- 1Department of Neurology, Kiel University, Kiel, Germany
- 2Department of Neurology, Research Center Neurosensory Science, School of Medicine and Health Sciences, University of Oldenburg, Oldenburg, Germany
Task specific dystonia belongs to the group of focal dystonias. They are debilitating movement disorders that present with co-contraction of antagonist muscles during a specific task. The most common one is writer’s cramp. Botulinum toxin is the symptomatic standard treatment. Its response rate is 50% after 1 year, and the overall efficacy limited due to unwanted weakness in not injected muscles. The pathophysiology of writer’s cramp remains unclear, but genetic and additional environmental causes have been proposed. A possible underlying mechanism may be maladaptive reorganization in the sensorimotor cortex. Based on this background alternative treatment strategies were developed such as several different sensory and motor training programs that have been applied to reverse these brain abnormalities. In some studies, sensory and motor training were combined and adjunct with fitness exercises. They were conducted either as an outpatient setting or were established home based. Clinical outcome was measured with different clinical scales such as the writer’s cramp rating scale, the arm dystonia rating scale or the Burke, Fahn Marsden Scale. For objective assessment, kinematic handwriting parameters were analyzed. Functional or structural changes of the sensorimotor cortex were estimated using functional magnetic tomography, magnetencephalography and voxel-based morphometry. The results of these training programs were promising; however, one drawback is that the number of patients studied were small and the programs were not controlled since it is difficult to establish a control training to conduct a randomized controlled study.
Introduction
Writer’s cramp (WC) is the most common task specific form of dystonia. With a prevalence of 3.8–80/1,000,000 people it ranks among the orphan diseases (1). The mean age of onset is 38 years. Clinically, patients present with co-contraction of antagonist muscles during writing. In some cases, involuntary flexion of one or several fingers and/or the wrist are the main complaints. Others show extension of their fingers and/or the wrist during writing or the abnormal posture may be accompanied by tremor (2) (see Figure 1). Mirror dystonia may occur in up to 44.6% of patients in the affected, resting hand, when they are asked to write with their contralateral, non - affected hand (3). In simple writer’s cramp co-contraction presents mainly during writing, while in more complex forms other fine motor tasks are also affected (4). In the literature, the complex forms of writer’s cramp have also been referred to as dystonic writer’s cramp because of the occurrence of dystonia during other activities (5). Patients with writer’s cramp often use a high pen pressure and increased axial pressure during writing (6–8). The writing speed and frequency are often reduced and the writing movement is irregular (9). Moreover, with high sensitivity and specificity abnormal word legibility and peak accelerations have been discovered when using a handwriting recognition software to record word legibility in an automated manner (10).
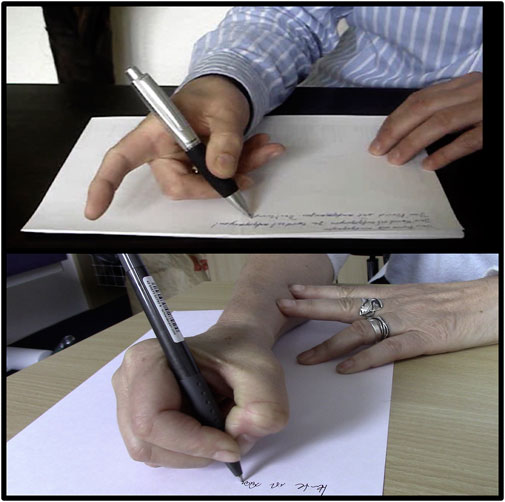
FIGURE 1. Figure shows two different types of writer’s cramp. The upper patient presents with extension of the index finger during writing. In the bottom part of the figure the patient has flexion of the thumb with contraction of flexor pollicis longus muscle.
Task specific dystonia may also affect other professions such as musician’s, golfer’s, typists or hairdressers. In musician’s dystonia the fingers are most commonly affected, rather than the hand. Task specific dystonia impacts patient’s livelihood and has substantial socio-economic impact, causing many patients to give up their profession (11). The standard treatment includes botulinum toxin (BoNT) injections into affected muscles. However, BoNT injections are not always helpful and may cause a number of side effects (12). As alternative treatment approaches training programs have been developed (13). In this review we will focus on these treatment strategies that are based on the current neurophysiological findings and discuss their methodology and outcome. We will also discuss future perspectives.
Pathophysiology of writer’s cramp
The pathophysiology is multifactorial and has been linked to environmental and genetic factors (14–17). It is considered a motor network disorder including the sensorimotor cortex, basal ganglia, thalamus and cerebellum and provides a model for other forms of task specific dystonia areas (18–21). Several studies demonstrated impaired sensory function (13, 22–27), abnormal reorganization of the somatosensory cortex (S1) (28, 29) and sensorimotor integration (30) as result of maladaptive neural plasticity (31), alterations in motor planning with a lack of neuronal inhibition (13, 17), and cerebellar dysfunction (19, 32, 33). These areas are also involved during writing (34–36). Probably, not only the planning of a writing movement is affected (37), but it seems like the parieto-premotor-M1 network, which codes well-trained and skillful tasks, is deficient (38, 39).
The sensory system is deficient in writer’s cramp
Several structural and functional imaging as well as neurophysiological methodologies have been implemented to understand the deficient sensory system in writer’s cramp. The general view is that maladaptive reorganization of the somatotopic finger representations within the primary somatosensory cortex is a pathogenic feature in task specific dystonia.
For example functional connectivity was strengthened between the somatosensory cortical-putamen loop during a visuo-motor control task (40). The putamen seems to play a major role as its grey matter volume is elevated (39, 41). The thalamus, another area that contributes to sensory processing, is considered as a central dysfunctional hub integrating basal ganglia and cerebellar output and gating sensory streams (26). Here, grey matter decreases were found bilaterally in affected patients (42).
Beside imaging studies there are a number of neurophysiological studies that demonstrated abnormalities of the sensory system in writer’s cramp. First, proprioceptive processing was defective as shown in a tonic vibration task (43, 44). Second, contact heat-evoked potentials and pain rating were reduced using quantitative sensory testing (45). Third, tactile information processing was impaired (26). Specifically, temporal (26, 48) and spatial discrimination thresholds (SDT) (22, 48, 49) are increased. Fourth, fine force regulation was disturbed. Patients with writer’s cramp had not only deficits in the coordination of grip and lift load (50), but also in scaling of the precision grip force in a drawer opening task (51). A grip force overshoot during the initial lifts of an unfamiliar object has been described (52). Fifth, patients with writer’s cramp showed increased error, greater variability, and longer release in a force tracking task indicating a generalized deficit in sensorimotor integration (53). Finally, they presented with an abnormal rate of force production and relaxation during wrist movements when measuring peak torque output at the elbow joint and in multiple flexor and extensor muscle groups (54). They had difficulties in adjusting the pressure of their index finger to rather low force plateaus given by visual cues (55). In a probabilistic cued fine motor task writer’s cramp patients were capable of anticipatory adaptation of forces, but could not utilize the decision range in motor planning and adjust their force (23).
Writer’s cramp is a sensorimotor network disorder
Functional and structural imaging studies have advanced our understanding of the pathophysiology in WC (21). Studies examining the morphometric changes in focal dystonia detected either increased or decreased grey matter volume, in some studies bilaterally, in the putamen or globus pallidus (41, 56–59). In patients with writer’s cramp, voxel-based analysis showed larger grey matter volume bilateral in the posterior part of the putamen and globus pallidus (39). Conversely, decreased grey matter density was found in the hand area of the left primary sensorimotor cortex, bilaterally in the cerebellum, and subcortically in the thalamus in the same patient group (42). Diffusion tensor imaging (60) identified lower fractional anisotropy in the tracts between the middle frontal gyrus and putamen (61). In contrast, higher fractional anisotropy has been reported bilaterally between the posterior internal capsule and the ventroposteriolateral thalamic nucleus. Tractography demonstrated that changes involved fiber tracts connecting the primary sensorimotor or the brainstem (62). Finally, more recent work included diffusion-weighted imaging (DWI) and graph theoretical analysis to examine the structural connectome. The structural regional networks in dystonic patients showed a reduction in the number of nodes mainly in the bilateral putamen. In writer’s cramp, the abnormalities occurred bilateral in the insula and the anterior and middle cingulate cortex. The cerebellar vermis, the left cerebellar lobule VIII and the inferior temporal gyrus were also affected. Thus, structural changes in areas that are involved in dystonia represented nodes of a large structural network disruption that were related to areas responsible for sensorimotor planning and processing during writing (18).
Using functional imaging, patients with WC exhibited abnormal blood oxygenation level dependent (BOLD) activity in the sensorimotor cortex, cerebellum and possibly thalamus during writing in an fMRI study (63). The BOLD response was decreased in cortical and subcortical areas during a finger tapping tasks in patients with in simple and/or complex WC compared to controls (39, 64), the hippocampus (65) and the hippocampal-striatal functional connectivity reduced, while the activity was increased in the putamen (65). After practice, premotor-striatal areas, which connectivity correlated with motor performance, were overactive (65). Dynamic causal modelling techniques revealed that patients with WC used the same neural network as healthy individuals during finger tapping, but the effective connectivity patterns differed between both groups. Specifically, the effective connectivity between the globus pallidus’ inhibitory influence on the motor cortex (M1) was weakened while M1 inhibited the putamen stronger in WC. Furthermore, connectivity between M1 and the cerebellum and the cerebellum and the putamen was altered in WC (20). Functional network changes have further been explored using graph theoretical analysis approaches. Here, hub analysis revealed alterations in communication patterns of the primary motor cortex, the thalamus and the cerebellum. Especially the abnormal activity in the cerebellum had been attributed to compensatory rerouting at an early stage of the disease (33) (see Figure 2).
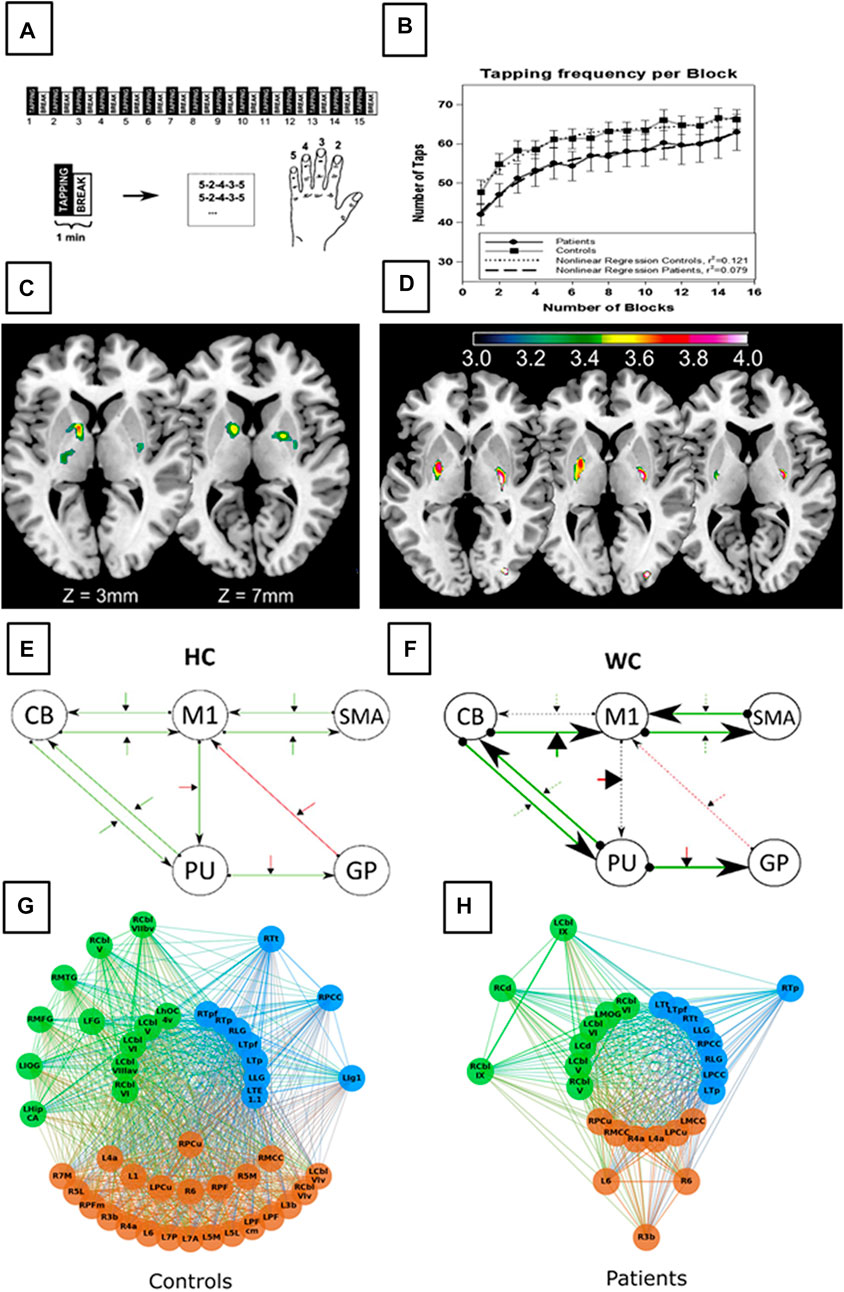
FIGURE 2. Figure displays morphometric alterations and functional disturbances in network communication in WC patients compared to healthy controls (HC). The task included a block-wise executed sequential finger-tapping task (A) of the non-dominate hand to avoid dystonic contraction during tapping. Behavioural results demonstrate comparable motor performance in HC and WC patients (B). Voxel based morphometry demonstrate increase grey matter density within the basal ganglia in WC patients (C). Functional magnetic resonance imaging revealed altered basal ganglia activation (D) in WC patients (putamen and globus pallidus). In addition, altered network functions in WC patients can be demonstrated using dynamic causal modeling (E,F) with stronger task activated connectivity in both, the cortico-cerebellar and the cortico-basal ganglia loops. Graph theorem analysis revealed altered modular hub formation in WC patients. The order of the circles display different hierarchical hubs, starting with the connector hubs in the innermost circle, provincial hubs in the intermediate circle and high influence nodes on the outer circle. WC patients network architecture differs in terms of the number of hubs per network (blue the front-occipital module, green the parietal module and brown a subcortical module) and a reduction in high influence nodes, and provincial hubs. These results together identifie WC as a network disorder even detectable in the non-dystonic hand (Figures 1A–D adapted from Zeuner et al. (39); Figures 1E, F adapted from Rothkirch et al. (20); Figures 1 G, F adapted from Schill et al. (33).
The influence of writing on the cortical and subcortical network
The premotor cortex is involved in planning and monitoring writing movements while the superior parietal cortex controls the somatosensory information and integration into a motor plan (34–36). In an executed writing compared to an imagined writing task the activated network was the same, although during writing the activation of the fronto–parieto-temporal network was clearly more pronounced during the execution of writing in a group of healthy individuals (Figure 3). In contrast, WC patients displayed reduced connectivity between the dorsal premotor and superior parietal cortex (66) that was positively correlated with the severity of dystonic symptoms. They demonstrated a more pronounced BOLD signal in the contralateral sensorimotor cortex, the supplementary- and dorsal premotor cortex as well as in the putamen and thalamus during motor imagery of writing (66). Motor imagery of grasping a pencil for writing lead to an elevated BOLD response in the supplementary-, dorsal pre- and motor cortex (BA 6) indicating alterations in planning a writing movement (37). In the task specific network of the dominant hand, WC patients showed a deficient parieto-premotor-M1 network, which codes well-trained and skillful tasks (38, 39). The cerebellum (vermis und lobulus VI) and the anterior, associative putamen showed decreased activation in writer’s cramp patients that were unrelated to the specific task (38).
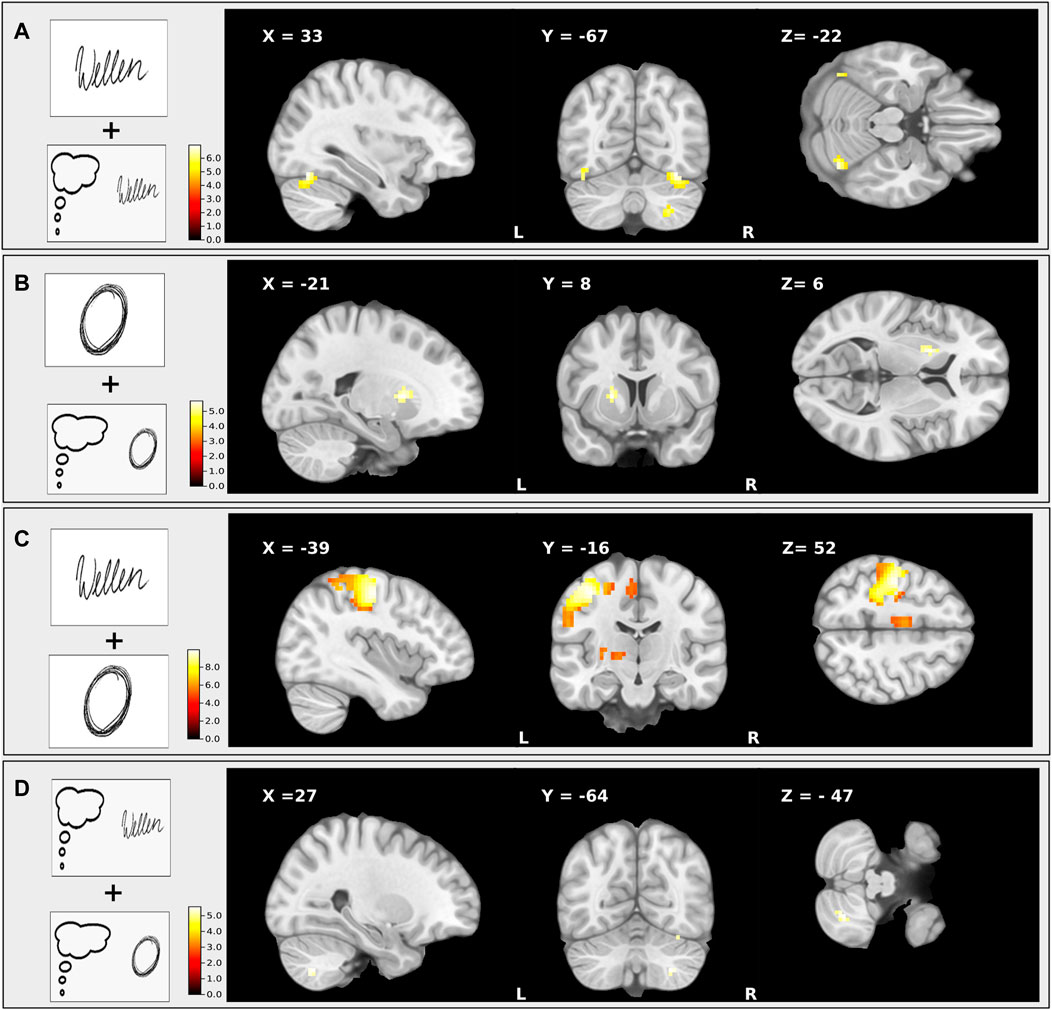
FIGURE 3. This figure shows the T-maps of four different conjunction contrasts: (A) executed writing and imagined writing were accompanied by an increased activity of the ipsilateral cerebellum and the contralateral sensorimotor cortex; (B) executed drawing and imagined drawing revealed elevated activity of a fronto–parieto-temporal network; (C) executed writing and executed drawing induced an enhanced activation of the left somatosensory and premotor area; (D) imagined writing and imagined drawing revealed a higher involvement of occipital activation during. The clusters were significant within the ROI analysis after FWE-correction on p < 0.05. This figure is adapted from Baumann et al. (36).
Increased excitability and loss of inhibition in writer’s cramp
Reduced inhibitory control over cortical motor areas might be responsible for sustained muscle contraction in patients with writer’s cramp (31). For decades, not only loss of inhibition, but also increased excitability at multiple levels have been discussed as an underlying pathophysiological feature in patients with writer’s cramp (67). This includes the motor cortex (M1), premotor cortex, somatosensory cortex (S1), and the cerebellum. Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter in the brain. GABAergic deficits have been described in the sensorimotor cortex and in the cerebellum and might be an explanation for the loss of inhibitory control resulting from maladaptive plasticity and abnormal surround inhibition (68).
Treatment of writer’s cramp
Standard treatment of writer’s cramp
The standardized therapy, botulinum neurotoxin (BoNT) injections into affected muscles, has been shown to be effective in a number of uncontrolled (69) and one randomized, placebo controlled study (12, 70). Kruisdjik et al. (2007) included 40 patients writer’s cramp (12). Their patients received either BoNT or placebo EMG guided in a randomized manner twice with 1 month apart in case they were unsatisfied with the treatment effect. The primary outcome measure was the patients’ decision to either continue or stop the injections after 3 months. They were followed for a year. Fourteen from 20 patients reported a positive effect from BoNT and chose to continue the treatment, while in the placebo group 13 of 19 patients wished to stop with the injections. The positive results were supported by improvements in the visual analogue scale (writing), the writer’s cramp rating scale, the writing speed and the symptom severity scale. Ideally, the application of the toxin should be performed with ultrasound or with stimulation through the EMG needle in order to target the correct muscle for injection (69, 71, 72). However, most patients display several ways to compensate their dystonic posture, so frequently it is difficult to identify the dystonic muscles (70). Hence, just 50% of patients, who were treated for 1 year (12) decided to continue with BoNT treatment. In a retrospective 10 years follow-up, only 20/214 patients continued this treatment. The reasons for discontinuation included a lack of treatment efficacy or involuntary paresis in not injected muscles probably caused by spreading of the toxin into neighboring muscles. Affected patients might then show a paresis in both, the injected and non-injected muscles, which can result in restraints in their daily activities that are more severe than the disability in writing (73).
Sensorimotor training based on pathophysiological concepts
Alternative treatment approaches based on the pathophysiology of writer’s cramp have been developed. This includes re-training programs with different concepts. The purpose of these training programs was to achieve a long-term effect.
One of the first training programs that had been developed aimed at re-organizing the disturbed somatosensory maladaptation and disorganization in the sensory cortex by implementing a sensory training with Braille reading (74). As an objective parameter the grating orientation task was used. Patients improved their writing and their performance in the grating orientation task, but a long-term effect remained only as long as they trained (75).
In subsequent motor training programs with (76) or without precedent immobilization (77, 78) patients learned individualized finger movements. Immobilization was carried out continuously with a splint for the wrist and the finger joints. Patients with writer’s cramp have difficulties in activating fingers individually, because they often present with co-contraction. Therefore, therapeutic putty has been used to teach patients moving each finger separately. Pathophysiologically the intention was to reverse cortical disorganization, enhance the training efficacy and normalize sensorimotor integration. Their efficacy on writing was measured with kinematic analyses of writing movements (6, 9, 76–78). The purpose of those motor training programs was to decrease the abnormally increased writing pressure (6), disturbed regularity of movement kinematics (9, 78) and dystonic symptoms (6, 76, 78). Training without immobilization led to mild improvement in simple handwriting parameters (77), while the combination of a 4 weeks forearm immobilization with subsequent re-training over 8 weeks improved the writing fluency, and patients exerted less vertical force on the digitizing tablet (76). In addition, there was significantly clinical improvement as indexed by a decrease in the writer’s cramp rating scale (79) and an increase in the arm dystonia rating scale (80). Transcranial magnetic stimulation (TMS) was implemented to test concurrent changes in regional excitability. Grey matter changes in the contralateral primary motor hand area (M1HAND) were evaluated utilizing voxel-based morphometry (VBM). Specifically, either suppression of regional excitability or grey matter decrease after immobilization or enhancement and grey matter increase after re-training of the right, dominant hand were analyzed in the contralateral left M1HAND area. While immobilization reduced corticomotor excitability and caused relative grey matter decrease in the contralateral left M1HAND, subsequent training reversed the effects of immobilization, causing an increase in regional grey matter density and excitability (41).
Sensory discriminative and motor training has also been combined with fitness and resulted in clinical gains of motor control, motor accuracy, sensory discrimination and physical performance (81, 82). After 6 months, all 11 patients were contacted. Ten out of 11 patients returned to work, and the improvement was scored 84%–90% for the specific task that was impaired. Patients, whose training was supervised, showed a better outcome (81). Enlarged and disorganized hand representations were reversed after prolonged rehabilitation of 5.5 months investigated with magnetencephalography (MEG) and 3D-MRI 3D brain reconstructions that were paralleled with clinical recovery and improved writing performance (83). There was, however no long-term follow up, so it remains unclear, whether patients continued their training, and how long the positive training effect lasted. That program included several different training aspects including relaxation techniques, elementary movement and postures correction, pen control. Several studies started with simple movements and progressed to more complex movements during the course of the training (6, 76, 78, 83).
Biofeedback to reverse abnormally high muscle activity
Biofeedback is another technique that had been used to treat writer’s cramp. This approach was based on faulty inhibition due to the abnormal reorganization in the sensorimotor cortex. Therefore, the training approach was to teach patients active inhibition of proximal muscles and thus reduce the overflow of motor areas pertaining to them. The authors implemented auditorial EMG feedback from affected muscles with abnormally high activity during writing. This feedback served as a control measure to teach patients writing in a relaxed manner (84). Patients received practice sessions once every 2 weeks and instructions for a home-based training program. On a visual analogue scale, nine of 10 patients reported an overall improvement ranging from 37.5% to 93.4% (84). To reverse decreased striatal D2 receptor-binding, as measured with single-photon emission computed tomography (SPECT) with [123I]iodobenzamide (IBZM), patients were instructed to visually maintain the EMG motor unit amplitude at the minimum possible level while writing exercises (85). The authors concluded from their data that with a biofeedback-based sensorimotor training it was possible to reorganize the activity in the nigrostriatal dopaminergic pathway (85).
A different biofeedback method that lead to a significant improvement in writing was auditory grip force feedback (86). In that study the authors used a pen wrapped with a force sensor matrix. Writer’s cramp patients received 7 h training sessions that was distributed over several weeks. They performed the specialized training with a standard pen over 50 min, and for the last 10 minutes with the force sensor matrix wrapped writing stylus to measure grip force and to give auditory grip force feedback. Patients improved their handwriting performance, the writing pressure, and reported to have less pain (86).
Non-invasive brain stimulation techniques to reverse abnormal excitability
Furthermore, non-invasive stimulation techniques have been investigated using repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) (67, 87–92). The purpose was to reduce deficient inhibition. Low- (<1 Hz) frequency TMS decreases cortical excitability and had been applied in patients with writer’s cramp to improve handwriting. The area that had been stimulated included the premotor cortex, the primary motor cortex and the supplementary motor area with the intention to improve handwriting and pen pressure (69, 87–91). In most studies showed short-term positive effects after 15–30 min and in some studies 10–15 days post stimulation (67). More recent studies imply that repetitive sessions over consecutive days are necessary to achieve therapeutic effects (67). In a tDCS study that applied anodal, cathodal, or sham tDCS to the cerebellum the authors reported improved writing kinematics with reduced cerebellar inhibition with anodal tDCS (92). However, there are a number of tDCS studies with negative results according to clinical parameters that were investigated (67).
Discussion and future directions
Writer’s cramp is a rare task specific form of dystonia. In their medical history, many affected patients report long daily writing hours and often they have professions, in which writing is required. Therefore, the impact on patients’ everyday life is tremendous, especially when they are working. BoNT offers one treatment possibility. However, over the years, a high percentage of patients refrain from BoNT injections. The main reasons are that this treatment form is not efficient for the patient’s daily life or the side effects are not tolerable. For example, severe paresis may occur in the injected muscles. Sometimes the toxin spreads to neighboring muscles causing involuntary paresis and can cause constraints in daily life, even if writing has improved.
Therefore, based on the pathophysiology, several rehabilitative treatment approaches have been suggested (69). Studies with re-training programs for several weeks were designed to reverse abnormal sensorimotor reorganization and to achieve long-lasting therapeutic effects. In a subsequent study biofeedback was combined with a re-training program to improve muscle co-contraction. Low-frequency rTMS studies have to be considered as short -term projects. Positive effects were measured during the first 30 min post-stimulation or a maximum of 15 days. There is ongoing discussion, whether the stimulation has to be repeated after a certain time to achieve long-term results in rTMS (67).
Currently, writer’s cramp is considered as a network disorder affecting the sensory system, the motor system, the basal ganglia and the cerebellum. Especially, maladaptive reorganization in the sensory cortex (30), increased excitation or faulty inhibition have been reported in a number of studies (13, 17, 31). However, this view has been challenged due to the development of new technologies for examining brain region reorganization and it is expected that further advances will be made leading to additional changes in our understanding. For example, normal digit representation maps between fingers in patients with musician’s dystonia have been reported very recently using an optimized spatial metric multivariate pattern analysis (93). So, perhaps, as we get a deeper understanding of the pathophysiology, it is necessary to reconsider current treatment approaches. This may also apply to the way, how writing has been evaluated. Mostly, a kinematic writing analysis has been used, and parameters such as increased axial pressure, decreased writing frequency, and irregular writing movements have been found to be characteristic features in writer’s camp (9, 86). On the other hand, a handwriting recognition software has been recently applied for writer’s cramp patients to evaluate word legibility in an automated manner. In that study, patients with writer’s cramp showed abnormal word legibility and peak accelerations (10) with high sensitivity and specificity, but mean axial pen pressure, average velocity, stroke length, or CV of peak vertical velocity were normal. Therefore, it is conceivable that re-training should focus on alternative aspects of writing.
One major problem that applies to all treatment studies is the small number of patients. It is not possible to compare any occupational treatment to normal controls, therefore a randomized controlled study is not feasible. Another challenge is to establish a control training. It is impossible to introduce a control motor training, because any motor training will always have an influence on the sensorimotor network, independently of its specificity. A control training that includes no motor aspects might unblind writer’s cramp patients, who expect some way of motor intervention to treat this motor disorder.
Therefore, future studies with new technologies have to be performed with larger sample sizes to either confirm or reject the current view of the pathophysiology. Depending on the results, the rehabilitative treatment approaches have to be reassessed and adapted. Ideally, future treatment studies should be performed in a multicentre setting with a higher number of patients and a control training needs to established.
Author contributions
KEZ developed the concept and wrote the first draft of the manuscript. AB and KW reviewed and revised the manuscript for content. In addition, they provided the figures.
Conflict of interest
KEZ has received research support from the Christa and Hans-Peter Thomsen Foundation, the German Research Foundation (DFG 5919/4-1) and from Strathmann GmbH & Co. KG. She reports speaker’s honoraria from Bayer Vital GmbH, BIAL, Alexion, AbbVie Allergan and Merz outside the submitted work. She has served as a consultant and received fees from Merz, Ipsen, Alexion and the German Federal Institute for Drugs and Medical Devices (BfArM). KW receives research support for the German Research Foundation (DFG GK 2783) and form STADAPHARM. He serves as a consultant for BIAL and receives speaker’s honoraria form BIAL, STADAPHARM and Boston Scientific.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Defazio, G, Berardelli, A, and Hallett, M. Do primary adult-onset focal dystonias share aetiological factors? Brain (2007) 130(5):1183–93. doi:10.1093/brain/awl355
3. Jedynak, PC, Tranchant, C, and de Beyl, DZ. Prospective clinical study of writer's cramp. Mov Disord (2001) 16(3):494–9. doi:10.1002/mds.1094
4. Stahl, CM, and Frucht, SJ. Focal task specific dystonia: A review and update. J Neurol (2017) 264(7):1536–41. doi:10.1007/s00415-016-8373-z
5. Sheehy, MP, and Marsden, CD. Writers' cramp-a focal dystonia. Brain (1982) 105(3):461–80. doi:10.1093/brain/105.3.461
6. Baur, B, Furholzer, W, Jasper, I, Marquardt, C, and Hermsdorfer, J. Effects of modified pen grip and handwriting training on writer's cramp. Arch Phys Med Rehabil (2009) 90(5):867–75. doi:10.1016/j.apmr.2008.10.015
7. Hermsdorfer, J, Marquardt, C, Schneider, AS, Furholzer, W, and Baur, B. Significance of finger forces and kinematics during handwriting in writer's cramp. Hum Mov Sci (2011) 30(4):807–17. doi:10.1016/j.humov.2010.04.004
8. Schneider, AS, Furholzer, W, Marquardt, C, and Hermsdorfer, J. Task specific grip force control in writer's cramp. Clin Neurophysiol (2014) 125(4):786–97. doi:10.1016/j.clinph.2013.09.043
9. Zeuner, KE, Peller, M, Knutzen, A, Holler, I, Munchau, A, Hallett, M, et al. How to assess motor impairment in writer's cramp. Mov Disord (2007) 22(8):1102–9. doi:10.1002/mds.21294
10. Bukhari-Parlakturk, N, Lutz, MW, Al-Khalidi, HR, Unnithan, S, Wang, JE, Scott, B, et al. Suitability of automated writing measures for clinical trial outcome in writer's cramp. Mov Disord (2022). doi:10.1002/mds.29237
11. Amini, D. Occupational therapy interventions for work-related injuries and conditions of the forearm, wrist, and hand: A systematic review. Am J Occup Ther (2011) 65(1):29–36. doi:10.5014/ajot.2011.09186
12. Kruisdijk, JJ, Koelman, JH, Ongerboer de Visser, BW, de Haan, RJ, and Speelman, JD. Botulinum toxin for writer's cramp: A randomised, placebo-controlled trial and 1-year follow-up. J Neurol Neurosurg Psychiatry (2007) 78(3):264–70. doi:10.1136/jnnp.2005.083170
13. Zeuner, KE, and Molloy, FM. Abnormal reorganization in focal hand dystonia--sensory and motor training programs to retrain cortical function. NeuroRehabilitation (2008) 23(1):43–53. doi:10.3233/nre-2008-23105
14. Lohmann, K, Schmidt, A, Schillert, A, Winkler, S, Albanese, A, Baas, F, et al. Genome-wide association study in musician's dystonia: A risk variant at the arylsulfatase G locus? Mov Disord (2014) 29(7):921–7. doi:10.1002/mds.25791
15. Lange, LM, Junker, J, Loens, S, Baumann, H, Olschewski, L, Schaake, S, et al. Genotype-phenotype relations for isolated dystonia genes: Mdsgene systematic review. Mov Disord (2021) 36(5):1086–103. doi:10.1002/mds.28485
16. Zeuner, KE, Acewicz, A, Knutzen, A, Dressler, D, Lohmann, K, and Witt, K. Dopamine Drd2 polymorphism (Drd2/Annk1-Taq1a) is not a significant risk factor in writer's cramp. J Neurogenet (2016) 30(3-4):276–9. doi:10.1080/01677063.2016.1238916
17. Hallett, M. Pathophysiology of writer's cramp. Hum Mov Sci (2006) 25(4-5):454–63. doi:10.1016/j.humov.2006.05.004
18. Hanekamp, S, and Simonyan, K. The large-scale structural connectome of task-specific focal dystonia. Hum Brain Mapp (2020) 41(12):3253–65. doi:10.1002/hbm.25012
19. Neychev, VK, Fan, X, Mitev, VI, Hess, EJ, and Jinnah, HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain (2008) 131(9):2499–509. doi:10.1093/brain/awn168
20. Rothkirch, I, Granert, O, Knutzen, A, Wolff, S, Govert, F, Pedersen, A, et al. Dynamic causal modeling revealed dysfunctional effective connectivity in both, the cortico-basal-ganglia and the cerebello-cortical motor network in writers' cramp. Neuroimage Clin (2018) 18:149–59. doi:10.1016/j.nicl.2018.01.015
21. Simonyan, K. Neuroimaging applications in dystonia. Int Rev Neurobiol (2018) 143:1–30. doi:10.1016/bs.irn.2018.09.007
22. Molloy, FM, Carr, TD, Zeuner, KE, Dambrosia, JM, and Hallett, M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain (2003) 126(10):2175–82. doi:10.1093/brain/awg219
23. Zeuner, KE, Knutzen, A, Granert, O, Trampenau, L, Baumann, A, Wolff, S, et al. Never too little: Grip and lift forces following probabilistic weight cues in patients with writer's cramp. Clin Neurophysiol (2021) 132(12):2937–47. doi:10.1016/j.clinph.2021.09.010
24. Todt, I, Baumann, A, Knutzen, A, Granert, O, Tzvi, E, Lindert, J, et al. Abnormal effective connectivity in the sensory network in writer's cramp. Neuroimage Clin (2021) 31:102761. doi:10.1016/j.nicl.2021.102761
25. Merchant, SHI, Frangos, E, Parker, J, Bradson, M, Wu, T, Vial-Undurraga, F, et al. The role of the inferior parietal lobule in writer's cramp. Brain (2020) 143(6):1766–79. doi:10.1093/brain/awaa138
26. Conte, A, Defazio, G, Hallett, M, Fabbrini, G, and Berardelli, A. The role of sensory information in the pathophysiology of focal dystonias. Nat Rev Neurol (2019) 15(4):224–33. doi:10.1038/s41582-019-0137-9
27. Conte, A, Ferrazzano, G, Belvisi, D, Manzo, N, Suppa, A, Fabbrini, G, et al. Does the somatosensory temporal discrimination threshold change over time in focal dystonia? Neural Plast (2017) 2017:9848070. doi:10.1155/2017/9848070
28. Garraux, G, Bauer, A, Hanakawa, T, Wu, T, Kansaku, K, and Hallett, M. Changes in brain anatomy in focal hand dystonia. Ann Neurol (2004) 55(5):736–9. doi:10.1002/ana.20113
29. Lerner, A, Shill, H, Hanakawa, T, Bushara, K, Goldfine, A, and Hallett, M. Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. Neuroimage (2004) 21(3):904–13. doi:10.1016/j.neuroimage.2003.10.019
30. Patel, N, Jankovic, J, and Hallett, M. Sensory aspects of movement disorders. Lancet Neurol (2014) 13(1):100–12. doi:10.1016/S1474-4422(13)70213-8
31. Quartarone, A, and Hallett, M. Emerging concepts in the physiological basis of dystonia. Mov Disord (2013) 28(7):958–67. doi:10.1002/mds.25532
32. Shakkottai, VG, Batla, A, Bhatia, K, Dauer, WT, Dresel, C, Niethammer, M, et al. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum (2017) 16(2):577–94. doi:10.1007/s12311-016-0825-6
33. Schill, J, Zeuner, KE, Knutzen, A, Todt, I, Simonyan, K, and Witt, K. Functional neural networks in writer's cramp as determined by graph-theoretical analysis. Front Neurol (2021) 12:744503. doi:10.3389/fneur.2021.744503
34. Planton, S, Jucla, M, Roux, FE, and Demonet, JF. The "handwriting brain": A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex (2013) 49(10):2772–87. doi:10.1016/j.cortex.2013.05.011
35. Planton, S, Longcamp, M, Peran, P, Demonet, JF, and Jucla, M. How specialized are writing-specific brain regions? An fmri study of writing, drawing and oral spelling. Cortex (2017) 88:66–80. doi:10.1016/j.cortex.2016.11.018
36. Baumann, A, Todt, I, Knutzen, A, Gless, CA, Granert, O, Wolff, S, et al. Neural correlates of executed compared to imagined writing and drawing movements: A functional magnetic resonance imaging study. Front Hum Neurosci (2022) 16:829576. doi:10.3389/fnhum.2022.829576
37. Delnooz, CC, Helmich, RC, Medendorp, WP, Van de Warrenburg, BP, and Toni, I. Writer's cramp: Increased dorsal premotor activity during intended writing. Hum Brain Mapp (2013) 34(3):613–25. doi:10.1002/hbm.21464
38. Gallea, C, Horovitz, SG, Najee-Ullah, M, and Hallett, M. Impairment of a parieto-premotor network specialized for handwriting in writer's cramp. Hum Brain Mapp (2016) 37(12):4363–75. doi:10.1002/hbm.23315
39. Zeuner, KE, Knutzen, A, Granert, O, Gotz, J, Wolff, S, Jansen, O, et al. Increased volume and impaired function: The role of the basal ganglia in writer's cramp. Brain Behav (2015) 5(2):e00301. doi:10.1002/brb3.301
40. Moore, RD, Gallea, C, Horovitz, SG, and Hallett, M. Individuated finger control in focal hand dystonia: An fmri study. Neuroimage (2012) 61(4):823–31. doi:10.1016/j.neuroimage.2012.03.066
41. Granert, O, Peller, M, Gaser, C, Groppa, S, Hallett, M, Knutzen, A, et al. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage (2011) 54(1):32–41. doi:10.1016/j.neuroimage.2010.08.013
42. Delmaire, C, Vidailhet, M, Elbaz, A, Bourdain, F, Bleton, JP, Sangla, S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology (2007) 69(4):376–80. doi:10.1212/01.wnl.0000266591.49624.1a
43. Kaji, R, Rothwell, JC, Katayama, M, Ikeda, T, Kubori, T, Kohara, N, et al. Tonic vibration reflex and muscle afferent block in writer's cramp. Ann Neurol (1995) 38(2):155–62. doi:10.1002/ana.410380206
44. Trompetto, C, Curra, A, Buccolieri, A, Suppa, A, Abbruzzese, G, and Berardelli, A. Botulinum toxin changes intrafusal feedback in dystonia: A study with the tonic vibration reflex. Mov Disord (2006) 21(6):777–82. doi:10.1002/mds.20801
45. Suttrup, I, Oberdiek, D, Suttrup, J, Osada, N, Evers, S, and Marziniak, M. Loss of sensory function in patients with idiopathic hand dystonia. Mov Disord (2011) 26(1):107–13. doi:10.1002/mds.23425
46. Conte, A, Rocchi, L, Ferrazzano, G, Leodori, G, Bologna, M, Li Voti, P, et al. Primary somatosensory cortical plasticity and tactile temporal discrimination in focal hand dystonia. Clin Neurophysiol (2014) 125(3):537–43. doi:10.1016/j.clinph.2013.08.006
47. Fiorio, M, Tinazzi, M, Bertolasi, L, and Aglioti, SM. Temporal processing of visuotactile and tactile stimuli in writer's cramp. Ann Neurol (2003) 53(5):630–5. doi:10.1002/ana.10525
48. Sanger, TD, Tarsy, D, and Pascual-Leone, A. Abnormalities of spatial and temporal sensory discrimination in writer's cramp. Mov Disord (2001) 16(1):94–9. doi:10.1002/1531-8257(200101)16:1<94::aid-mds1020>3.0.co;2-o
49. Bara-Jimenez, W, and Shelton, HM. Spatial discrimination is abnormal in focal hand dystonia. Neurology (2000) 55(12):1869–73. doi:10.1212/wnl.55.12.1869
50. Odergren, T, Iwasaki, N, Borg, J, and Forssberg, H. Impaired sensory-motor integration during grasping in writer's cramp. Brain (1996) 119(2):569–83. doi:10.1093/brain/119.2.569
51. Serrien, DJ, Burgunder, JM, and Wiesendanger, M. Disturbed sensorimotor processing during control of precision grip in patients with writer's cramp. Mov Disord (2000) 15(5):965–72. doi:10.1002/1531-8257(200009)15:5<965::aid-mds1030>3.0.co;2-0
52. Nowak, DA, and Hermsdorfer, J. Grip force behavior during object manipulation in neurological disorders: Toward an objective evaluation of manual performance deficits. Mov Disord (2005) 20(1):11–25. doi:10.1002/mds.20299
53. Bleton, JP, Teremetz, M, Vidailhet, M, Mesure, S, Maier, MA, and Lindberg, PG. Impaired force control in writer's cramp showing a bilateral deficit in sensorimotor integration. Mov Disord (2014) 29(1):130–4. doi:10.1002/mds.25690
54. Prodoehl, J, MacKinnon, CD, Comella, CL, and Corcos, DM. Rate of force production and relaxation is impaired in patients with focal hand dystonia. Parkinsonism Relat Disord (2006) 12(6):363–71. doi:10.1016/j.parkreldis.2006.01.008
55. Zeuner, KE, Knutzen, A, Pedack, L, Hallett, M, Deuschl, G, and Volkmann, J. Botulinum neurotoxin treatment improves force regulation in writer's cramp. Parkinsonism Relat Disord (2013) 19(6):611–6. doi:10.1016/j.parkreldis.2013.02.011
56. Bradley, D, Whelan, R, Walsh, R, Reilly, RB, Hutchinson, S, Molloy, F, et al. Temporal discrimination threshold: Vbm evidence for an endophenotype in adult onset primary torsion dystonia. Brain (2009) 132(9):2327–35. doi:10.1093/brain/awp156
57. Egger, K, Mueller, J, Schocke, M, Brenneis, C, Rinnerthaler, M, Seppi, K, et al. Voxel based morphometry reveals specific grey matter changes in primary dystonia. Mov Disord (2007) 22(11):1538–42. doi:10.1002/mds.21619
58. Etgen, T, Muhlau, M, Gaser, C, and Sander, D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry (2006) 77(9):1017–20. doi:10.1136/jnnp.2005.087148
59. Pantano, P, Totaro, P, Fabbrini, G, Raz, E, Contessa, GM, Tona, F, et al. A transverse and longitudinal mr imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR Am J Neuroradiol (2011) 32(1):81–4. doi:10.3174/ajnr.A2242
60. MacIver, CL, Tax, CMW, Jones, DK, and Peall, KJ. Structural magnetic resonance imaging in dystonia: A systematic review of methodological approaches and findings. Eur J Neurol (2022) 29(11):3418–48. doi:10.1111/ene.15483
61. Berndt, M, Li, Y, Gora-Stahlberg, G, Jochim, A, and Haslinger, B. Impaired white matter integrity between premotor cortex and basal ganglia in writer's cramp. Brain Behav (2018) 8(10):e01111. doi:10.1002/brb3.1111
62. Delmaire, C, Vidailhet, M, Wassermann, D, Descoteaux, M, Valabregue, R, Bourdain, F, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer's cramp. Arch Neurol (2009) 66(4):502–8. doi:10.1001/archneurol.2009.8
63. Preibisch, C, Berg, D, Hofmann, E, Solymosi, L, and Naumann, M. Cerebral activation patterns in patients with writer's cramp: A functional magnetic resonance imaging study. J Neurol (2001) 248(1):10–7. doi:10.1007/s004150170263
64. Wu, CC, Fairhall, SL, McNair, NA, Hamm, JP, Kirk, IJ, Cunnington, R, et al. Impaired sensorimotor integration in focal hand dystonia patients in the absence of symptoms. J Neurol Neurosurg Psychiatry (2010) 81(6):659–65. doi:10.1136/jnnp.2009.185637
65. Gallea, C, Balas, M, Bertasi, E, Valabregue, R, Garcia-Lorenzo, D, Coynel, D, et al. Increased cortico-striatal connectivity during motor practice contributes to the consolidation of motor memory in writer's cramp patients. Neuroimage Clin (2015) 8:180–92. doi:10.1016/j.nicl.2015.04.013
66. Castrop, F, Dresel, C, Hennenlotter, A, Zimmer, C, and Haslinger, B. Basal ganglia-premotor dysfunction during movement imagination in writer’s cramp. Mov Disord (2012) 27(11):1432–9. doi:10.1002/mds.24944
67. Cho, HJ, and Hallett, M. Non-invasive brain stimulation for treatment of focal hand dystonia: Update and future direction. J Mov Disord (2016) 9(2):55–62. doi:10.14802/jmd.16014
68. Gallea, C, Herath, P, Voon, V, Lerner, A, Ostuni, J, Saad, Z, et al. Loss of inhibition in sensorimotor networks in focal hand dystonia. Neuroimage Clin (2018) 17:90–7. doi:10.1016/j.nicl.2017.10.011
69. Gupta, N, and Pandey, S. Treatment of focal hand dystonia: Current status. Neurol Sci (2021) 42(9):3561–84. doi:10.1007/s10072-021-05432-7
70. Hallett, M, Benecke, R, Blitzer, A, and Comella, CL. Treatment of focal dystonias with botulinum neurotoxin. Toxicon (2009) 54(5):628–33. doi:10.1016/j.toxicon.2008.12.008
71. Lungu, C, Nmashie, A, George, MC, Karp, BI, Alter, K, Shin, S, et al. Comparison of ultrasound and electrical stimulation guidance for onabotulinum toxin-a injections: A randomized crossover study. Mov Disord Clin Pract (2022) 9(8):1055–61. doi:10.1002/mdc3.13546
72. Zakin, E, and Simpson, DM. Botulinum toxin therapy in writer's cramp and musician's dystonia. Toxins (Basel) (2021) 13(12):899. doi:10.3390/toxins13120899
73. Lungu, C, Karp, BI, Alter, K, Zolbrod, R, and Hallett, M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: Outcome at 10 Years or more. Mov Disord (2011) 26(4):750–3. doi:10.1002/mds.23504
74. Zeuner, KE, Bara-Jimenez, W, Noguchi, PS, Goldstein, SR, Dambrosia, JM, and Hallett, M. Sensory training for patients with focal hand dystonia. Ann Neurol (2002) 51(5):593–8. doi:10.1002/ana.10174
75. Zeuner, KE, and Hallett, M. Sensory training as treatment for focal hand dystonia: A 1-year follow-up. Mov Disord (2003) 18(9):1044–7. doi:10.1002/mds.10490
76. Zeuner, KE, Peller, M, Knutzen, A, Hallett, M, Deuschl, G, and Siebner, HR. Motor Re-training does not need to Be task specific to improve writer's cramp. Mov Disord (2008) 23(16):2319–27. doi:10.1002/mds.22222
77. Zeuner, KE, Shill, HA, Sohn, YH, Molloy, FM, Thornton, BC, Dambrosia, JM, et al. Motor training as treatment in focal hand dystonia. Mov Disord (2005) 20(3):335–41. doi:10.1002/mds.20314
78. Schenk, T, Bauer, B, Steidle, B, and Marquardt, C. Does training improve writer's cramp? An evaluation of a behavioral treatment approach using kinematic analysis. J Hand Ther (2004) 17(3):349–63. doi:10.1197/j.jht.2004.04.005
79. Wissel, J, Kabus, C, Wenzel, R, Klepsch, S, Schwarz, U, Nebe, A, et al. Botulinum toxin in writer's cramp: Objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry (1996) 61(2):172–5. doi:10.1136/jnnp.61.2.172
80. Fahn, S. Assessment of the primary dystonias. In: T Munsat, editor. The quantification of neurologic deficit. Boston: Butterworths (1989). p. 241–70.
81. Byl, NN, Archer, ES, and McKenzie, A. Focal hand dystonia: Effectiveness of a home program of fitness and learning-based sensorimotor and memory training. J Hand Ther (2009) 22(2):183–97. doi:10.1016/j.jht.2008.12.003
82. McKenzie, AL, Goldman, S, Barrango, C, Shrime, M, Wong, T, and Byl, N. Differences in physical characteristics and response to rehabilitation for patients with hand dystonia: Musicians' cramp compared to writers' cramp. J Hand Ther (2009) 22(2):172–81. doi:10.1016/j.jht.2008.12.006
83. Bleton, JP, Vidailhet, M, Bourdain, F, Ducorps, A, Schwartz, D, Delmaire, C, et al. Somatosensory cortical remodelling after rehabilitation and clinical benefit of in writer's cramp. J Neurol Neurosurg Psychiatry (2011) 82(5):574–7. doi:10.1136/jnnp.2009.192476
84. Deepak, KK, and Behari, M. Specific muscle emg biofeedback for hand dystonia. Appl Psychophysiol Biofeedback (1999) 24(4):267–80. doi:10.1023/a:1022239014808
85. Berger, HJ, van der Werf, SP, Horstink, CA, Cools, AR, Oyen, WJ, and Horstink, MW. Writer's cramp: Restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism Relat Disord (2007) 13(3):170–3. doi:10.1016/j.parkreldis.2006.09.003
86. Baur, B, Furholzer, W, Marquardt, C, and Hermsdorfer, J. Auditory grip force feedback in the treatment of writer's cramp. J Hand Ther (2009) 22(2):163–70. doi:10.1016/j.jht.2008.11.001
87. Siebner, HR, Tormos, JM, Ceballos-Baumann, AO, Auer, C, Catala, MD, Conrad, B, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology (1999) 52(3):529–37. doi:10.1212/wnl.52.3.529
88. Murase, N, Rothwell, JC, Kaji, R, Urushihara, R, Nakamura, K, Murayama, N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain (2005) 128(1):104–15. doi:10.1093/brain/awh315
89. Havrankova, P, Jech, R, Walker, ND, Operto, G, Tauchmanova, J, Vymazal, J, et al. Repetitive tms of the somatosensory cortex improves writer's cramp and enhances cortical activity. Neuro Endocrinol Lett (2010) 31(1):73–86.
90. Huang, YZ, Lu, CS, Rothwell, JC, Lo, CC, Chuang, WL, Weng, YH, et al. Modulation of the disturbed motor network in dystonia by multisession suppression of premotor cortex. PLoS One (2012) 7(10):e47574. doi:10.1371/journal.pone.0047574
91. Kimberley, TJ, Borich, MR, Arora, S, and Siebner, HR. Multiple sessions of low-frequency repetitive transcranial magnetic stimulation in focal hand dystonia: Clinical and physiological effects. Restor Neurol Neurosci (2013) 31(5):533–42. doi:10.3233/RNN-120259
92. Bradnam, LV, Graetz, LJ, McDonnell, MN, and Ridding, MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci (2015) 9:286. doi:10.3389/fnhum.2015.00286
Keywords: writer’s cramp, treatment, pathophysiology, sensorimotor disorganization, structural and functional imaging
Citation: Zeuner KE, Baumann A and Witt K (2023) Treatment of writer’s cramp based on current pathophysiological concepts. Dystonia 2:11067. doi: 10.3389/dyst.2023.11067
Received: 18 November 2022; Accepted: 23 January 2023;
Published: 09 February 2023.
Edited by:
Aasef Shaikh, Case Western Reserve University, United StatesCopyright © 2023 Zeuner, Baumann and Witt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten E. Zeuner, ay56ZXVuZXJAbmV1cm9sb2dpZS51bmkta2llbC5kZQ==
 Kirsten E. Zeuner
Kirsten E. Zeuner Alexander Baumann
Alexander Baumann Karsten Witt2
Karsten Witt2