- 1Medical Scientist Training Program, Duke University School of Medicine, Duke University, Durham, NC, United States
- 2Duke Institute for Brain Sciences, Duke University, Durham, NC, United States
- 3Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Duke University, Durham, NC, United States
- 4Department of Electrical and Computer Engineering, Duke University, Durham, NC, United States
- 5Department of Neurosurgery, Duke University School of Medicine, Duke University, Durham, NC, United States
- 6Department of Biomedical Engineering, Duke University, Durham, NC, United States
- 7Department of Neurology, Duke University School of Medicine, Duke University, Durham, NC, United States
- 8Department of Neurobiology, Duke University School of Medicine, Duke University, Durham, NC, United States
- 9Department of Cell Biology, Duke University School of Medicine, Duke University, Durham, NC, United States
Despite many research studies, transcranial magnetic stimulation (TMS) is not yet an FDA-approved clinical therapy for dystonia patients. This review describes the four major challenges that have historically hindered the clinical translation of TMS. The four challenges described are limited types of clinical trial designs, limited evidence on objective behavioral measures, variability in the TMS clinical response, and the extensive TMS parameters to optimize for clinical therapy. Progress has been made to diversify the types of clinical trial design available to clinical researchers, identify evidence-based objective behavioral measures, and reduce the variability in TMS clinical response. Future studies should identify objective behavioral measures for other dystonia subtypes and expand the optimal TMS stimulation parameters for clinical therapy. Our review highlights the key progress made to overcome these barriers and gaps that remain for TMS to develop into a long-lasting clinical therapy for dystonia patients.
Introduction
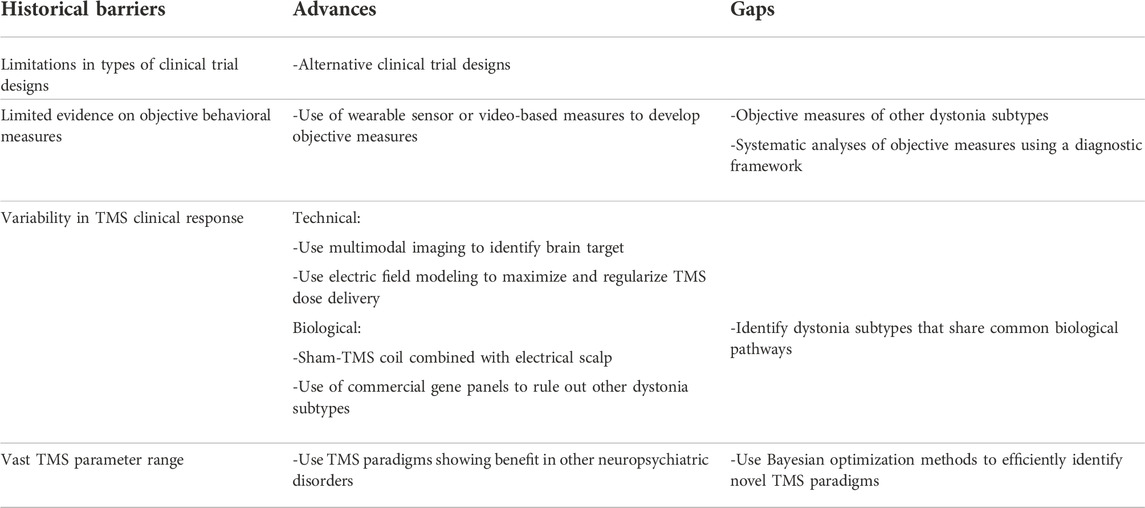
TMS is a noninvasive brain stimulation technique currently FDA-approved for treating depression (including anxiety symptoms), obsessive compulsive disorder, migraine, and smoking addiction [1–3]. TMS has been studied as a potential clinical therapy for dystonia since the early 1990s [4]. However, no TMS paradigm is currently FDA-approved for clinical therapy in any subtype of dystonia. In this review, we discuss the four historical challenges that have delayed the progress of TMS as a clinical therapy for dystonia, the progress made in the last three decades to overcome these barriers, and the work that remains for future dystonia researchers. The four challenges discussed are limited types of clinical trial designs, limited evidence on objective behavioral measures, variability in the TMS clinical response, and the vast TMS parameter space to optimize for clinical therapy (Table 1).
Limited types of clinical trial designs
With a prevalence of 405 per million, dystonia is a rare brain disorder [5, 6]. Traditional clinical trials that require large samples to power a randomized and placebo-controlled design adequately are not possible. As a result, rare disorders such as dystonia have historically struggled to meet the bar for FDA approval of clinical therapies. Indeed, deep brain stimulation (DBS), a long-standing clinical therapy for dystonia, is not FDA-approved [7]. Instead, DBS carries a humanitarian device exemption to permit its application in patients with generalized and cervical dystonia [7]. In the past few decades, there has been a growing rejection of the “one-size-fits-all” approach to clinical trial design. Instead, organizations such as the rare disease network have proposed alternative clinical trial designs, and policies such as the Orphan Drug Act have successfully allowed rare disorders to receive FDA approval for drugs [8]. Readers are referred to another source for a comprehensive review of alternative clinical trial designs in rare diseases [9]. Among these alternative designs, three clinical trial designs may be particularly suited for testing TMS effects in dystonia subtypes. Cross-over trial compares the effects of two or more treatments by allocating each participant to each treatment in a sequential but randomized order [9]. This approach allows the comparison of multiple TMS conditions while minimizing the cost and sample size in a powered study. For example, a single arm cross-over study design was previously employed to compare the behavioral effects of three different TMS conditions in focal hand dystonia [10]. To negate order effects, all six possible orders of the three conditions were included. By allowing patients to serve as their own comparison through a cross-over design, sufficient biological data was collected to power the study while minimizing sample size. An important drawback of a cross-over study design is the potential for carryover effects between the different conditions [9]. This issue can be reduced by ensuring a sufficient washout period between each TMS condition. If a washout period is unknown, data on TMS effects can be collected at pre- and post-intervals for all TMS conditions to adjust for the cumulative or additive effects of multiple conditions over time.
An enriched enrollment, randomized withdrawal design was previously used in a TMS study of dystonia patients [11]. The research design has two phases: the first enrichment phase is an open-label trial where all patients receive the active TMS condition. TMS responders are identified and enrolled in the second randomized withdrawal phase. In the second phase, responders are randomly assigned to the active or sham TMS intervention. Study analysis is only conducted using the second phase of the study [9]. Given the variability in TMS response, this design allows the enrichment of the study cohort with TMS responders, thereby increasing the power of the study. An inherent limitation of the enriched study design is that the findings cannot be generalized to the dystonia patient population as a whole but are limited to TMS responders of the dystonia population being studied [9]. Nevertheless, the enriched study design allows for a powered evaluation of the efficacy of a TMS intervention in a rare disorder.
Response adaptive randomization design uses data analysis at planned interim breaks throughout the study to adjust the study design [9]. For example, an adaptive trial may change the randomization scheme to favor the better-performing arm of the TMS study based on the interim analysis. Response adaptive trials, therefore, maximize the number of participants assigned to the more effective TMS condition while minimizing overall study recruitment. A major limitation of the study is that the imbalance in treatment assignment may lead to unblinding for study investigators and selection bias. To overcome this limitation, a two-staged randomized and nonrandomized phase of the response adaptive trial has been proposed [9].
In conclusion, the study design of a clinical trial is critical to its success. Alternative clinical trial designs are important advancements for clinical research studies in rare disorders such as dystonia. Researchers should carefully consider the diversity of clinical trial designs for rare disorders to ensure that an optimal design is selected to successfully carry out a TMS study in dystonia patients.
Limited evidence on objective behavioral measures
In order to develop TMS as a clinical therapy for dystonia, evidence-based objective behavioral measures are much needed. Historically, patient-reported outcome measures were available to assess response to clinical treatments [7]. However, these measures are highly susceptible to placebo effects. As a result, while patient-reported measures are essential to include in trials to understand the clinical meaningfulness of study findings, they present major limitations that make them less suitable as primary outcomes in an intervention trial. In the last few decades, extensive progress has been made in creating clinician-rated scales to quantify the observed dystonic movements, such as the Burke-Fahn-Marsden dystonia scale, writer’s cramp rating scale, and Toronto Spasmodic Torticollis scale (TWSTR) [12–14]. Unfortunately, these rating scales require specialized practitioners and are susceptible to high inter-rater variability. Furthermore, the data collected are ultimately categorical rather than continuous, reducing the analytic power.
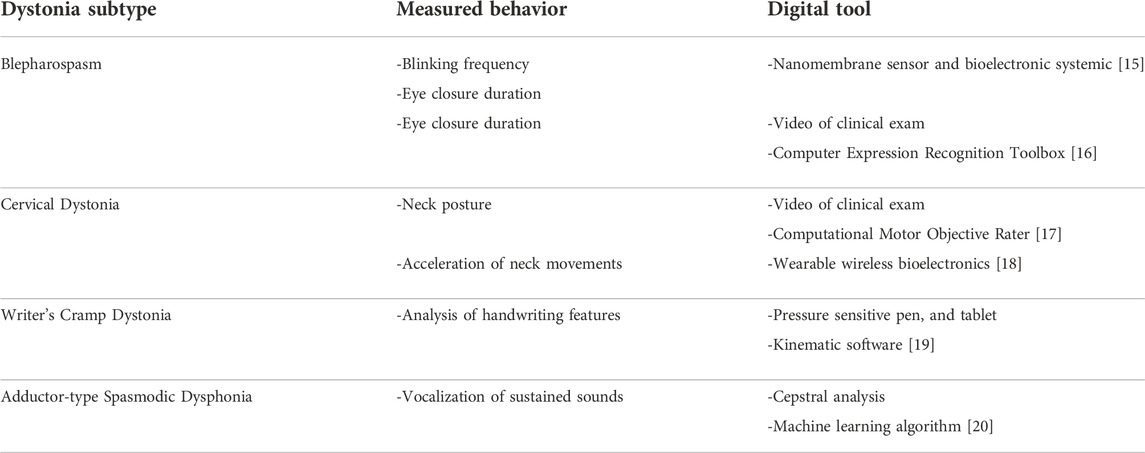
Using modern technology such as digital tablets, research groups in neurological disorders are developing objective behavioral assays to overcome the shortcomings of these types of patient and clinician-rated categorical datasets (Table 2). For example, research studies of adult focal dystonias such as writer’s cramp, cervical dystonia, blepharospasm, and spasmodic dysphonia increasingly use wearable sensors and video-based quantification to capture dystonic movements [15–20]. These digital behavioral measures are continuous data, thereby improving the analytic power. Another advantage of these digital measures is that most of these devices and software are accessible commercially without extensive clinical or engineering expertise needed. Although objective behavioral measures are increasingly being used to study the effect of TMS in dystonia, clear evidence on the diagnostic potential of these digital measures is lacking. As a result, it remains unclear if these objective measures can capture the behavioral response to TMS intervention in dystonia patients [21, 22].
Systematic analyses are, therefore, needed to understand the diagnostic potential of these objective behavioral measures. Specifically, studies are needed to understand the sensitivity and specificity of these objective measures to detect changes in behavior, retest reliability, and the power needed to differentiate behavioral signals from noise. Objective behavioral measures that pass a systematic analysis should then be correlated with the other two data types (patient-reported outcome measures and clinician-rated measures) to understand the clinical meaning of these objective behavioral measures. Measures that pass these systematic diagnostic evaluations should then be used as primary outcomes in TMS interventional studies of dystonia patients.
For example, multiple prior TMS studies used kinematic features of writing, such as pen pressure and writing velocity, to quantify TMS effects in writer’s cramp dystonia [22–24]. However, the evidence for using these kinematic measures in clinical research studies of dystonia patients was sparse. As a result, prior to initiating a TMS study in focal hand dystonia, our group systematically analyzed 23 kinematic writing measures, including pen pressure and writing velocity. We demonstrated that these previously used kinematic measures did not display the diagnostic evidence (sensitivity, specificity, reliability) to serve as a primary outcome in a clinical intervention study of focal hand dystonia. Instead, we identified a novel behavioral measure (peak accelerations) that could serve as a primary outcome in our TMS study of focal hand dystonia [19]. Although identifying objective behavioral outcome measures delayed the initiation of the primary TMS study by a year, findings from this study were critical to understanding the effects of TMS in focal hand dystonia. Using an evidence-based, objective behavioral measure allowed us to detect a medium-effect behavioral response to TMS in our study [10]. Furthermore, the objective behavioral measure also allowed us to understand the relationship between motor symptoms in dystonia and brain connections [25]. These insights will inform future TMS clinical research studies in focal hand dystonia. More studies are needed to identify evidence-based objective measures for use in clinical research studies of other dystonia subtypes. One of the missions of the Dystonia Coalition is to incorporate technologies such as a video capture system to develop objective behavioral measures for use in clinical research studies.
Variability in TMS clinical response
Technical variability
Another historical challenge to developing TMS as a clinical therapy for dystonia has been the variability in clinical response. Variability in clinical response may arise from both technical and biological aspects. Technical variability can result from “one-size-fits-all” approaches to brain targeting and a lack of tools to individualize stimulation delivery [26]. Historically, identifying brain targets for TMS delivery was performed using anatomic landmarks on the scalp or structural MRI scans [26]. In recent years, a new field has emerged, with researchers using neuroimaging (fMRI, DTI, PET) and neurophysiology (EEG, EMG) to improve the precision of brain targeting [2, 26, 27]. These techniques aim to deliver TMS to a precise brain region relevant to the disease of interest and are customized to the individual subject’s brain. The accurate delivery of the electric field induced by TMS is also contingent on accounting for the distance between the TMS coil and the brain target. The accurate delivery of the electric field must therefore adjust for the effect of the individual skull anatomy and different tissue conductivities between the TMS coil and relevant brain target. To address this challenge, electrical field modeling was developed to adjust coil position and maximize the electric field induced by TMS in the brain target of interest [28, 29]. Software pipelines such as TAP (Targeting and Analysis Pipeline) have synthesized the electric field modeling steps with identification of a brain target using neuroimaging or neurophysiology tools to improve brain targeting efficiency [30, 31]. These pipelines have the added advantage of retrospectively providing analyses on the accuracy of TMS delivery by calculating the predicted electric field delivered to the brain target of interest based on the individual’s resting motor threshold and coil deviations during the TMS session [31]. A retrospective measure of the accuracy of TMS delivery is a vital quality control measure in an interventional clinical trial design of TMS. In sum, recent advances in prospective brain target selection and electric field modeling combined with retrospective TMS delivery analysis can help to address the technical variability in TMS clinical response.
Biological variability
The variability in clinical response to TMS can also have biological origins. One source of biological variability is the placebo effect. Historically, prior studies in psychiatry have reported that placebo responses to TMS can be as high as 25% [2]. As a result, study subjects are now routinely blinded to TMS treatment arms, using a sham TMS coil combined with electrical scalp stimulation to mimic active TMS without stimulating the brain directly [32]. Biological variability may also be due to an incorrect diagnosis. Neurologists and study investigators, therefore, must do a comprehensive workup to ensure that another disease does not confound study participants. For example, TMS has historically been studied in patients with writer’s cramp and cervical dystonia. However, similar dystonia symptoms can manifest in patients with Parkinson’s disease. Monogenic forms of dystonia, such as DYT1, also show variable penetrance, and silent carriers of this gene may manifest in the adult age with writer’s cramp symptoms [33]. Ensuring that dystonias are homogenous in etiology is, therefore, an important quality control step for clinical trials. Since dystonia gene panels are now commercially available and more affordable, studies enrolling patients with specific dystonia subtypes can use these options to ensure the study cohort has the same etiology on Axis II of the dystonia classification system. Alternatively, there is a growing understanding that multiple genetic forms of dystonia converge in shared biological pathways [34]. Updating the dystonia classification system to group dystonia syndromes by shared biological pathways may expand future studies to allow all dystonia patients who share a common biological pathway to be included in a clinical trial, thus opening the studies to a larger subgroup of dystonia patients.
Wide TMS parameter range
The fourth historical barrier to developing TMS as a clinical therapy has been the wide range of possible stimulation parameters to test. For example, in repetitive TMS (rTMS), researchers need to decide not only on the brain target and frequency of stimulation but also the intensity, the number of pulse trains, intertrain interval, the number of sessions and their frequency, among other parameters [35–37]. As a result, many clinical trials in dystonia have used the same stimulation parameters shown to transiently benefit patients in previous studies. For example, 1 Hz rTMS has been extensively studied in patients with focal hand dystonia [38]. While this approach is logical and builds on prior work in the field, it can also deter new discoveries of stimulation parameters. Keeping an eye on successful TMS paradigms for other diseases, such as intermittent theta burst stimulation for treatment-resistant depression, may inspire the next iteration of TMS parameters for dystonia therapy [39, 40]. Understanding the effects of different TMS parameters on the same brain region of interest, regardless of the targeted disease, could identify new TMS paradigms for dystonia. The daunting task of parameter selection is further complicated by brain state dependence: for example, whether to deliver TMS at rest or during a dystonia-evoking task [41]. Prior neuroimaging studies of dystonia patients report abnormalities in the brain networks during both rest and performance of the dystonic motor task [42–44]. Understanding how these abnormalities link with the manifestation of dystonic behavior is the next step in understanding the ideal brain state for delivering neuromodulation therapy. Recent work by our group evaluates these relationships [25]. More studies are needed to understand the relationship between brain abnormalities and dystonic behavior to generate further insight into the ideal brain state to deliver neuromodulation therapy.
As knowledge of TMS effects on dystonia subtypes is still growing, it may be the case that different dystonia subtypes may have distinct brain targets and TMS parameters. For example, a prior TMS study targeting the cerebellum in writer’s cramp failed to show a motor response, while another TMS study targeting the cerebellum in cervical dystonia showed improvement in motor symptoms [45, 46]. Similarly, the TMS stimulation parameters for different dystonia subtypes may be distinct. For example, intermittent theta burst stimulation previously failed to show a behavioral response in patients with focal hand dystonia but showed behavioral improvement in cervical dystonia [45, 47]. As knowledge of dystonia pathophysiology advances, it is possible that dystonia subtypes with different biological pathways may show distinct brain targets and TMS parameters for an optimal behavioral response.
Considering the continued challenge of identifying the optimal TMS parameters for dystonia, we might envision a roadmap to rationally select TMS stimulation parameters for dystonia. First, the critical brain network in dystonia pathophysiology, such as the motor network during a relevant brain state, should be identified. It is preferable to select a brain network associated with a behavioral abnormality captured on an objective behavioral measure such as motor movements. This critical brain network would serve as the TMS target. Advanced neuroimaging or neurophysiology analyses at the individual and group levels of the dystonia study cohort should be used to identify a superficial cortical region associated with the abnormal brain network. This node would be the access point for TMS delivery to the targeted brain network since TMS directly activates the superficial cortical regions only [48]. Then, the TMS stimulation parameters for modifying the brain network of interest must be determined. To understand the effects of different TMS parameters on dystonia, it is important to report TMS parameters that show a clinical effect and those that do not. For example, a novel stimulation approach called accelerated TMS consists of multiple daily 10-minute TMS sessions interspersed with 50-minute intervals of rest [39, 40]. This accelerated TMS has recently shown high remission rates and secured FDA clearance in refractory depression [39]. However, rest intervals of less than 50 min have not shown similar behavioral improvement in depression patients [39]. These negative findings led the authors to conclude that the minimum of a 50-minute rest interval may be important for the process of synaptic strengthening and, ultimately, TMS’s cumulative therapeutic effects. Reporting both positive and negative clinical responses to new TMS parameters is, therefore, highly informative to our understanding of the effects of TMS parameters on brain physiology.
Computational tools such as closed-loop Bayesian optimization may also reduce the TMS selection parameters to test in future research studies [49]. Although our knowledge of optimal TMS parameter selection remains underdeveloped, maximizing the existing knowledge across TMS paradigms and diseases while incorporating novel computational tools can further narrow down effective parameters to consider for developing TMS as a clinical therapy in dystonia.
Conclusion
Over the last 30 years, significant progress has been made to diversify clinical trial designs for rare disorders, develop novel objective behavioral outcome measures to capture dystonic movements, improve the technical delivery of TMS, and reduce the variability in the biological response to TMS. Future studies should identify objective behavioral measures for other dystonia subtypes, test the effect of different TMS stimulation parameters on behavioral symptoms of dystonia. Given the extensive TMS parameters to develop, testing TMS paradigms used in other brain disorders in conjunction with computational modeling can be helpful. The goal is to identify the optimal TMS parameters that provide long lasting clinical benefits in different dystonia subtypes. Our review highlights the key progress made to overcome some of the historical barriers and the remaining gaps in the field that should be the focus of future research to advance TMS as a clinical therapy for dystonia patients.
Author contributions
PM assisted with manuscript writing. AP and NC reviewed and critiqued the manuscript. NB-P conceptualized the review, wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants to NB-P from Dystonia Medical Research Foundation (Clinical Fellowship Training Program from), Doris Duke Charitable Foundation (Fund to Retain Clinician Scientists), American Academy of Neurology (career development award) and NIH NCATS (1KL2TR002554). NB-P was also supported by a career development award from the Dystonia Coalition (NS065701, TR001456, NS116025) which is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported by the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Institute of Neurological Diseases and Stroke (NINDS). AP’s contributions were supported in part by the National Institutes of Health (R01MH128422, R01NS117405).
Conflict of interest
NB-P reports funding from AAN. NB-P also serves as a member of the DMRF Medical and Scientific Advisory Council. AP is an inventor on patents on TMS technology, has equity options and serves on the scientific advisory board of Ampa, and has received patent royalties, consulting fees, and equipment loans from Rogue Research, MagVenture, Neuronetics, Soterix Medical, Magnetic Tides, and Ampa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
1. Lipton, RB, and Pearlman, SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics (2010) 7(2):204–12. doi:10.1016/j.nurt.2010.03.002
2. Perera, T, George, MS, Grammer, G, Janicak, PG, Pascual-Leone, A, and Wirecki, TS. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul (2016) 9(3):336–46. doi:10.1016/j.brs.2016.03.010
3. Zangen, A, Moshe, H, Martinez, D, Barnea-Ygael, N, Vapnik, T, Bystritsky, A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry (2021) 20(3):397–404. doi:10.1002/wps.20905
4. Siebner, HR, Tormos, JM, Ceballos-Baumann, AO, Auer, C, Catala, MD, Conrad, B, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology (1999) 52(3):529–37. doi:10.1212/wnl.52.3.529
5.Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J Neurol (2000) 247(10):787–92. doi:10.1007/s004150070094
6. Ortiz, RM, Scheperjans, F, and Pekkonen, E. Deep brain stimulation for dystonia in Finland during 2007-2016. BMC Neurol (2019) 19(1):137. doi:10.1186/s12883-019-1370-y
7. Copeland, BJ. Principles and practice of movement disorders. 3rd ed. London: Elsevier (2022). p. 436. Journal of the Neurological Sciences.
8. Roberts, AD, and Wadhwa, R. Orphan drug approval laws. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2023).
9. Abrahamyan, L, Feldman, BM, Tomlinson, G, Faughnan, ME, Johnson, SR, Diamond, IR, et al. Alternative designs for clinical trials in rare diseases. Am J Med Genet C Semin Med Genet (2016) 172(4):313–31. doi:10.1002/ajmg.c.31533
10. Bukhari-Parlakturk, N, Mulcahey, P, Lutz, M, Ghazi, R, Huang, Z, Dannhauer, M, et al. Functional MRI-guided individualized TMS modifies motor network and reduces writing dysfluency in Focal Hand Dystonia. In: Samuel Belzberg 6th International Dystonia Symposium; 1st – 3rd June 2023; Dublin, Ireland (2023).
11. Havrankova, P, Jech, R, Walker, ND, Operto, G, Tauchmanova, J, Vymazal, J, et al. Repetitive TMS of the somatosensory cortex improves writer's cramp and enhances cortical activity. Neuro Endocrinol Lett (2010) 31(1):73–86.
12. Burke, RE, Fahn, S, Marsden, CD, Bressman, SB, Moskowitz, C, and Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology (1985) 35(1):73–7. doi:10.1212/wnl.35.1.73
13. Espay, AJ, Trosch, R, Suarez, G, Johnson, J, Marchese, D, and Comella, C. Minimal clinically important change in the Toronto western spasmodic Torticollis rating scale. Parkinsonism Relat Disord (2018) 52:94–7. doi:10.1016/j.parkreldis.2018.03.002
14. Wissel, J, Kabus, C, Wenzel, R, Klepsch, S, Schwarz, U, Nebe, A, et al. Botulinum toxin in writer's cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry (1996) 61(2):172–5. doi:10.1136/jnnp.61.2.172
15. Mahmood, M, Kwon, S, Berkmen, GK, Kim, YS, Scorr, L, Jinnah, HA, et al. Soft nanomembrane sensors and flexible hybrid bioelectronics for wireless quantification of blepharospasm. IEEE Trans Biomed Eng (2020) 67(11):3094–100. doi:10.1109/TBME.2020.2975773
16. Peterson, DA, Littlewort, GC, Bartlett, MS, Macerollo, A, Perlmutter, JS, Jinnah, HA, et al. Objective, computerized video-based rating of blepharospasm severity. Neurology (2016) 87(20):2146–53. doi:10.1212/WNL.0000000000003336
17. Zhang, Z, Cisneros, E, Lee, HY, Vu, JP, Chen, Q, Benadof, CN, et al. Hold that pose: capturing cervical dystonia's head deviation severity from video. Ann Clin Transl Neurol (2022) 9(5):684–94. doi:10.1002/acn3.51549
18. Kwon, YT, Lee, Y, Berkmen, GK, Lim, HR, Scorr, L, Jinnah, HA, et al. Soft material-enabled, active wireless, thin-film bioelectronics for quantitative diagnostics of cervical dystonia. Adv Mater Technol (2019) 4(10):1900458. doi:10.1002/admt.201900458
19. Bukhari-Parlakturk, N, Lutz, MW, Al-Khalidi, HR, Unnithan, S, Wang, JEH, Scott, B, et al. Suitability of automated writing measures for clinical trial outcome in writer's cramp. Mov Disord (2023) 38(1):123–32. doi:10.1002/mds.29237
20. Suppa, A, Asci, F, Saggio, G, Marsili, L, Casali, D, Zarezadeh, Z, et al. Voice analysis in adductor spasmodic dysphonia: objective diagnosis and response to botulinum toxin. Parkinsonism Relat Disord (2020) 73:23–30. doi:10.1016/j.parkreldis.2020.03.012
21. Bologna, M, Paparella, G, Fabbrini, A, Leodori, G, Rocchi, L, Hallett, M, et al. Effects of cerebellar theta-burst stimulation on arm and neck movement kinematics in patients with focal dystonia. Clin Neurophysiol (2016) 127(11):3472–9. doi:10.1016/j.clinph.2016.09.008
22. Kimberley, TJ, Schmidt, RLS, Chen, M, Dykstra, DD, and Buetefisch, CM. Mixed effectiveness of rTMS and retraining in the treatment of focal hand dystonia. Front Hum Neurosci (2015) 9:385. doi:10.3389/fnhum.2015.00385
23. Hermsdorfer, J, Marquardt, C, Schneider, AS, Fürholzer, W, and Baur, B. Significance of finger forces and kinematics during handwriting in writer's cramp. Hum Mov Sci (2011) 30(4):807–17. doi:10.1016/j.humov.2010.04.004
24. Zeuner, KE, Shill, HA, Sohn, YH, Molloy, FM, Thornton, BC, Dambrosia, JM, et al. Motor training as treatment in focal hand dystonia. Mov Disord (2005) 20(3):335–41. doi:10.1002/mds.20314
25. Bukhari-Parlakturk, N, Fei, M, Voyvodic, J, and Michael, A. Data driven exploration of network connectivity in task-FMRI of focal hand dystonia. MedRxiv (2022). doi:10.1101/2021.05.14.21257239
26. Rossi, S, Hallett, M, Rossini, PM, and Pascual-Leone, A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol (2009) 120(12):2008–39. doi:10.1016/j.clinph.2009.08.016
27. Rossi, S, Antal, A, Bestmann, S, Bikson, M, Brewer, C, Brockmöller, J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin Neurophysiol (2021) 132(1):269–306. doi:10.1016/j.clinph.2020.10.003
28. Gomez, LJ, Dannhauer, M, and Peterchev, AV. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage (2021) 228:117696. doi:10.1016/j.neuroimage.2020.117696
29. Opitz, A, Fox, MD, Craddock, RC, Colcombe, S, and Milham, MP. An integrated framework for targeting functional networks via transcranial magnetic stimulation. Neuroimage (2016) 127:86–96. doi:10.1016/j.neuroimage.2015.11.040
30. Beynel, L, Davis, SW, Crowell, CA, Dannhauer, M, Lim, W, Palmer, H, et al. Site-specific effects of online rTMS during a working memory task in healthy older adults. Brain Sci (2020) 10(5):255. doi:10.3390/brainsci10050255
31. Dannhauer, M, Huang, Z, Beynel, L, Wood, E, Bukhari-Parlakturk, N, and Peterchev, AV. Tap: targeting and analysis pipeline for optimization and verification of coil placement in transcranial magnetic stimulation. J Neural Eng (2022) 19(2):026050. doi:10.1088/1741-2552/ac63a4
32. Smith, JE, and Peterchev, AV. Electric field measurement of two commercial active/sham coils for transcranial magnetic stimulation. J Neural Eng (2018) 15(5):054001. doi:10.1088/1741-2552/aace89
33. van den Bos, M, Marotta, R, Goldup, S, Chataway, T, Firgaira, F, and Thyagarajan, D. Writer's cramp in an Australian pedigree with DYT1 dystonia. J Clin Neurosci (2004) 11(5):537–9. doi:10.1016/S0967-5868(03)00226-1
34. Gonzalez-Latapi, P, Marotta, N, and Mencacci, NE. Emerging and converging molecular mechanisms in dystonia. J Neural Transm (Vienna) (2021) 128(4):483–98. doi:10.1007/s00702-020-02290-z
35. Hallett, M. Transcranial magnetic stimulation: a primer. Neuron (2007) 55(2):187–99. doi:10.1016/j.neuron.2007.06.026
36. Peterchev, AV, Wagner, TA, Miranda, PC, Nitsche, MA, Paulus, W, Lisanby, SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul (2012) 5(4):435–53. doi:10.1016/j.brs.2011.10.001
37. Rothwell, J. Transcranial magnetic stimulation as a method for investigating the plasticity of the brain in Parkinson's disease and dystonia. Parkinsonism Relat Disord (2007) 13(3):S417–20. doi:10.1016/S1353-8020(08)70040-3
38. Cho, HJ, and Hallett, M. Non-invasive brain stimulation for treatment of focal hand dystonia: update and future direction. J Mov Disord (2016) 9(2):55–62. doi:10.14802/jmd.16014
39. Cole, EJ, Phillips, AL, Bentzley, BS, Stimpson, KH, Nejad, R, Barmak, F, et al. Stanford neuromodulation therapy (snt): a double-blind randomized controlled trial. Am J Psychiatry (2022) 179(2):132–41. doi:10.1176/appi.ajp.2021.20101429
40. Cole, EJ, Stimpson, KH, Bentzley, BS, Gulser, M, Cherian, K, Tischler, C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry (2020) 177(8):716–26. doi:10.1176/appi.ajp.2019.19070720
41. Hallett, M. Smart brain stimulation. Clin Neurophysiol (2017) 128(5):839–40. doi:10.1016/j.clinph.2017.02.002
42. Gallea, C, Horovitz, SG, Najee-Ullah, M'A, and Hallett, M. Impairment of a parieto-premotor network specialized for handwriting in writer's cramp. Hum Brain Mapp (2016) 37(12):4363–75. doi:10.1002/hbm.23315
43. Mantel, T, Meindl, T, Li, Y, Jochim, A, Gora-Stahlberg, G, Kräenbring, J, et al. Network-specific resting-state connectivity changes in the premotor-parietal axis in writer's cramp. Neuroimage Clin (2018) 17:137–44. doi:10.1016/j.nicl.2017.10.001
44. Norris, SA, Morris, AE, Campbell, MC, Karimi, M, Adeyemo, B, Paniello, RC, et al. Regional, not global, functional connectivity contributes to isolated focal dystonia. Neurology (2020) 95(16):e2246–e2258. doi:10.1212/WNL.0000000000010791
45. Bradnam, LV, McDonnell, MN, and Ridding, MC. Cerebellar intermittent theta-burst stimulation and motor control training in individuals with cervical dystonia. Brain Sci (2016) 6(4):56. doi:10.3390/brainsci6040056
46. Sadnicka, A, Hamada, M, Bhatia, KP, Rothwell, JC, and Edwards, MJ. Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov Disord (2014) 29(10):1304–7. doi:10.1002/mds.25881
47. Belvisi, D, Suppa, A, Marsili, L, Di Stasio, F, Parvez, AK, Agostino, R, et al. Abnormal experimentally- and behaviorally-induced LTP-like plasticity in focal hand dystonia. Exp Neurol (2013) 240:64–74. doi:10.1016/j.expneurol.2012.11.003
48. Aberra, AS, Wang, B, Grill, WM, and Peterchev, AV. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul (2020) 13(1):175–89. doi:10.1016/j.brs.2019.10.002
Keywords: transcranial magnetic stimulation, dystonia, kinematics, clinical trial design, TMS parameters
Citation: Mulcahey PJ, Peterchev AV, Calakos N and Bukhari-Parlakturk N (2023) Transcranial magnetic stimulation: the road to clinical therapy for dystonia. Dystonia 2:11660. doi: 10.3389/dyst.2023.11660
Received: 07 June 2023; Accepted: 04 August 2023;
Published: 16 August 2023.
Edited by:
Alfonso Fasano, Toronto Western Hospital, CanadaCopyright © 2023 Mulcahey, Peterchev, Calakos and Bukhari-Parlakturk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noreen Bukhari-Parlakturk, bm9yZWVuLmJ1a2hhcmlAZHVrZS5lZHU=
 Patrick J. Mulcahey
Patrick J. Mulcahey Angel V. Peterchev
Angel V. Peterchev Nicole Calakos
Nicole Calakos Noreen Bukhari-Parlakturk
Noreen Bukhari-Parlakturk