Abstract
The Dystonia Medical Research Foundation organized an expert virtual workshop in March 2023 to review the evidence on non-motor symptoms across the spectrum of dystonia, discuss existing assessment methods, need for their harmonisation and roadmap to achieve this, and evaluate potential treatment approaches. Albeit the most investigated non-motor domains, experts highlighted the need to identify the most accurate screening procedure for depression and anxiety, clarify their mechanistic origin and quantify their response to already available therapies. Future exploration of sleep disruption in dystonia should include determining the accuracy and feasibility of wearable devices, understanding the contribution of psychotropic medication to its occurrence, and defining the interaction between maladaptive plasticity and abnormal sleep patterns. Despite recent advances in the assessment of pain in dystonia, more research is needed to elucidate the relative importance of different mechanisms called into play to explain this impactful sensory feature and the most appropriate treatments. Amongst the different non-motor features investigated in dystonia, cognitive dysfunction and fatigue require an in-depth observation to evaluate their functional impact, their clinical profile and assessment methods and, in the case of cognition, whether impairment represents a prodrome of dementia. Finally, experts identified the development and field validation of a self-rated screening tool encompassing the full spectrum of non-motor symptoms as the most urgent step towards incorporating the management of these features into routine clinical practice.
Introduction
The nature and frequency of non-motor symptoms in dystonia have gained increased recognition and research focus over the past 10 years, despite not forming part of the current dystonia classification system [1]. Investigation of non-motor symptoms have primarily focused on specific subtypes, predominantly psychiatric symptoms, with fewer studies examining sleep, pain, cognition, and fatigue (Figure 1). Variable cohort diagnoses, size and methodological approaches have contributed to highly heterogenous findings, suggesting a need for more consistent and standardised approaches. However, systematic evaluation and management of non-motor symptoms do not currently form part of routine clinical care for patients with dystonia, and beyond standardised practices for botulinum neurotoxin (BoNT) and deep brain stimulation (DBS) therapies, a comprehensive model of care remains to be developed for dystonia. These limitations provided the rationale for the Dystonia Medical Research Foundation to convene a workshop aiming to 1) examine the evidence to date for non-motor symptoms across the spectrum of dystonia, 2) identify different methodological approaches to assessing non-motor symptoms, 3) determine whether these approaches differ across children, young people, and adults, and whether there is need for harmonisation, 4) develop a roadmap to integrate non-motor symptom evaluation into routine clinical care, and 5) evaluate potential therapeutic interventions or whether existing therapies can be repurposed and tested in clinical trials for treatment of non-motor symptoms. Here, we provide an overview of each of the subject areas discussed—psychiatric symptoms, sleep disruption, pain, fatigue, cognitive dysfunction—differences and commonalities between childhood and adult non-motor assessment approaches, and development of models of care to better incorporate non-motor symptoms.
FIGURE 1
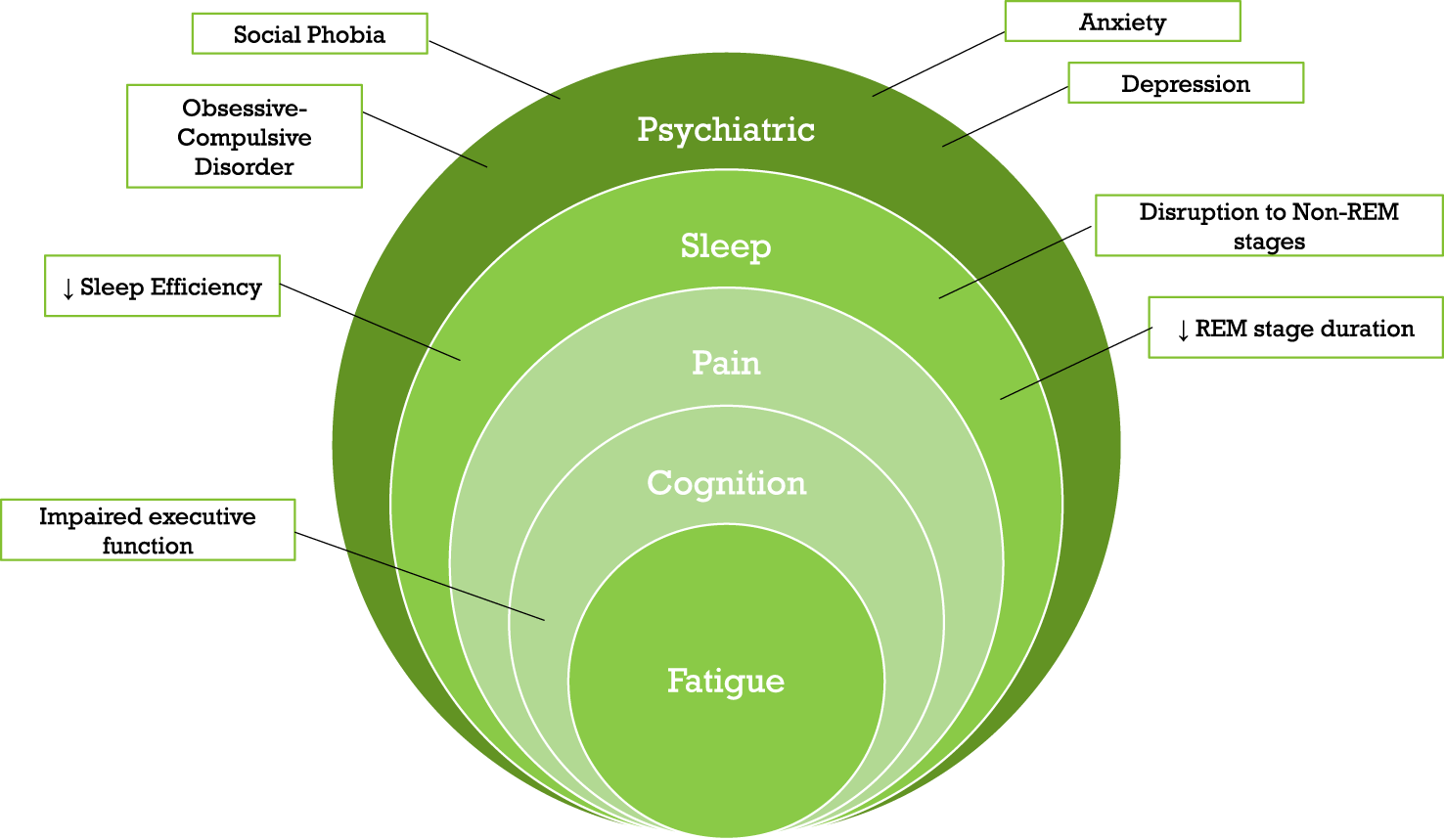
Schematic representation of the non-motor symptoms discussed and reviewed in this workshop, coupled with key examples from each symptom type. Key: REM, Rapid Eye Movement.
Methods: workshop details
The workshop was held virtually on 1st and 2nd March 2023, with the co-authors of this manuscript in attendance. Talks were given by those with expertise in each of the fields: psychiatric (BB, YW), pain (MT, NB), sleep (SPR, AWS), cognition (GD), pediatric assessments (HG, ER), models of care (DM, KP) and co-chaired by the corresponding authors. Time was given to discussion after each set of presentations focused on a single symptom group, with summary sessions held at the end of each day. The manuscript below summarises the detail of the presentations coupled with the associated discussion, with Table 1 developed as a summary item from the workshop.
TABLE 1
| Non-motor domain | Phenomenology | Assessment | Mechanism | Treatment |
|---|---|---|---|---|
| Depression Anxiety | What is the profile of mood and anxiety disorders in pediatric dystonia? | What is the most accurate screening tool for depressive and/or anxiety symptoms in dystonia? | Are depression and anxiety in dystonia associated with dysfunction of different brain networks compared to depression/anxiety in other movement disorders or unrelated to movement disorders? | Can we predict when established treatments for dystonia will improve depression and anxiety? |
| What prevalence and impact does suicidal behaviour have? | How much does stigma account for the severity of depression/anxiety in dystonia? | Do depression and anxiety in dystonia respond to anti-depressants? | ||
| How do emotional symptoms impact cognition and sleep in dystonia? | Which patients with depression/anxiety and dystonia are more likely to respond to behavioural therapies? | |||
| Is there a role for non-invasive brain stimulation to treat depression in dystonia? | ||||
| Sleep | What prevalence and impact does sleep disruption have in pediatric dystonia? | What is the accuracy of wearable devices in detecting sleep disruption in dystonia? | What are the brain networks associated with sleep disruption in dystonia? | Can established treatments for dystonia improve sleep disruption? And in whom? |
| What is the comorbidity profile of sleep disorders in dystonia in general? | When is it necessary to perform PSG in people with dystonia? | Could sleep disruption in dystonia contribute to maladaptive plasticity or other mechanisms that promote the motor symptoms of dystonia? | In which patients with dystonia should we use sleep-inducing treatments? Are people with dystonia at higher risk for sleep-inducing medication overuse? | |
| What is the accuracy of sleep questionnaires in detecting clinically relevant sleep disruption in dystonia? | What is the impact of psychotropic medications, depression, anxiety, and pain on sleep disruption in dystonia? | Are circadian rhythm profiles preserved in dystonia? | ||
| Pain | Can we distinguish dystonia-related pain from other sources of pain? | Should we use a common rating instrument for pain across all forms of dystonia, or do different forms require different assessment strategies? | What is the relative contribution of myogenic vs. peripheral, non-myogenic vs. central mechanisms to pain in the different forms of dystonia? | Is the effect of BNTx on pain supported by the same mechanism(s) that support(s) the effect on motor symptoms? |
| How can we categorize uncomfortable, but not painful, sensory symptoms reported by people with dystonia? | What is the contribution of emotional symptoms and stigma to pain perception and interpretation by people with dystonia? | What analgesic medications are most likely to help pain in dystonia? | ||
| Are people with dystonia at higher risk for analgesics’ overuse? | ||||
| Cognitive impairment | Do different forms of isolated dystonia differ by cognitive profile? | What type of neuropsychometric assessment is preferable in dystonia, according to age group? | Is cognitive dysfunction in dystonia associated with dysfunction of different brain networks compared to cognitive impairment in other movement disorders or unrelated to movement disorders? | Are people with dystonia and cognitive impairment more at risk to undergo DBS? |
| Is cognitive impairment in dystonia a prodrome of major neurocognitive decline? | How much does depression account for cognitive impairment in dystonia? | Is there a role for non-invasive brain stimulation to treat cognitive dysfunction in dystonia? | ||
| Fatigue | What is the functional impact of fatigue in people with dystonia? | What is the most valid and reliable fatigue measure in dystonia? | What is the difference between fatigue mechanisms in Parkinson’s disease and other movement disorders and the mechanisms underlying fatigue in dystonia? | Can we predict when established treatments for dystonia will improve fatigue? |
| Is fatigue prevalent in some forms of dystonia more than others? | Is there a physiological marker of fatigue in dystonia? | Can exercise help improve fatigue in dystonia? | ||
| Can we differentiate between “physical” and “mental” fatigue in dystonia? | What is the contribution of emotional symptoms, sleep disruption, pain and cognitive difficulties to fatigue in people with dystonia? | Is there a role for non-invasive brain stimulation to treat fatigue in dystonia? |
Key research questions related to non-motor domains in dystonia.
Psychiatric symptoms
Evidence evaluation was divided into that relating to isolated dystonias, most commonly adult-onset forms, and monogenic forms of dystonia. Most publications have focused on the type and rate of psychiatric symptoms, and whether these are significantly more prevalent compared to that of a control population or those affected by another disorder, for example, hemifacial spasm. Driven by anecdotal clinical observation and larger case series, this work has mostly focused on anxiety-related disorders and depression, primarily involving prospectively recruited adult-onset idiopathic dystonia cohorts, and identifying higher rates of mood and anxiety disorders across a range of dystonia subtypes [2, 3]. More specifically in those with cervical dystonia (CD), rates of reported anxiety related symptoms range from 40% to 83.6%, and 37%–53% for mood disorders, including major depressive disorder [4, 5], while in blepharospasm (BSP) rates of anxiety are twice that of hemifacial spasm [6]. Cohorts examining those with spasmodic dysphonia and focal hand dystonia have tended to be smaller with 52% of the former meeting diagnostic criteria for any psychiatric disorder, compared to 20% for those with vocal fold paralysis [7], and a study of 39 cases diagnosed with writer’s cramp or musician’s dystonia, found rates of anxiety disorder and recurrent depression to be 26% and 18%, respectively [8]. More recently, a systematic review and meta-analysis of previous studies found odds ratios for anxiety disorders in idiopathic dystonia to be 6.73 compared to unaffected controls, and 5.77 compared to those with other medical co-morbidities, such as alopecia areata. By contrast, odds ratios for depression were 4.86 and 3.62 for the same group comparisons, respectively [9]. Recently, results from the Dystonia Coalition dataset (n = 478) have shown higher rates of anxiety in CD and laryngeal dystonia, compared to those with cranial or limb involvement, while no differences in rates of depression were observed across the motor phenotypes [10]. Another, more recent study from the Dystonia Coalition focused on oromandibular dystonia (n = 727) where, through use of standardised scales, 34% of the cohort had scores reaching symptomatic thresholds for depression, and 44% for social anxiety [11].
Exploration of psychiatric symptoms in monogenic forms of dystonia has focused on early onset torsion dystonia (TOR1A), myoclonus dystonia (SGCE) and rapid-onset dystonia-parkinsonism (ATP1A3), with a recent meta-analysis (SGCE, n = 240, 6 studies; TOR1A, n = 96, 2 studies; ATP1A3, n = 29, 1 study), evaluating the cumulative odds ratio across the multiple studies [9]. The overall odds ratio for psychiatric symptoms in DYT11 was 5.68, with this value increasing for more specific disorders including obsessive-compulsive disorder (OCD), anxiety disorders and alcohol dependence. Further focus on those harbouring SGCE variants highlighted longitudinal studies demonstrating stable motor symptoms but a change to psychiatric burden over time [12]. By contrast, a follow-up study of those who had received DBS found that anxiety and depression scores normalised after intervention [13]. Others have suggested a higher frequency of impulse control disorders and motor impulsivity, indexed by experimental tasks, in the SGCE population [13]. Imaging studies have suggested that the proposed lack of inhibitory control underpinning motor impulsivity is related to aberrant activation of the motor cortex-cerebellum network and insula [14]. Others have focused on genetic forms of dystonia directly linked to the dopaminergic metabolic pathways, with a larger case series of those harbouring GCH1 mutations (DYT5) demonstrating a significant overall excess of psychiatric symptoms (p < 0.05), and more specifically generalised anxiety disorder and agoraphobia [14].
A key discussion related to whether these psychiatric symptoms represented primary or secondary phenotypes, with the prevailing view being that both were likely true, with variation dependent on type of dystonia and nature of the psychiatric symptoms. Several studies have demonstrated pre-motor onset of psychiatric symptoms, apparent in ∼60% of an Italian registry study, and prominent in those with CD and dystonic tremor in another study [15, 16]. Familial studies have also indicated a higher rate of psychiatric symptoms amongst siblings of those diagnosed with dystonia, suggesting common underlying biological processes, rather than secondary sequelae [17]. Furthermore, the increased prevalence of early-onset recurrent major depression in TOR1A pathogenic variant carriers in the absence of motor features of dystonia, highlights the potential for independent occurrence of non-motor symptoms in dystonia [18].
The pathophysiological basis of these symptoms in dystonia remains to be determined however, an unbiased systems-biology approach to investigate the cellular specificity of known dystonia causing genes and whether a functional relationship was shared with neuropsychiatric disorders identified enrichment within the putamen, frontal cortex, and white matter modules [19]. These regions are consistent with the network-based model for dystonia, in which evidence for involvement of the basal ganglia has been seen in murine studies and human imaging analyses [20], as well as involvement of the cerebral cortex in human electrophysiological studies and patient-derived stem cell models [21, 22], Few studies have directly addressed the treatment of psychiatric symptoms in dystonia, although population level linked clinical data studies have identified almost half of dystonia cohorts to have been prescribed at least one anti-depressant or anxiolytic oral medical therapy [15]. Alternative approaches have included in person delivery of group cognitive behavioural and mindfulness therapies, as well as internet-based, self-guided cognitive behavioural therapy, both of which provided indications of symptomatic improvement in limited size study populations [23, 24].
These discussions led to a debate on the potential need for dystonia-specific tools and scales to better define and provide greater sensitivity to change in severity of psychiatric symptoms. Tools used to date have included standardised questionnaires based on DSM-IV diagnostic categories including, for example, the Hamilton Depression Rating Scale and Patient Health Questionnaire (PHQ-9) for depression [25], Beck Anxiety Inventory [26] and State Trait Anxiety Scale for anxiety [27], the Yale-Brown Obsessive-Compulsive Scale for Obsessive-Compulsive Disorder (OCD) [28, 29], coupled with broader diagnostic screening questionnaires such as the MINI International Neuropsychiatric Inventory (M.I.N.I) [30]. However, these tools have largely been developed either as a brief mechanism for screening within the general population, or as a means of quantifying symptom severity in psychiatric services. Additionally, the tools used have varied across individual studies in dystonia, with the added complexity as to whether they are designed for participant self-completion (either in paper form or digitally) or researcher delivered. Analogy was made to Parkinson’s disease (PD), where use of generic scales lacked sensitivity in identifying symptoms of anxiety thereby leading to the development of a PD specific anxiety scale [31]. While some agreement was reached that non-motor symptom specific scales would be of benefit, the group agreed that use and/or generation of any scales should be applied across all dystonia subtypes to allow for uniformity of approach. Points were raised as to whether more relevant screening instruments were needed for dystonia, noting the recent Dystonia Non-Motor Symptoms Questionnaire (DNMSQuest), although the focus here on individual non-motor symptom types is limited [32].
Pain
The negative impact of pain on quality of life and daily functioning of people with dystonia is well recognized. Pain is the most frequently reported non-motor symptom by CD patients, with a prevalence of 54.6%–88.9% [33], and primarily involving the neck and shoulders, although this may spread to the upper back, cranial region on the side of head deviation, or the ipsilateral upper limb. Approximately 10%–30% of those diagnosed with CD also report chronic headache, predominantly in the occipital (79%) or nuchal (73%) regions. People with BSP typically report ocular dysesthesia, occasionally described as pain, or photo-oculodynia. More than a third of patients with upper or lower limb dystonia report clinically significant pain, whereas systematic data collection on jaw and throat pain in oromandibular and laryngeal dystonia is lacking. In CD specifically, pain correlates with motor severity and there is a 2.6-fold higher frequency of alleviating manoeuvres in CD with pain compared to those without. The TWSTRS and its revised version (TWSTRS-2) [34] have been the only disease-specific instruments (focused on CD) to include a pain sub-scale for decades, scoring the patient’s usual, worst, and best pain severity in the previous week, and a pain-related disability score. Recently, Bruno et al. developed the Pain in Dystonia Scale (PIDS) [35], the first disease-specific instrument to evaluate pain across different body regions, and applicable to all patients with adult-onset isolated dystonia. Validated to date only in CD, the PIDS includes a severity scale, a functional impact measure and a questionnaire collecting data on pain-modulating factors. Another group have recently developed and tested a “patient-relevant” classification framework for chronic pain in dystonia [the Dystonia Pain Classification System (Dystonia-PCS)]. This scale focuses more on the relationship between pain and dystonia, specifically evaluating whether the pain is directly related to, aggravated by, or unrelated to dystonia [36].
Pain has also been reported in non-dystonic muscles of those diagnosed with dystonia, with pressure algometry showing no correlation with the degree of contraction of dystonic muscles [37]. Some experts claim that localising and targeting pain increases the success of botulinum neurotoxin treatment in those with CD [38]. Tinazzi et al. applied a conditioned pain modulation protocol, demonstrating defective inhibition in CD patients, both with and without pain, suggesting a primary deficit of the endogenous descending inhibitory pain system, a feature not observed in those with BSP [39]. The involvement of sensory processing in the mechanisms of pain in dystonia is also supported by preliminary findings of pain improvement in CD using sensory modulation techniques, e.g., kinesiotaping, although results differ between studies [40, 41]. The group highlighted the need for future work to elucidate the relationship between pain and other non-motor features of dystonia, in particular emotional dysregulation. In CD patients, a Bayesian approach applied to cluster analyses demonstrated a link between greater pain acceptance and lower degree of perceived pain, whereas the presence of relevant depressive and anxiety symptoms correlated with a catastrophic interpretation of pain [42].
Limited evidence exists for the systematic evaluation of pain therapies in dystonia however, studies have demonstrated superior efficacy of injectable botulinum toxin, particularly in the treatment of cervical dystonia, over oral medical therapies, such as trihexyphenidyl [43]. However, observational studies have indicated 45% of cervical dystonia cohorts receiving botulinum toxin treatment to be additionally taking oral analgesic medication [44], In this setting the majority were taking non-steroidal anti-inflammatory medication, of importance given a further study from the Dystonia Coalition identified rates of 11% for substance abuse amongst cohorts of patients with CD, with these symptoms were higher amongst those using opiate analgesia for pain management [45].
Sleep disruption
Observational studies of sleep have consistently documented disturbance in people with adult-onset dystonia, predominantly CD (40%–70%), with limited evidence from pediatric cohorts [46, 47]. Questionnaire- or survey-based studies have found reduced sleep quality using non-disease-specific instruments (primarily, the Pittsburgh Sleep Quality Index). Preliminary qualitative data from the Dystonia Coalition Patient-Centered Outcomes Project show that CD patients rate an improvement of 32% as the smallest meaningful improvement for sleep disruption (Pirio Richardson, et al., in preparation). Overall, studies to date of sleep physiology and quality in dystonia are limited by small sample sizes, heterogeneity of patient population, lack of control groups in some studies, inclusion of patients receiving medical treatment for sleep disturbance, and a paucity of studies combining self-reported measures and polysomnographic recordings. The latter has been used in assessment of a small cohorts of those diagnosed with CD, BSP, segmental craniofacial dystonia and dopa-responsive dystonia (GCH1), consistently showing reduced sleep efficiency, increased sleep latency, increased duration of N1 sleep phase and decreased duration of REM sleep [48–50]. Overnight recordings have also shown a significant decrease in signal amplitude from the more affected sternocleidomastoid muscle in CD patients during N3 phase of sleep, possibly suggesting sleep-promoted homeostatic balance, whereby “over-active” muscles during the day need “deeper” rest at night. Brain slow wave activity overnight is considered an important physiological change, allowing for synaptic downscaling and memory consolidation, and thus promoting brain plasticity. One study demonstrated a decrease in the power of physiological slow-wave activity in individuals with CD, potentially suggesting impaired synaptic downscaling resulting in impaired cortical plasticity [51]. Two recent murine models of dystonia also demonstrate sleep abnormalities (increased time awake and REM latency, and aberrant sleep architecture), suggesting multiple avenues for further investigation [52].
Selecting the most informative and feasible method of sleep assessment in people with dystonia remains a crucial goal. A recent multi-modal study of sleep quality used 1-week remote monitoring of 50 people with CD and 50 control participants through a consumer-grade wrist triaxial accelerometer, coupling it to daily sleep diary and other non-motor symptom questionnaires [53]. Accelerometry showed significantly longer total sleep time and time spent in NREM sleep in CD patients compared to control subjects, however, also evident was discrepancy between self-report and wearable data, significant for both dystonia and control subjects. However, future studies can build on this as data from wearable devices improves, improving validity with mathematical analysis of both accelerometric and polysomnographic data. Quantitative measures of sleep could use similar methodologies to those of physical activity, allowing a more accurate exploration of the association of sleep and activity levels with motor and other non-motor features of dystonia (e.g., depression, anxiety, pain). Few studies have examined sleep in interventional studies, demonstrating that motor improvement with BoNT or DBS is not paralleled by improvement in sleep quality [54, 55]. The impact of important potential contributors to sleep disruption, e.g., use of medications such as benzodiazepines, pain, depression, or other impactful non-motor symptoms, also remains under-explored.
Fatigue
Fatigue, intended as perception of physical or mental effort, remains poorly investigated in dystonia. Cross-sectional observational studies have applied both generic fatigue rating instruments, e.g., the Fatigue Severity Scale, and the DNMSQuest, consistently reporting a rates of 40%–50% in those with idiopathic dystonia, and a similar burden in dopa-responsive dystonias and myoclonus dystonia [56–58]. Fatigue severity has been shown to significantly correlate with quality of life in those with CD, and appears independent of psychiatric and motor symptom severity [57]. Beyond this, very little is known on the mechanisms of fatigue in dystonia and the effectiveness of different treatment approaches used in other disorders, such as PD. Future work should identify specific criteria to define and characterize fatigue in dystonia, with qualitative studies (interviews, focus groups) involving patients and health professionals, while physiological markers may be determined using techniques such as functional imaging techniques.
Cognitive dysfunction
Discussion of cognitive abnormalities focused primarily on isolated forms of dystonia, particularly adult-onset forms. Available studies have, however, generated mixed findings, which hinder the identification of a consistent cognitive profile. This has also been coupled with a high level of variation in the tools used to assess cognitive function, for example, the Wisconsin Card Sorting Test, multiple components of the adult and child versions of the Wechsler Intelligence Scale, Trail Making Test, Ray Auditory Verbal Learning Task, and the Florida Affect Battery [59–62]. A critical review of the literature demonstrated that most case-control observational studies, comparing isolated adult-onset dystonia to unaffected control, report poorer performance in those with dystonia [63, 64]. Interestingly, studies that failed to detect significant differences between groups were smaller in sample size, and involved those diagnosed with BSP or CD. The only cognitive domain in which consistent changes have been identified, is executive functions (also the most investigated domain), with inconsistent findings from other domains. Changes to executive dysfunction have most consistently been demonstrated in CD and DYT1 (TOR1A) forms of dystonia, while studies focused on other forms, for example, BSP, have shown deficits in sustained attention, complex movement planning and visuospatial working memory [65, 66]. When investigated, there was no significant correlation between cognitive impairment and severity of motor symptoms or depressive/anxiety symptoms, suggesting that these cognitive changes are not merely secondary to motor or mood disturbance, instead aligning with the view that executive dysfunction might stem from abnormal connectivity within fronto-striatal networks. In a previous study pre- and post-pallidal DBS stimulation in isolated dystonia, cognitive functions were reported to “improve” post-intervention, interpreted as a “re-allocation of mental resources.” Twelve months post-surgery free recall improved, with significant reduction in the number of errors in the WCS [67]. Consideration should also be given to the involvement of cerebellar pathways in the genesis of cognitive deficits in dystonia, with evidence suggesting a key role for these pathways in several cognitive functions, e.g., attention and language. Finally, available observational studies have used a cross-sectional design, therefore limiting insight of the longitudinal course of cognitive dysfunction.
Several objectives for future research in this area were discussed and proposed. First, there are limited data on the difference in cognitive profile between different forms of isolated adult-onset dystonia. Performance on broad cognitive assessment forms such as the Addenbrooke’s Cognitive Examination Revised (ACE-R) found worse performance in people with adult-onset dystonia compared to unaffected controls, but no differences between individual dystonia types [68]. A more recent study of social behaviour, one of the key domains of social cognition, reported a deficit in CD but not BSP. Overall, there is a need for more research on the motor-cognitive correlation, and a refinement—for example, through combining several established and standardised cognitive assessment to create a recognised battery of dystonia cognitive assessments—of the tools used to allow for greater across cohort comparison. Ideally these should be coupled with imaging studies exploring structural and functional connectivity patterns. Understanding this relationship will aid clinicians in tailoring cognitive assessments to specific motor presentations of dystonia, as well as better understand and monitor the cognitive risk of different motor symptom treatments, including DBS [69].
Pediatric dystonia—a different phenotypic group
The group began the discussion of NMS in pediatric dystonia questioning whether assessments should consider children/young people and adults distinctly, or whether there were approaches to capturing non-motor symptoms that would allow continuity while retaining sensitivity to early developmental change. Initial discussions focused on the need to recognise that ‘normal’ varies across developmental stages with specific examples provided for sleep-wake duration, sleep patterns, susceptibility and frequency of disorders which depend on age. For example, NREM parasomnias, such as night terrors, are more common in children, while restless leg syndrome is more common in adults. Similar differences can be seen in the diagnostic inference of psychiatric symptoms, where depression in adults involves description of feelings of sadness and despair, while in children is more likely to manifest in the form of anger and irritability. Additionally, age at symptom onset or diagnosis is also of importance in terms of likely diagnoses, e.g., anxiety, OCD and depression can arise pre-puberty and continue into adult life, whereas schizophrenia rarely begins pre-puberty. Key challenging questions emerging from this initial discussion included: if non-motor tools were to be used throughout the lifespan, would they sufficiently capture the same/similar symptoms, and whether there are advantages to considering all ages collectively, or whether greater understanding would be gained from independent investigation?
Although several studies have identified evidence of non-motor symptoms in childhood-onset dystonia, at present there are no validated outcome measures [70]. The studies that have been undertaken to date are often small and heterogenous regarding dystonia aetiology, task performance across individuals being frequently unpredictable and not reliably assessed with single timepoint measures [71]. Intervention and evaluation has instead focused on tools used in rehabilitation services, rather than symptom checklists. One such framework is the International Classification of Functioning, Disability and Health (ICF), a World Health Organisation (WHO) classification of health and health-related domains. This examines impairments to body function, limitation to activities, restriction to participation, while the Canadian Occupational Performance Measure (COPM), for use at all ages, allows the individual to prioritise daily issues that may restrict activity participation. Strategies developed for intervention include those focused on a cognitive approach, namely, the Cognitive Orientation to daily Occupational Performance (CO-OP), demonstrated to be effective in its delivery across therapists of multiple clinical backgrounds [72–74]. The CO-OP approach seeks to apply cognitive and motor learning theories and meta-cognition to everyday activities, measuring performance over time. Multiple studies within this framework, however, have demonstrated the need for repeated longitudinal assessments, rather than single evaluations, due to the fluctuant nature of performance. Given this potential for performance and functional variability, “n-of-1” trials have been proposed as a more effective approach to evaluating interventions within highly heterogenous pediatric dystonia cohorts, with further work needed to establish a consensus set of outcome measures considered valid for this patient population.
Discussion
Models of care–the need for new approaches and treatment pathways in dystonia
Transitioning improved research understanding into clinical services formed the final discussion section, identifying the urgent need for updated models of care that comprehensively address both motor and non-motor symptoms. The current model of care, predominantly applied to adult-onset dystonia, is centered on the motor features of dystonia and involves frequent (quarterly) but brief clinic consultations for delivery of BoNT injections. The temporal and spatial constraints of these services hinder screening, assessment, and active management of non-motor symptoms, often leaving these tasks to primary care physicians and increasing the risk that non-motor symptoms are under-recognized by specialists and passively accepted by patients. Overall, this generates patient dissatisfaction, who have openly criticized these limitations in the small number of qualitative studies available. This problem appears to be considerably less relevant in pediatric dystonia, in which a more comprehensive work-up and related management plan are typically initiated, supporting the need for transition services from childhood/adolescent care to adult services [75].
Multiple opportunities exist for learning from other movement disorders, such as Tourette’s Syndrome, a disorder with well recognised motor and psychiatric symptoms, in which clinic consultations typically involve extensive batteries of symptom checklists or screening tools, often undertaken electronically in advance of the clinic appointment. Consensus around symptomatic screening represents step one in this process, and while digital technology, such as online or app delivered questionnaires and use of wearable devices, represent attractive approaches for data collection, any care model would ideally be implemented internationally, with a desire to avoid widening differences between higher and lower income countries. However, care models need to extend beyond data capture, and involve a framework for delivery of assessment outcomes to patients, and any clinical intervention they might drive, such as anxiolytic or anti-depressant medication, or cognitive behavioural therapy [24, 76]. To date there has been little research focus on establishing effective interventions for management of these non-motor symptoms, with sparse available data even for the evaluation of commonly prescribed medication, such as selective serotonin reuptake inhibitors (SSRIs), in the treatment of anxiety and depression in dystonia [77]. A more standardised approach to non-motor symptom capture would provide the framework necessary to evaluate future interventions and establish clinical trials.
Evidence from qualitative studies, which have focused more on psychiatric features, supports the use of one (or more) self-administered screening instrument(s), coupled with an evaluation of functional impairment deriving from these symptoms [78, 79]. A single instrument covering the full spectrum of non-motor symptoms would appear to be the most efficient solution for clinical screening, and although the development of the DNMSQuest has contributed to raised awareness of non-motor symptoms in dystonia, this instrument is a simple yes/no questionnaire that merges non-motor and motor (gait, swallowing) features without providing detail of their severity or functional impact [32]. Different strategies could be considered to optimize clinical screening, including revision/expansion of the DNMSQuest to involve judgement of clinical relevance to guide further action, or creation of a comprehensive instrument through the collation of items from pre-existing questionnaires that demonstrate the greatest accuracy in identifying non-motor features. Moreover, questionnaire-based screening could be integrated with data capture from wearable sensors or combined with patient-centered outcomes to achieve a more informed clinical decision. Screening repetition should take place at an interval that is clinically meaningful and practically actionable, such as an annual assessment that could be integrated with other activities such as enrolment in clinical or research registries. For pediatric forms, a more detailed assessment that considers multiple psychiatric screening tools, multi-domain cognitive assessment, videotaped motor examination, and pain assessment is likely to be necessary.
Where should we start to make an impactful change?
As outlined above, clinical characterization and knowledge of assessment methods differ across the various non-motor features associated with dystonia, with the key knowledge gaps highlighted in the workshop summarized in Table 1. Whereas the clinical characteristics, frequency, and related impact on quality of life have been outlined in some depth for psychiatric features and pain, evaluation of the impact of sleep disturbance, cognitive impairment, and fatigue in patients with isolated dystonia should still be considered in progress. Although dystonia-specific instruments have been developed for psychiatric symptoms (but only in CD) and pain, there are no guidelines on which disease-specific or non-disease-specific rating instruments are most suitable for dystonia in either research or clinical settings, and there are no recommendations on how best to screen for these symptoms in clinical practice. This uncertainty is even greater for those features that require more elaborate phenotyping such as sleep or cognitive dysfunction. The difference in natural history, phenomenology and functional impact between pediatric and adult dystonia suggests an independent approach to non-motor symptoms for these two distinct life stages is necessary. Adult-onset dystonia, at the same time, also appears to be very heterogeneous with respect to motor and non-motor features, and it seems likely that assessment of non-motor features in clinical practice will eventually need to be diversified based on the prominent motor phenotype. We also lack evidence on how responsive non-motor symptoms are to therapies for mood, anxiety, pain, and sleep disruption repurposed “non-specifically” in people with dystonia.
Conclusion
The multifaceted nature of the “non-motor” dimension of dystonia complicates identification of the most promising strategy to tackle the clinical problem. Work should begin collaboratively to facilitate progress toward providing improved care with clinicians needing a practical and sufficiently informative screening tool that encompasses the “non-motor spectrum” of dystonia as we currently understand it. Therefore, the first objective selected by the workshop group, is the development and field validation of a self-administered screening tool that could easily be incorporated into specialist movement disorders clinics. As we strive towards better understanding of mechanisms and personalized therapies for non-motor symptoms, we are confident that this simple but effective advance in screening capacity will increase awareness of the frequency and impact of these symptoms amongst clinicians and patients. Such an advance is critical to a sustainable improvement of quality of care for people with lived experience of this highly impactful and complex neurological condition.
Statements
Data availability statement
No new data was created during this study.
Author contributions
KP, DM, and JT were involved in organising the workshop, KP and DM chaired the workshop and wrote the original draft of the manuscript. All authors contributed to the workshop, article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Albanese A Bhatia K Bressman SB Delong MR Fahn S Fung VSC et al Phenomenology and classification of dystonia: a consensus update. Mov Disord (2013) 28:863–73. 10.1002/mds.25475
2.
Moraru E Schnider P Wimmer A Wenzel T Birner P Griengl H et al Relation between depression and anxiety in dystonic patients: implications for clinical management. Depress Anxiety (2002) 16:100–3. 10.1002/da.10039
3.
Fabbrini G Berardelli I Moretti G Pasquini M Bloise M Colosimo C et al Psychiatric disorders in adult-onset focal dystonia: a case-control study. Mov Disord (2010) 25:459–65. 10.1002/mds.22983
4.
Wenzel T Schnider P Wimmer A Steinhoff N Moraru E Auff E . Psychiatric comorbidity in patients with spasmodic torticollis. J Psychosom Res (1998) 44:687–90. 10.1016/s0022-3999(97)00229-8
5.
Gundel H Wolf A Xidara V Busch R Ceballos-Baumann AO Social phobia in spasmodic torticollis. J Neurol Neurosurg Psychiatry (2001) 71:499–504. 10.1136/jnnp.71.4.499
6.
Hall TA McGwin G Searcey K Xie A Hupp SL Owsley C et al Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol (2006) 124:116–9. 10.1001/archopht.124.1.116
7.
Gundel H Busch R Ceballos-Baumann A Seifert E Psychiatric comorbidity in patients with spasmodic dysphonia: a controlled study. J Neurol Neurosurg Psychiatry (2007) 78:1398–400. 10.1136/jnnp.2007.121699
8.
Voon V Butler TR Ekanayake V Gallea C Ameli R Murphy DL et al Psychiatric symptoms associated with focal hand dystonia. Mov Disord (2010) 25:2249–52. 10.1002/mds.23250
9.
Lane V Lane M Sturrock A Rickards H Understanding psychiatric disorders in idiopathic and inherited (monogenic) forms of isolated and combined dystonia: a systematic review. J Neuropsychiatry Clin Neurosci (2021) 33:295–306. 10.1176/appi.neuropsych.20110293
10.
Berman BD Junker J Shelton E Sillau SH Jinnah HA Perlmutter JS et al Psychiatric associations of adult-onset focal dystonia phenotypes. J Neurol Neurosurg Psychiatry (2017) 88:595–602. 10.1136/jnnp-2016-315461
11.
Scorr LM Factor SA Parra SP Kaye R Paniello RC Norris SA et al Oromandibular dystonia: a clinical examination of 2,020 cases. Front Neurol (2021) 12:700714. 10.3389/fneur.2021.700714
12.
Timmers ER Peall KJ Dijk JM Zutt R Tijssen CC Bergmans B et al Natural course of myoclonus-dystonia in adulthood: stable motor signs but increased psychiatry. Mov Disord (2020) 35:1077–8. 10.1002/mds.28033
13.
Kosutzka Z Tisch S Bonnet C Ruiz M Hainque E Welter ML et al Long-term GPi-DBS improves motor features in myoclonus-dystonia and enhances social adjustment. Mov Disord (2019) 34:87–94. 10.1002/mds.27474
14.
Timmers ER Kuiper A Smit M Bartels AL Kamphuis DJ Wolf NI et al Non-motor symptoms and quality of life in dopa-responsive dystonia patients. Parkinsonism Relat Disord (2017) 45:57–62. 10.1016/j.parkreldis.2017.10.005
15.
Bailey GA Rawlings A Torabi F Pickrell WO Peall KJ Longitudinal analysis of the relationship between motor and psychiatric symptoms in idiopathic dystonia. Eur J Neurol (2022) 29:3513–27. 10.1111/ene.15530
16.
Berardelli I Ferrazzano G Pasquini M Biondi M Berardelli A Fabbrini G . Clinical course of psychiatric disorders in patients with cervical dystonia. Psychiatry Res (2015) 229:583–5. 10.1016/j.psychres.2015.07.076
17.
Martino D Brander G Svenningsson P Larsson H de la Cruz LF Association and familial coaggregation of idiopathic dystonia with psychiatric outcomes. Mov Disord (2020) 35:2270–8. 10.1002/mds.28257
18.
Heiman GA Ottman R Saunders-Pullman RJ Ozelius LJ Risch NJ Bressman SB . Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology (2004) 63:631–7. 10.1212/01.wnl.0000137113.39225.fa
19.
Mencacci NE Reynolds R Ruiz SG Vandrovcova J Forabosco P Sánchez-Ferrer A et al Dystonia genes functionally converge in specific neurons and share neurobiology with psychiatric disorders. Brain (2020) 143:2771–87. 10.1093/brain/awaa217
20.
Wilson BK Hess EJ Animal models for dystonia. Mov Disord (2013) 28:982–9. 10.1002/mds.25526
21.
Tseng YJ Chen RS Hsu WY Hsiao FJ Lin YY Reduced motor cortex deactivation in individuals who suffer from writer's cramp. PLoS One (2014) 9:e97561. 10.1371/journal.pone.0097561
22.
Sperandeo A Tamburini C Noakes Z de la Fuente DC Keefe F Petter O et al Cortical neuronal hyperexcitability and synaptic changes in SGCE mutation-positive myoclonus dystonia. Brain (2023) 146:1523–41. 10.1093/brain/awac365
23.
Sandhu H Bernstein CJ Davies G Tang NKY Belhag M Tingle A et al Combined cognitive-behavioural and mindfulness programme for people living with dystonia: a proof-of-concept study. BMJ Open (2016) 6:e011495. 10.1136/bmjopen-2016-011495
24.
Wadon ME MacIver C Winter M Peall KJ Internet-based cognitive behavioural therapy as a feasible treatment of adult-onset, focal, isolated, idiopathic cervical dystonia. Clin Park Relat Disord (2021) 5:100121. 10.1016/j.prdoa.2021.100121
25.
Kroenke K Spitzer RL Williams JB The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med (2001) 16:606–13. 10.1046/j.1525-1497.2001.016009606.x
26.
Beck AT Epstein N Brown G Steer RA An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol (1988) 56:893–7. 10.1037//0022-006x.56.6.893
27.
Tluczek A Henriques JB Brown RL Support for the reliability and validity of a six-item state anxiety scale derived from the state-trait anxiety inventory. J Nurs Meas (2009) 17:19–28. 10.1891/1061-3749.17.1.19
28.
Goodman WK Price LH Rasmussen SA Mazure C Delgado P Heninger GR et al The yale-brown obsessive compulsive scale. II. validity. Arch Gen Psychiatry (1989) 46:1012–6. 10.1001/archpsyc.1989.01810110054008
29.
Goodman WK Price LH Rasmussen SA Mazure C Fleischmann RL Hill CL et al The yale-brown obsessive compulsive scale. I. development, use, and reliability. Arch Gen Psychiatry (1989) 46:1006–11. 10.1001/archpsyc.1989.01810110048007
30.
Sheehan DV Lecrubier Y Sheehan KH Amorim P Janavs J Weiller E et al The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry (1998) 59(20):22–57.
31.
Leentjens AF Dujardin K Pontone GM Starkstein SE Weintraub D Martinez-Martin P . The Parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov Disord (2014) 29:1035–43. 10.1002/mds.25919
32.
Klingelhoefer L Chaudhuri KR Kamm C Martinez-Martin P Bhatia K Sauerbier A et al Validation of a self-completed dystonia non-motor symptoms questionnaire. Ann Clin Transl Neurol (2019) 6:2054–65. 10.1002/acn3.50900
33.
Avenali M De Icco R Tinazzi M Defazio G Tronconi L Sandrini G et al Pain in focal dystonias - a focused review to address an important component of the disease. Parkinsonism Relat Disord (2018) 54:17–24. 10.1016/j.parkreldis.2018.04.030
34.
Comella CL Perlmutter JS Jinnah HA Waliczek TA Rosen AR Galpern WR et al Clinimetric testing of the comprehensive cervical dystonia rating scale. Mov Disord (2016) 31:563–9. 10.1002/mds.26534
35.
Bruno V Achen B Morgante F Erro R Fox SH Edwards MJ et al The pain in dystonia scale (PIDS)-development and validation in cervical dystonia. Mov Disord (2023) 38:1175–86. 10.1002/mds.29452
36.
Listik C Listik E de Paiva Santos Rolim F Meneses Cury Portela DM Perez Lloret S de Alves Araújo NR et al Development and validation of the dystonia-pain classification system: a multicenter study. Mov Disord (2023) 38:1163–74. 10.1002/mds.29423
37.
Morgante F Matinella A Andrenelli E Ricciardi L Allegra C Terranova C et al Pain processing in functional and idiopathic dystonia: an exploratory study. Mov Disord (2018) 33:1340–8. 10.1002/mds.27402
38.
Camargo CH Cattai L Teive HA Pain relief in cervical dystonia with botulinum toxin treatment. Toxins (Basel) (2015) 7:2321–35. 10.3390/toxins7062321
39.
Tinazzi M Squintani GM Bhatia KP Segatti A Donato F Valeriani M et al Pain in cervical dystonia: evidence of abnormal inhibitory control. Parkinsonism Relat Disord (2019) 65:252–5. 10.1016/j.parkreldis.2019.06.009
40.
Pelosin E Avanzino L Marchese R Stramesi P Bilanci M Trompetto C et al Kinesiotaping reduces pain and modulates sensory function in patients with focal dystonia: a randomized crossover pilot study. Neurorehabil Neural Repair (2013) 27:722–31. 10.1177/1545968313491010
41.
Dec-Cwiek M Porębska K Sawczyńska K Kubala M Witkowska M Zmijewska K et al Kinesiotaping after botulinum toxin type A for cervical dystonia in adult patients. Brain Behav (2022) 12:e2541. 10.1002/brb3.2541
42.
Wadon ME Bailey GA Yilmaz Z Hubbard E AlSaeed M Robinson A et al Non-motor phenotypic subgroups in adult-onset idiopathic, isolated, focal cervical dystonia. Brain Behav (2021) 11:e2292. 10.1002/brb3.2292
43.
Brans JW Lindeboom R Snoek JW Zwarts MJ van Weerden TW Brunt ER et al Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double-blind controlled trial. Neurology (1996) 46:1066–72. 10.1212/wnl.46.4.1066
44.
Trosch RM Espay AJ Truong D Gil R Singer C LeWitt PA et al Multicenter observational study of abobotulinumtoxin a neurotoxin in cervical dystonia: the ANCHOR-CD registry. J Neurol Sci (2017) 376:84–90. 10.1016/j.jns.2017.02.042
45.
Mahajan A Jankovic J Marsh L Patel A Jinnah HA Comella C et al Cervical dystonia and substance abuse. J Neurol (2018) 265:970–5. 10.1007/s00415-018-8840-9
46.
Hertenstein E Tang NKY Bernstein CJ Nissen C Underwood MR Sandhu HK . Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev (2016) 26:95–107. 10.1016/j.smrv.2015.04.004
47.
Bailey GA Hubbard EK Fasano A Tijssen MA Lynch T Anderson KN et al Sleep disturbance in movement disorders: insights, treatments and challenges. J Neurol Neurosurg Psychiatry (2021) 92:723–36. 10.1136/jnnp-2020-325546
48.
Sforza E Montagna P Defazio G Lugaresi E Sleep and cranial dystonia. Electroencephalogr Clin Neurophysiol (1991) 79:166–9. 10.1016/0013-4694(91)90135-q
49.
Antelmi E Ferri R Provini F Scaglione CML Mignani F Rundo F et al Modulation of the muscle activity during sleep in cervical dystonia. Sleep (2017) 40 (7), zsx088. 10.1093/sleep/zsx088
50.
Bruggemann N Stiller S Tadic V Kasten M Münchau A Graf J et al Non-motor phenotype of dopa-responsive dystonia and quality of life assessment. Parkinsonism Relat Disord (2014) 20:428–31. 10.1016/j.parkreldis.2013.12.014
51.
Caverzasio S Amato N Chiaro G Staedler C Kaelin-Lang A Galati S . Impairment of sleep homeostasis in cervical dystonia patients. Sci Rep (2022) 12:6866. 10.1038/s41598-022-10802-y
52.
Leon LES Sillitoe RV Disrupted sleep in dystonia depends on cerebellar function but not motor symptoms in mice (2023). bioRxiv, Available at: https://www.biorxiv.org/content/biorxiv/early/2023/02/10/2023.02.09.527916.full.pdf (Accessed February 10 2023).
53.
Bailey GA Matthews C Szewczyk-Krolikowski K Moore P Komarzynski S Davies EH et al Use of remote monitoring and integrated platform for the evaluation of sleep quality in adult-onset idiopathic cervical dystonia. J Neurol (2023) 270:1759–69. 10.1007/s00415-022-11490-4
54.
Costanzo M Belvisi D Berardelli I Maraone A Baione V Ferrazzano G et al Effect of botulinum toxin on non-motor symptoms in cervical dystonia. Toxins (Basel) (2021) 13:647. 10.3390/toxins13090647
55.
Ouyang J Hao Q Zhu R Wu G Ding H Wang D et al Subthalamic nucleus deep brain stimulation in primary meige syndrome: a 1-year follow-up study. Neuromodulation (2021) 24:293–9. 10.1111/ner.13174
56.
Zhang L Hou Y Lin J Yang J Cao B Wei Q et al Comprehensive analysis of non-motor symptoms and their association with quality of life in writer's cramp. Parkinsonism Relat Disord (2022) 100:37–40. 10.1016/j.parkreldis.2022.05.025
57.
Wagle Shukla A Brown R Heese K Jones J Rodriguez RL Malaty IM et al High rates of fatigue and sleep disturbances in dystonia. Int J Neurosci (2016) 126:928–35. 10.3109/00207454.2015.1085035
58.
Timmers ER Smit M Kuiper A Bartels AL van der Veen S van der Stouwe AMM et al Myoclonus-dystonia: distinctive motor and non-motor phenotype from other dystonia syndromes. Parkinsonism Relat Disord (2019) 69:85–90. 10.1016/j.parkreldis.2019.10.015
59.
Burke T Monaghan R McCormack D Cogley C Pinto-Grau M O'Connor S et al Social cognition in cervical dystonia: a case-control study. Clin Park Relat Disord (2020) 3:100072. 10.1016/j.prdoa.2020.100072
60.
Coenen MA Eggink H Spikman JM Tijssen MA Cognition in children and young adults with myoclonus dystonia - a case control study. Parkinsonism Relat Disord (2021) 89:162–6. 10.1016/j.parkreldis.2021.07.016
61.
Maggi G D'Iorio A Mautone G Peluso S Manganelli F Dubbioso R et al Cognitive correlates of prospective memory in dystonia. Parkinsonism Relat Disord (2019) 66:51–5. 10.1016/j.parkreldis.2019.06.027
62.
Niccolai L Aita SL Walker HC Martin RC Clay OJ Crowe M et al An examination of the neurocognitive profile and base rate of performance impairment in primary dystonia. J Clin Neurosci (2020) 74:1–5. 10.1016/j.jocn.2019.12.050
63.
Bailey GA Martin E Peall KJ Cognitive and neuropsychiatric impairment in dystonia. Curr Neurol Neurosci Rep (2022) 22:699–708. 10.1007/s11910-022-01233-3
64.
O'Connor S Hevey D Burke T Rafee S Pender N O'Keeffe F A systematic review of cognition in cervical dystonia. Neuropsychol Rev (2023). 10.1007/s11065-022-09558-z
65.
Jahanshahi M Torkamani M The cognitive features of idiopathic and DYT1 dystonia. Mov Disord (2017) 32:1348–55. 10.1002/mds.27048
66.
Aleman GG de Erausquin GA Micheli F Cognitive disturbances in primary blepharospasm. Mov Disord (2009) 24:2112–20. 10.1002/mds.22736
67.
Pillon B Ardouin C Dujardin K Vittini P Pelissolo A Cottencin O et al Preservation of cognitive function in dystonia treated by pallidal stimulation. Neurology (2006) 66:1556–8. 10.1212/01.wnl.0000216131.41563.24
68.
Yang J Shao N Song W Wei Q Ou R Wu Y et al Nonmotor symptoms in primary adult-onset cervical dystonia and blepharospasm. Brain Behav (2017) 7:e00592. 10.1002/brb3.592
69.
Coenen MA Eggink H van Egmond ME Oterdoom DLM van Dijk JMC van Laar T et al Deep brain stimulation in dystonia: the added value of neuropsychological assessments. J Neuropsychol (2023). 10.1111/jnp.12331
70.
Peall KJ Kuiper A de Koning TJ Tijssen MA Non-motor symptoms in genetically defined dystonia: homogenous groups require systematic assessment. Parkinsonism Relat Disord (2015) 21:1031–40. 10.1016/j.parkreldis.2015.07.003
71.
Bates L Taylor M Lin JP Gimeno H Kingston J Rudebeck SR . Mental health and behaviour in children with dystonia: anxiety, challenging behaviour and the relationship to pain and self-esteem. Eur J Paediatr Neurol (2021) 35:40–8. 10.1016/j.ejpn.2021.09.002
72.
Butchereit K Manzini M Polatajko HJ Lin JP McClelland VM Gimeno H . Harnessing cognitive strategy use for functional problems and proposed underlying mechanisms in childhood-onset dystonia. Eur J Paediatr Neurol (2022) 41:1–7. 10.1016/j.ejpn.2022.08.007
73.
Gimeno H Polatajko HJ Lin JP Cornelius V Brown RG Cognitive strategy training in childhood-onset movement disorders: replication across therapists. Front Pediatr (2020) 8:600337. 10.3389/fped.2020.600337
74.
Gimeno H Brown RG Lin JP Cornelius V Polatajko HJ Cognitive approach to rehabilitation in children with hyperkinetic movement disorders post-DBS. Neurology (2019) 92:e1212–24. 10.1212/WNL.0000000000007092
75.
Mc Govern EM Maillart E Bourgninaud M Manzato E Guillonnet C Mochel F et al Making a 'JUMP' from paediatric to adult healthcare: a transitional program for young adults with chronic neurological disease. J Neurol Sci (2018) 395:77–83. 10.1016/j.jns.2018.09.030
76.
Wadon ME Winter M Peall KJ Internet-based cognitive behavioural therapy programme as an intervention for people diagnosed with adult-onset, focal, isolated, idiopathic cervical dystonia: a feasibility study protocol. Pilot Feasibility Stud (2020) 6:100. 10.1186/s40814-020-00641-x
77.
Zoons E Booij J Delnooz CCS Dijk JM Dreissen YEM Koelman JHTM et al Randomised controlled trial of escitalopram for cervical dystonia with dystonic jerks/tremor. J Neurol Neurosurg Psychiatry (2018) 89:579–85. 10.1136/jnnp-2017-317352
78.
Martino D Nosratmirshekarlou E Cothros N Medina Escobar A Goodarzi Z Development of a new care pathway for depression and anxiety in adult-onset isolated dystonia. Mov Disord Clin Pract (2023) 10:415–26. 10.1002/mdc3.13655
79.
Morgan A Eccles FJR Greasley P Experiences of living with dystonia. Disabil Rehabil (2021) 43:944–52. 10.1080/09638288.2019.1645217
Summary
Keywords
dystonia, non-motor symptoms, psychiatric illness, screening, assessment
Citation
Peall KJ, Berman BD, Bruggemann N, Defazio G, Gimeno H, Jinnah HA, Perlmutter JS, Richardson SEP, Roze E, Schrag A, Tinazzi M, Vidailhet M, Wagle Shukla A, Worbe Y, Teller JK and Martino D (2023) Non-motor symptoms in dystonia: from diagnosis to treatment. Dystonia 2:11860. doi: 10.3389/dyst.2023.11860
Received
27 July 2023
Accepted
13 October 2023
Published
24 October 2023
Volume
2 - 2023
Edited by
Sanjay Pandey, Amrita Hospitals Faridabad, India
Updates
Copyright
© 2023 Peall, Berman, Bruggemann, Defazio, Gimeno, Jinnah, Perlmutter, Richardson, Roze, Schrag, Tinazzi, Vidailhet, Wagle Shukla, Worbe, Teller and Martino.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathryn J. Peall, peallkj@cardiff.ac.uk; Davide Martino, davide.martino@ucalgary.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.