (Basel) (2021) 11(6):477. doi:10.3390/life11060477
107. Sako, W, Goto, S, Shimazu, H, Murase, N, Matsuzaki, K, Tamura, T, et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord (2008) 23(13):1929–31. doi:10.1002/mds.22100
108. Kefalopoulou, Z, Paschali, A, Markaki, E, Vassilakos, P, Ellul, J, and Constantoyannis, C. A double-blind study on a patient with tardive dyskinesia treated with pallidal deep brain stimulation. Acta Neurol Scand (2009) 119(4):269–73. doi:10.1111/j.1600-0404.2008.01115.x
109. Gruber, D, Trottenberg, T, Kivi, A, Schoenecker, T, Kopp, UA, Hoffmann, KT, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology (2009) 73(1):53–8. doi:10.1212/WNL.0b013e3181aaea01
110. Capelle, HH, Blahak, C, Schrader, C, Baezner, H, Kinfe, TM, Herzog, J, et al. Chronic deep brain stimulation in patients with tardive dystonia without a history of major psychosis. Mov Disord (2010) 25(10):1477–81. doi:10.1002/mds.23123
111. Kovacs, N, Balas, I, Janszky, J, Simon, M, Fekete, S, and Komoly, S. Status dystonicus in tardive dystonia successfully treated by bilateral deep brain stimulation. Clin Neurol Neurosurg (2011) 113(9):808–9. doi:10.1016/j.clineuro.2011.08.003
112. Trinh, B, Ha, AD, Mahant, N, Kim, SD, Owler, B, and Fung, VS. Dramatic improvement of truncal tardive dystonia following globus pallidus pars interna deep brain stimulation. J Clin Neurosci (2014) 21(3):515–7. doi:10.1016/j.jocn.2013.03.035
113. Woo, PY, Chan, DT, Zhu, XL, Yeung, JH, Chan, AY, Au, AC, et al. Pallidal deep brain stimulation: An effective treatment in Chinese patients with tardive dystonia. Hong Kong Med J (2014) 20(5):455–9. doi:10.12809/hkmj134082
114. Shaikh, AG, Mewes, K, DeLong, MR, Gross, RE, Triche, SD, Jinnah, HA, et al. Temporal profile of improvement of tardive dystonia after globus pallidus deep brain stimulation. Parkinsonism Relat Disord (2015) 21(2):116–9. doi:10.1016/j.parkreldis.2014.11.013
115. Sobstyl, M, Zabek, M, Mossakowski, Z, and Zaczynski, A. Deep brain stimulation of the internal globus pallidus for disabling haloperidol-induced tardive dystonia. Report of two cases. Neurol Neurochir Pol (2016) 50(4):258–61. doi:10.1016/j.pjnns.2016.04.006
116. Pouclet-Courtemanche, H, Rouaud, T, Thobois, S, Nguyen, JM, Brefel-Courbon, C, Chereau, I, et al. Long-term efficacy and tolerability of bilateral pallidal stimulation to treat tardive dyskinesia. Neurology (2016) 86(7):651–9. doi:10.1212/WNL.0000000000002370
117. Gruber, D, Sudmeyer, M, Deuschl, G, Falk, D, Krauss, JK, Mueller, J, et al. Neurostimulation in tardive dystonia/dyskinesia: A delayed start, sham stimulation-controlled randomized trial. Brain Stimul (2018) 11(6):1368–77. doi:10.1016/j.brs.2018.08.006
118. Sharma, VD, Bezchlibnyk, YB, Isbaine, F, Naik, KB, Cheng, J, Gale, JT, et al. Clinical outcomes of pallidal deep brain stimulation for dystonia implanted using intraoperative MRI. J Neurosurg (2019) 133:1582–94. doi:10.3171/2019.6.JNS19548
119. Oliveira, ML, Yan, H, Algarni, M, Elias, JBG, Germann, J, Boutet, A, et al. Probabilistic characterisation of deep brain stimulation in patients with tardive syndromes. J Neurol Neurosurg Psychiatry (2021) 92:909–11324270. doi:10.1136/jnnp-2020-324270
120. Tambirajoo, K, Furlanetti, L, Samuel, M, and Ashkan, K. Globus pallidus internus deep brain stimulation for dystonic opisthotonus in adult-onset dystonia: A personalized approach. Front Hum Neurosci (2021) 15:683545. doi:10.3389/fnhum.2021.683545
121. Bove, F, Piano, C, Bentivoglio, AR, Chiurazzi, P, and Tufo, T. Deep brain stimulation in Fragile X syndrome with tardive dystonia. Neurol Sci (2021) 42(7):2987–9. doi:10.1007/s10072-021-05112-6
122. Krause, P, Kroneberg, D, Gruber, D, Koch, K, Schneider, GH, and Kuhn, AA. Long-term effects of pallidal deep brain stimulation in tardive dystonia: A follow-up of 5-14 years. J Neurol (2022) 269(7):3563–8. doi:10.1007/s00415-022-10965-8
123. Boulogne, S, Danaila, T, Polo, G, Broussolle, E, and Thobois, S. Relapse of tardive dystonia after globus pallidus deep-brain stimulation discontinuation. J Neurol (2014) 261(8):1636–7. doi:10.1007/s00415-014-7404-x
124. M Oliveira, L, Hodaie, M, Yan, H, Algarni, M, J B Elias, G, Germann, J, et al. Probabilistic characterisation of deep brain stimulation in patients with tardive syndromes. J Neurol Neurosurg Psychiatry (2021) 92:909–11. doi:10.1136/jnnp-2020-324270
125. Sun, B, Chen, S, Zhan, S, Le, W, and Krahl, SE. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir Suppl (2007) 97(2):207–14. doi:10.1007/978-3-211-33081-4_23
126. Deng, ZD, Li, DY, Zhang, CC, Pan, YX, Zhang, J, Jin, H, et al. Long-term follow-up of bilateral subthalamic deep brain stimulation for refractory tardive dystonia. Parkinsonism Relat Disord (2017) 41:58–65. doi:10.1016/j.parkreldis.2017.05.010
127. Zhang, JG, Zhang, K, Wang, ZC, Ge, M, and Ma, Y. Deep brain stimulation in the treatment of secondary dystonia. Chin Med J (Engl) (2006) 119(24):2069–74. doi:10.1097/00029330-200612020-00008
128. Zhang, JG, Zhang, K, and Wang, ZC. Deep brain stimulation in the treatment of tardive dystonia. Chin Med J (Engl) (2006) 119(9):789–92. doi:10.1097/00029330-200605010-00017
129. Tai, CH, Tseng, SH, Liu, HM, and Wu, RM. Bilateral deep brain stimulation of subthalamic nucleus alleviates tardive dystonia. Neurology (2006) 66(11):1778–9. doi:10.1212/01.wnl.0000218300.38599.a0
130. Meng, DW, Liu, HG, Yang, AC, Zhang, K, and Zhang, JG. Long-term effects of subthalamic nucleus deep brain stimulation in tardive dystonia. Chin Med J (Engl) (2016) 129(10):1257–8. doi:10.4103/0366-6999.181977
131. Hashimoto, T, Naito, K, Kitazawa, K, Imai, S, and Goto, T. Pallidotomy for severe tardive jaw-opening dystonia. Stereotact Funct Neurosurg (2010) 88(2):105–8. doi:10.1159/000280822
132. Horisawa, S, Oka, M, Kohara, K, Kawamata, T, and Taira, T. Staged bilateral pallidotomy for dystonic camptocormia: Case report. J Neurosurg (2018) 131(3):839–42. doi:10.3171/2018.5.JNS1840
133. Peroski, MS, Chu, MM, Doddi, SR, and Regenold, WT. The safety of electroconvulsive therapy in patients with implanted deep brain stimulators: A review of the literature and case report. J ECT (2019) 35(2):84–90. doi:10.1097/YCT.0000000000000554
134. Weetman, J, Anderson, IM, Gregory, RP, and Gill, SS. Bilateral posteroventral pallidotomy for severe antipsychotic induced tardive dyskinesia and dystonia. J Neurol Neurosurg Psychiatry (1997) 63(4):554–6. doi:10.1136/jnnp.63.4.554a
135. Kohara, K, Taira, T, Horisawa, S, Hanada, T, and Kawamata, T. Bilateral pallidotomy for tardive dystonia:A case report. No Shinkei Geka (2017) 45(11):971–6. doi:10.11477/mf.1436203631
136. Lenders, MW, Buschman, HP, Vergouwen, MD, Steur, EN, Kolling, P, and Hariz, M. Long term results of unilateral posteroventral pallidotomy for antipsychotic drug induced tardive dyskinesia. J Neurol Neurosurg Psychiatry (2005) 76(7):1039. doi:10.1136/jnnp.2004.044438
Keywords: dystonia, tardive, clozapine, botulinum, stimulation
Citation: Testini P and Factor SA (2023) Treatment of tardive dystonia: A review. Dystonia 2:10957. doi: 10.3389/dyst.2023.10957
Received: 05 October 2022; Accepted: 12 January 2023;
Published: 06 February 2023.
Edited by:
Aasef Shaikh, Case Western Reserve University, United StatesCopyright © 2023 Testini and Factor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stewart A. Factor, c2ZhY3RvckBlbW9yeS5lZHU=
 Paola Testini
Paola Testini Stewart A. Factor
Stewart A. Factor