- Department of Neurology and Institute of Neurology, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: The etiology and motor presentation differs between pediatric- and adult-onset dystonia. Emerging evidence has demonstrated that non-motor symptoms are frequent in adult dystonia, which affect the quality of life. By contrast, little is known about the frequency and severity of such presentations in pediatric-onset individuals. Here, we investigated the motor and non-motor symptoms in a large cohort of Chinese patients with isolated dystonia and compared between pediatric-onset and adult-onset groups.
Methods: In this retrospective study, 34 pediatric-onset patients and 197 adult-onset patients with isolated dystonia were recruited. Motor impairment was assessed by the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS). Non-motor symptoms were evaluated through several validated scales, including fatigue (by Fatigue Severity Scale, FSS), excessive daytime sleepiness (by Epworth Sleepiness Scale, ESS), sleep disturbance (by Pittsburgh Sleep Quality Index, PSQI), anxiety (by Beck Anxiety Inventory, BAI) and depression (by Beck Depression Inventory 21, BDI-21).
Results: Generalized dystonia was more common in pediatric-onset patients and focal dystonia was more common in adult-onset patients (p < 0.001). Generally, the BFMDRS score in total pediatric-onset group was higher than adult-onset group (p = 0.002). No differences was found in BFMDRS score between pediatric-onset and adult-onset patients with cervical and multifocal subtype dystonia. Compared with adult-onset group, pediatric-onset group had a lower rate of sleep disturbance (p < 0.0001) and similar rates of fatigue, excessive daytime sleepiness, depression and anxiety. Logistic regression analysis on patients with cervical dystonia indicated that the adult-onset and motor severity were independently associated with increased odds of sleep disturbance (p = 0.03) and depression (p = 0.01), respectively.
Conclusion: Pediatric-onset dystonia patients were less likely to display focal dystonia. Most non-motor symptoms in pediatric-onset patients were comparable to their adult-onset counterparts. Non-motor presentations may to some extent correlate with motor symptoms, but their underlying pathophysiology need to be investigated further.
Introduction
Dystonia is the third most prevalent movement disorder characterized by involuntary muscle contractions that lead to abnormal movement (often twisting and repetitive), postures, or both. While the pathogenesis of dystonia still remains uncertain, for dystonia occurring at childhood and adolescence, here referred to as pediatric-onset dystonia, the cause is more detectable than adult-onset dystonia, which could be attributable to a list of known genetic and non-genetic factors [1].
Emerging evidence has demonstrated that it is of great importance in clinical practice to dissect the dystonia according to patients’ onset age [1, 2]. In adult-onset patients, dystonia often remains focal, whereas in pediatric-onset patients, dystonia is more likely to progress from one body region to a generalized body involvement, which may pose burden on their development and daily life [2]. Apart from motor impairment, a sizable proportion of adult-onset dystonia patients have been shown to suffer from various non-motor symptoms, such as neuropsychiatric disturbance, sleep problems, sensory disorder and cognitive decline, some of them has been found to associate with the decreased quality of life [3–5].
Although non-motor symptoms have already been investigated among adult-onset dystonia patients by a number of studies, little is known about the frequency and severity of such presentations and their clinical relevance with motor impairment for pediatric-onset individuals. The largest retrospective study on 50 childhood dystonia patients has observed a higher incidence of anxiety and prosocial difficulties in young patients compared with their age-matched peers, suggesting that pediatric-onset dystonia patients also suffer from non-motor disorders [6]. To our knowledge, no study has compared the differences between pediatric-onset and adult-onset dystonia patients in terms of non-motor presentations so far.
Here, we report the motor and non-motor features in a large cohort of Chinese patients with isolated dystonia and compare the differences between pediatric-onset and adult-onset groups. The correlation between motor and non-motor impairment is also evaluated.
Methods
Patients
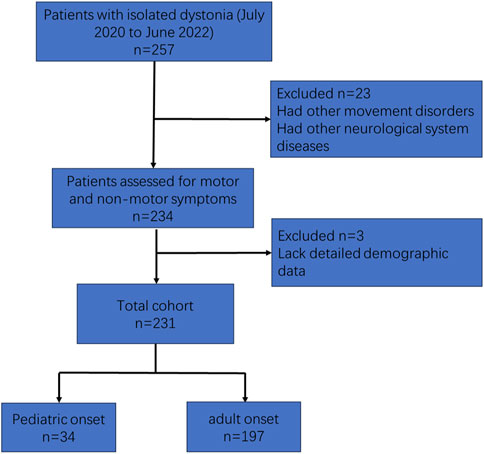
Isolated dystonia patients who were followed at the Movement Center of Ruijin Hospital (Shanghai Jiao Tong University School of Medicine, Shanghai) during July 2020 and June 2022 were enrolled in the present study. The diagnostic criterion of dystonia was based on the 2013 Consensus of Movement Disorder Society [1]. Clinical data was collected from hospital records. A series of standardized scales were used to evaluate the motor and non-motor symptoms at their latest visits. Exclusion criteria were: 1) had other movement disorders, such as parkinsonism. 2) had other neurological system diseases. 3) lack detailed demographic data. The flow chart of patient selection was presented in Figure 1. The study was approved by the Ethics Committee of Ruijin Hospital. Written informed consent was obtained from all participants.
Motor and non-motor symptoms assessment
The Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) was used to assess the severity of motor symptoms. Non-motor symptoms were determined by the following validated scales: Fatigue Severity Scale (FSS) was applied for fatigue; excessive daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS); the Pittsburgh Sleep Quality Index (PSQI) was used to measure individuals’ sleep disturbance; the Beck Anxiety Inventory (BAI) and the Beck Depression Inventory 21 (BDI-21) were implied to evaluate self-reported anxiety and depression, respectively.
Patients under 10 years old were not evaluated in this study.
Statistical analyses
Statistical analyses were performed on the SPSS version 26.0 (SPSS, Chicago, IL, United States). Continuous variables were presented as median (interquartile range [IQR]). Mann-Whitney U test, χ2 test and the Fisher’s exact test were used to compare the differences between pediatric-onset and adult-onset groups. The Pearson correlation analysis was use to explore the correlation between motor severity and the severity of non-motor symptoms. Logistic regression analysis was applied to assess the risk factors of non-motor symptoms, including fatigue (FSS ≥ 36), excessive daytime sleepiness (ESS > 10), sleep disturbance (PSQI > 5), anxiety (BAI > 7), and depression (BDI-21 > 13). Variables of clinical interests were included in univariable analysis, and variables with p ≤ 0.20 were then entered into further multivariable analyses. A two-tail p-value < 0.05 was defined as statistically significant.
Results
Clinical characteristics of patients with pediatric-onset and adult-onset dystonia
A total of 34 pediatric-onset and 197 adult-onset patients were recruited in the present study. Table 1 showed the demographic and clinical features in patients with isolated dystonia. In the pediatric-onset group, the median age of onset was 15 years (range: 10–18 years) with a gender ratio (F/M) of 1.4:1. Median disease duration was 4.5 years (range: 0.92–13.75 years). The median age at the time of evaluation for the pediatric-onset onset group was 21 years (range: 16–29 years). One patient’s age was less than 10 years old. The most prevalent phenotype of dystonia in pediatric-onset patients was focal dystonia (44.12%), which was followed by the generalized dystonia (29.41%), multi-focal dystonia (17.65%), segmental dystonia (5.88%) and hemi-dystonia (2.94%). Compared to their adult-onset counterparts, pediatric-onset patients were more likely to develop generalized dystonia and less likely to present with focal dystonia (p < 0.001). The median BFMDRS score in pediatric-onset patients was 12.0 (range: 6.0–33.0), which was markedly higher than that in adult-onset subjects (median: 7.0 (range: 4.6–11.0); p < 0.001).
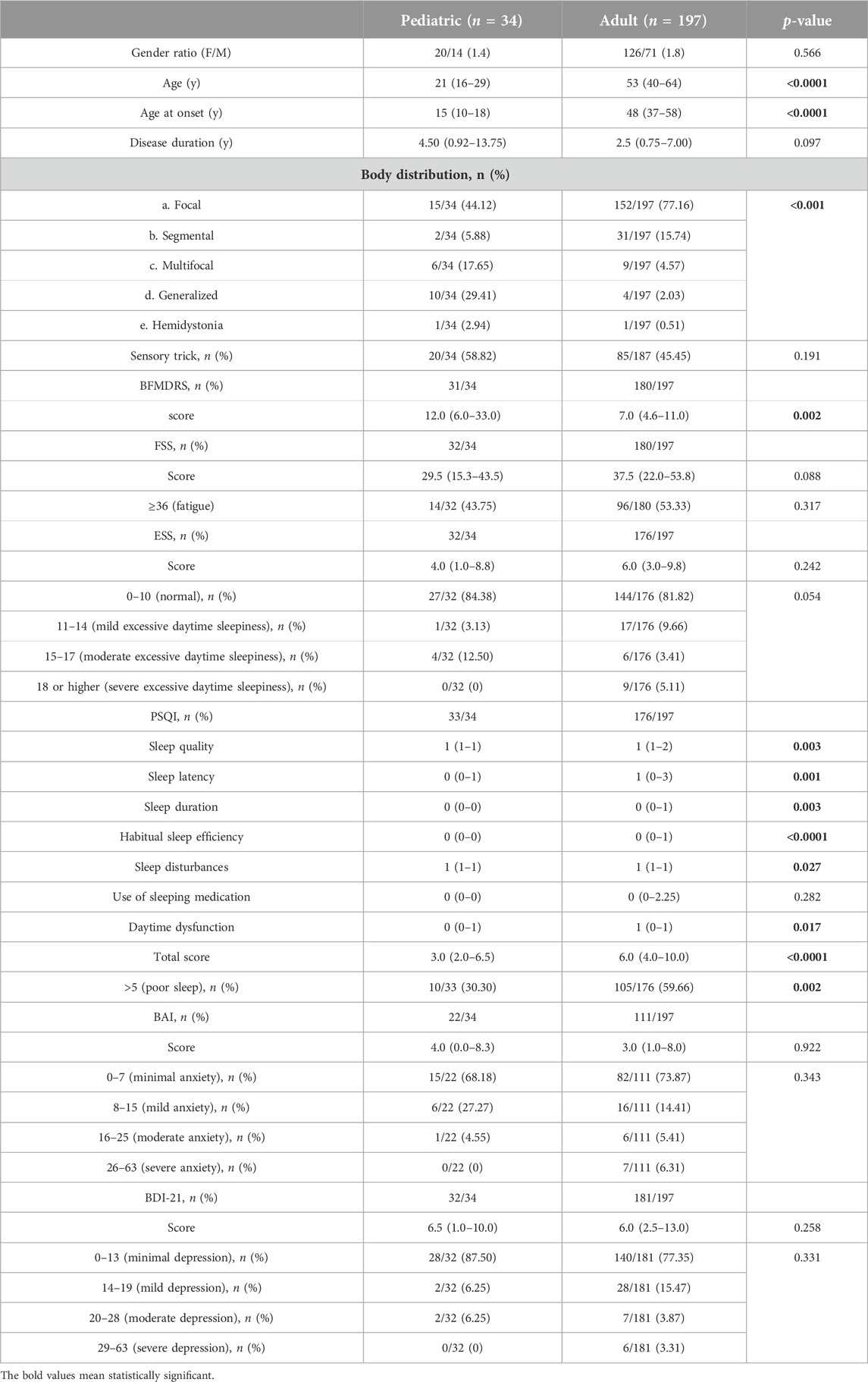
TABLE 1. Demographic and clinical features of pediatric-onset and adult-onset patients with isolated dystonia.
In terms of non-motor symptoms, fatigue determined by the FSS score ≥ 36 was observed in nearly half of pediatric-onset cases (43.75%). The percentage of pediatric-onset patients with excessive daytime sleepiness (ESS > 10) was 15.63%. No difference was found in FSS and ESS between pediatric-onset and adult-onset groups. Approximately one-third of pediatric-onset cases had poor sleep (PSQI > 5), and its frequency was predominately lower compared with adult-onset patients (30.30% vs. 59.66%, p = 0.002). In addition, the level of PSQI global score (p < 0.0001) and the sub-components of PSQI, including sleep quality (p = 0.003), sleep latency (p = 0.001), sleep duration (p = 0.0035), habitual sleep efficiency (p < 0.0001), sleep disturbance (p = 0.027) and daytime dysfunction (p = 0.0173) in pediatric-onset group were all milder than those in adult-onset group. Anxiety (BAI > 7) and depression (BDI-21 > 13) was found in 31.82% and 12.5% of pediatric-onset patients, respectively. No difference was found in BAI and BDI-21 between two age groups.
Table 2 presented the motor and non-motor symptoms in pediatric-onset and adult-onset patients with different dystonia subtypes (n ≥ 5 in each group). The BFMDRS scores in pediatric-onset patients with cervical (focal) and multifocal dystonia were comparable to their adult-onset counterparts. The PSQI global score and the percentage of patients with poor sleep (PSQI > 5) were significantly higher in pediatric-onset cervical (focal) patients compared with adult-onset cervical (focal) patients (p = 0.0016 and p = 0.0269, respectively).
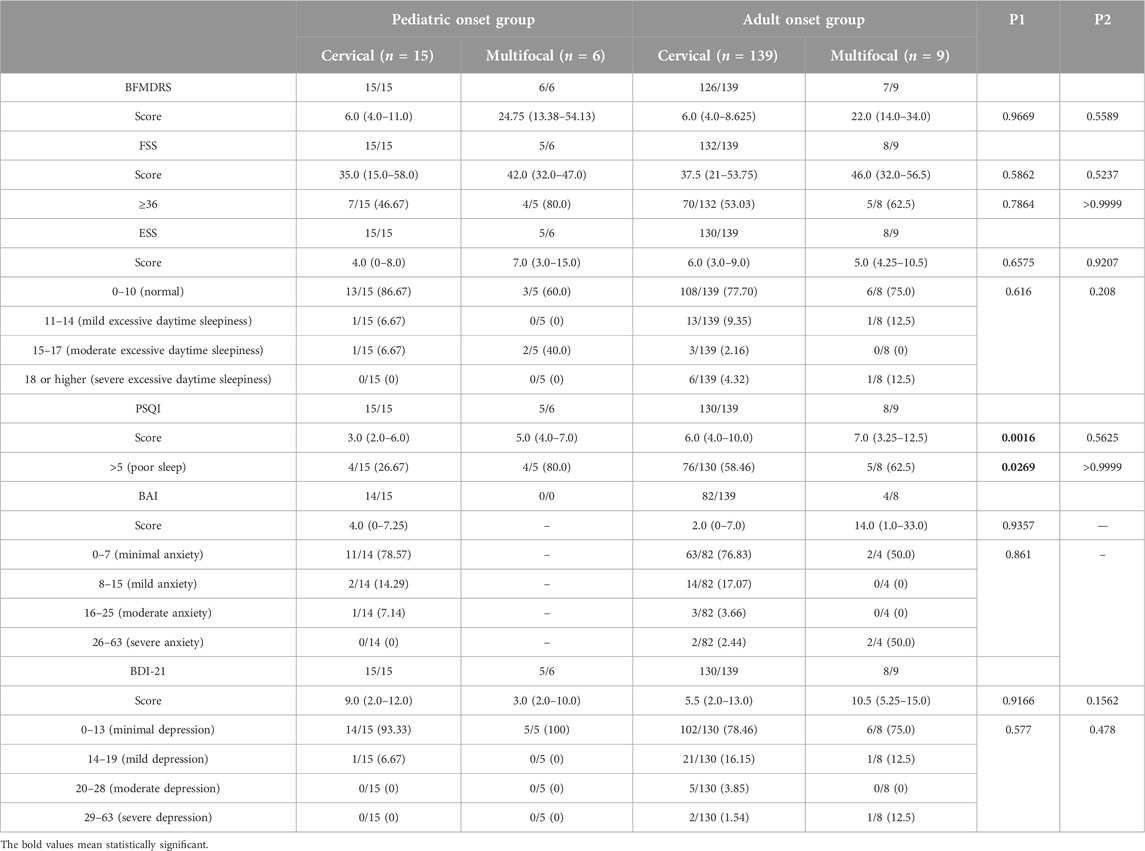
TABLE 2. Motor and non-motor symptoms in pediatric-onset and adult-onset patients with different dystonia subtypes.
Correlation between motor and non-motor symptoms
To identify the association between motor and non-motor impairments, we analyzed the correlation between BFMDRS score and non-motor score measured by different validated scales. In pediatric-onset group, no correlation was found between the BFMDRS score and any non-motor related score. However, in the adult-onset group, BFMDRS score had a positive correlation with the level of PSQI (r = 0.278, p < 0.01), BAI (r = 0.384, p < 0.01) and BDI-21 (r = 0.354, p < 0.01).
The risk factors of non-motor presentations
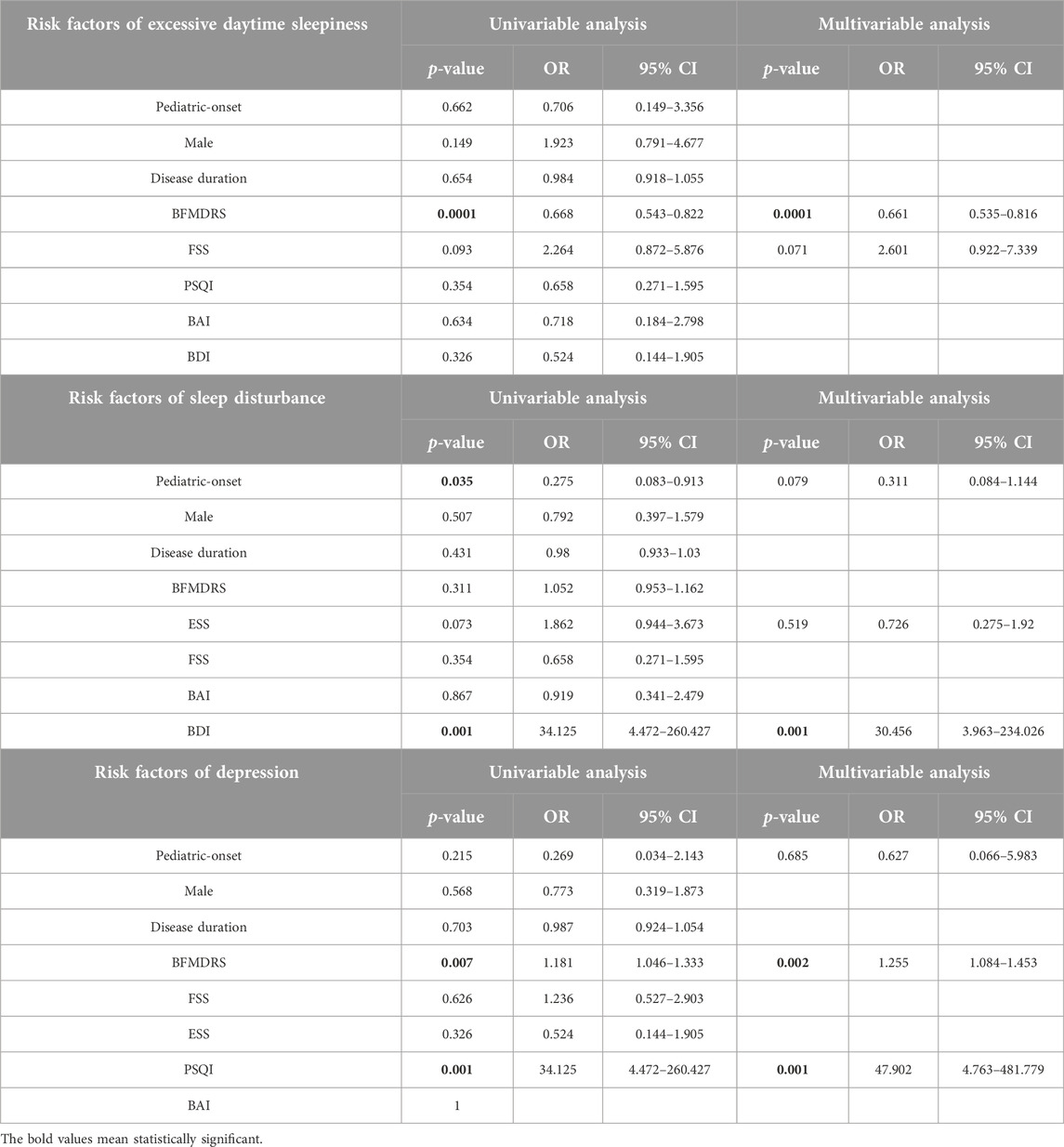
Finally, logistic regression models were performed to explore potential risk factors associated with different non-motor disorders (Table 3 and Supplementary Table S1). Since dystonia phenotype may exert an impact on motor as well as non-motor impairments, only patients with cervical (focal) dystonia (including 15 pediatric-onset and 139 adult-onset cases) were included in the analysis. Mono-variable model indicated that motor severity was associated with the decreased odds of excessive daytime sleepiness (ESS>10) (p < 0.001; OR: 0.668, 95% CI: 0.543–0.822). A pediatric-onset was associated with the decreased odds of poor sleep (PSQI > 5) (p = 0.035; OR: 0.275, 95% CI: 0.083–0.913), whereas depression (BDI-21 > 13) was associated with the increased odds of poor sleep (PSQI > 5) (p = 0.001; OR:34.125, 95% CI: 4.472–260.427). Moreover, there was a significantly positive correlation between motor severity and depression (BDI-21 > 13) (p = 0.007; OR: 1.181, 95% CI: 1.046–1.333).
Multi-variable analyses suggested that the motor severity was independently correlated with excessive daytime sleepiness (ESS > 10) (p = 0.000; OR: 0.661, 95% CI: 0.535–0.816) and depression (BDI-21 > 13) (p = 0.002; OR: 1.255, 95% CI: 1.084–1.453).
And the presence of poor sleep (PSQI > 5) was independently associated with depression (BDI-21 > 13) (0.001; OR: 30.456, 95% CI: 3.963–234.026).
Discussion
Our study highlighted three points: first, there was a high frequency of non-motor symptoms in Chinese pediatric-onset and adult-onset patients with isolated dystonia; second, compared to the adult-onset patients, pediatric-onset patients were less likely to suffer from sleep problems; third, although pediatric-onset patients was more likely to develop generalized dystonia, the frequency and severity of fatigue, anxiety and depression, were similar as compared to their adult-onset counterparts, suggesting that these non-motor presentations may to some extent be a primary deficit rather than a consequence of motor impairment.
As with other movement disorders, sleep problem is commonly seen in patients with dystonia. Recently, increased frequency of sleep disturbance has been reported in patients with cervical dystonia when compared with healthy subjects, and has been shown to be a risk factor of the quality of life [3, 7]. In the current study, sleep disturbance and excessive daytime sleepiness, as assessed by the PSQI and ESS scales, were observed in approximately 59% and 19% of adult-onset dystonia patients, respectively, which was in accordance with previous observation studies, which showed a high rate of sleep disturbance (∼50%) in patients with focal dystonia whereas the frequency of excessive daytime sleepiness was relatively uncommon (6%–21%) [7–10]. Surprisingly, our study demonstrated that pediatric-onset dystonia patients exhibited a significantly lower rate of sleep disturbance than adult-onset dystonia patients (30% vs. 59%). Moreover, a pediatric-onset-onset was shown to be independently associated with decreased odds of poor sleep in patients with cervical dystonia. Since pediatric-onset dystonia patients usually suffered from greater dystonia severity, we then analyzed the association between sleep disturbance and motor impairment. No correlation was noted between the severity of sleep disturbance and the severity of motor impairment in pediatric-onset group. There was a correlation between the degrees of sleep disturbance and motor impairment in adult-onset patients, however, in the logistic regression analysis that only included cervical dystonia cases, we found that the sleep disturbance had no correlation with motor severity but a correlation with depression and age of onset. Therefore, it is reasonable to assume that sleep disorder might be different among various dystonia phenotypes. As sleep problem has been suggested to impair the quality of life, more clinical and basic investigations, including polysomnographic recording, neurotransmitter and neuronal circuit studies are warranted to explore the mechanism of sleep disturbance for both pediatric-onset and adult-onset dystonia patients.
Furthermore, our results reinforced previous observations that sleep disturbance was positively correlated with depression, which suggested that the sleep disturbance might be partly secondary to the depression [8, 11]. In addition to the depression, bruxism, restless legs syndrome (RLS) and female gender have been identified as risk factors of sleep problem in patients with focal dystonia [11]. Taken together, whether sleep disturbance is a primary or secondary abnormality in dystonia individuals need to be explored further.
Recently, an excess of neuropsychiatric presentations among adult-onset dystonia patients has been elucidated by many studies [12, 13]. By contrast, there is scarce data to analyses neuropsychiatric symptoms in pediatric-onset dystonia patients. In our pediatric-onset cohort, co-existence of anxiety and depression were found to be 31% and 13%, by the BAI and BDI-21 scales, respectively. The high frequency of anxiety was in accordance with the study by Rudebeck et al, in which 48% of childhood dystonia patients aged 7–17 years were found to experience anxiety [6]. Given that neuropsychiatric comorbidity has been shown to be a predictor of health-related quality of life, rather than the motor severity, in adult-onset patients with cervical dystonia, it would be of clinical importance to integrate mental health into the management of dystonia [14].
To date, the pathophysiology underlying neuropsychiatric symptoms remains to be poorly understood, but has been considered to be related to the disruption in cortical-limbic-striatal circuits [15]. Consistent with previous studies, which found a correlation between neuropsychiatric and motor symptoms in adult-onset dystonia patients, our results observed a positive association between depression severity and motor severity in adult-onset group, and interestingly, not in pediatric-onset group [16, 17]. The discrepancies between two groups may be attributable to the differences in their dystonia phenotype and genetic susceptibility. This hypothesis could be supported by recent evidence which detected a higher frequency of depression and anxiety in patients with blepharospasm than in patients with cervical dystonia and writer’s cramp [18]. Moreover, Berman et al. observed a higher rate of anxiety in cervical and laryngeal groups and lower rate of anxiety in upper cranial group among adult-onset dystonia subjects [17]. In addition, some dystonia related mutations also play a role in neuropsychiatric disorders. For instance, DYT1 dystonia mutation carriers have been shown to confer an increased risk for recurrent major depression [19]. As genetically defined dystonia is more common for pediatric-onset cases, it would be more meaningful to investigate gene mutations and their relationship with non-motor presentations in our study in future. Notably, though the pediatric-onset patients displayed higher BFMDRS scores, the frequency and severity of depression and anxiety were comparable between pediatric-onset and adult-onset dystonia groups. This finding lent evidence that these neuropsychiatric disorders were unlikely to be a simple reaction to the disability induced by dystonia. Furthermore, previous studies have indicated that the occurrence of depression and anxiety could precede the onset of dystonia for many years in the majority of adult-onsets dystonia patients, suggesting that neuropsychiatric disorders may be part of the clinical spectrum of dystonia [20]. Taken together, our results uphold the notion that neuropsychiatric symptoms are primary endophenotypic deficits but can correlate with the motor symptoms in dystonia.
Our study had several limitations. Due to the retrospective design, sampling bias might restrict the interpretation of the results. The predominant recruitment of patients in the adult center may have led to the younger age dystonia individuals and children with mild symptoms being underrepresented. Some pediatric-onset and adult-onset patients were unwilling to complete all the scales, especially for BAI. Thus, we cannot exclude the possibility that these patients may lack corresponding symptoms to trigger screening, which would cause an overestimation of the prevalence of this symptom. No differences were found in the frequency and severity of most non-motor symptoms between the pediatric-onset and adult-onset groups. This may be due to a lack of statistical power because the number of pediatric-onset cases were relatively small. In addition, dystonia in children can be part of a metabolic disease with other neurological and psychiatric symptoms associated, which could influence the non-motor symptoms and should be considered. Further studies are required to take the causes and subtypes of dystonia into account for pediatric population. Finally, though the widely used scales for evaluating non-motor symptoms in our study have been applied for adult-onsets and adolescents in a number of studies [21–25], it would strengthen the ascertained conclusions if we could add age-matched controls because some features in people of different ages such as global cognitive and physical state may to some extent influence the score of scale.
In conclusion, most non-motor symptoms in pediatric-onset dystonia patients were comparable to that in adult-onsets in frequency and severity, with the exception of sleep disturbance. The pathophysiology of non-motor symptoms is complex and cannot be simply attributable to the motor impairment. Prospective and multicenter studies are needed to determine the prevalence and importance of non-motor presentations in pediatric-onset dystonia patients.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving humans was approved by Ethics Committee of Ruijin Hospital. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from all participants.
Author contributions
YW designed the study and recruited patients with dystonia. YZ and LW drafted the manuscript. HL assisted in survey. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/dyst.2024.11468/full#supplementary-material.
References
1. Albanese, A, Bhatia, K, Bressman, SB, Delong, MR, Fahn, S, Fung, VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord (2013) 28:863–73. doi:10.1002/mds.25475
2. van Egmond, ME, Kuiper, A, Eggink, H, Sinke, RJ, Brouwer, OF, Verschuuren-Bemelmans, CC, et al. Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry (2015) 86:774–81. doi:10.1136/jnnp-2014-309106
3. Han, V, Skorvanek, M, Smit, M, Turcanova, KM, Hoekstra, T, van Dijk, JP, et al. Prevalence of non-motor symptoms and their association with quality of life in cervical dystonia. Acta Neurol Scand (2020) 142:613–22. doi:10.1111/ane.13304
4. Junker, J, Berman, BD, Hall, J, Wahba, DW, Brandt, V, Perlmutter, JS, et al. Quality of life in isolated dystonia: non-motor manifestations matter. J Neurol Neurosurg Psychiatry (2021) 92:622–8. doi:10.1136/jnnp-2020-325193
5. Lee, S, Chung, SJ, and Shin, HW. Neuropsychiatric symptoms and quality of life in patients with adult-onset idiopathic focal dystonia and essential tremor. Front Neurol (2020) 11:1030. doi:10.3389/fneur.2020.01030
6. Bates, L, Taylor, M, Lin, JP, Gimeno, H, Kingston, J, and Rudebeck, SR. Mental health and behaviour in children with dystonia: anxiety, challenging behaviour and the relationship to pain and self-esteem. Eur J Paediatr Neurol (2021) 35:40–8. doi:10.1016/j.ejpn.2021.09.002
7. Liang, Y, Lin, J, Hou, Y, Zhang, L, Ou, R, Li, C, et al. Health-related quality of life in cervical dystonia using EQ-5D-5L: a large cross-sectional study in China. Front Neurol (2022) 13:895272. doi:10.3389/fneur.2022.895272
8. Avanzino, L, Martino, D, Marchese, R, Aniello, MS, Minafra, B, Superbo, M, et al. Quality of sleep in primary focal dystonia: a case-control study. Eur J Neurol (2010) 17:576–81. doi:10.1111/j.1468-1331.2009.02884.x
9. Eichenseer, SR, Stebbins, GT, and Comella, CL. Beyond a motor disorder: a prospective evaluation of sleep quality in cervical dystonia. Parkinsonism Relat Disord (2014) 20:405–8. doi:10.1016/j.parkreldis.2014.01.004
10. Trotti, LM, Esper, CD, Feustel, PJ, Bliwise, DL, and Factor, SA. Excessive daytime sleepiness in cervical dystonia. Parkinsonism Relat Disord (2009) 15:784–6. doi:10.1016/j.parkreldis.2009.04.007
11. Paus, S, Gross, J, Moll-Muller, M, Hentschel, F, Spottke, A, Wabbels, B, et al. Impaired sleep quality and restless legs syndrome in idiopathic focal dystonia: a controlled study. J Neurol (2011) 258:1835–40. doi:10.1007/s00415-011-6029-6
12. Wadon, ME, Fenner, E, Kendall, KM, Bailey, GA, Sandor, C, Rees, E, et al. Clinical and genotypic analysis in determining dystonia non-motor phenotypic heterogeneity: a UK Biobank study. J Neurol (2022) 269:6436–51. doi:10.1007/s00415-022-11307-4
13. Lehn, A, Mellick, G, and Boyle, R. Psychiatric disorders in idiopathic-isolated focal dystonia. J Neurol (2014) 261:668–74. doi:10.1007/s00415-014-7244-8
14. Smit, M, Kuiper, A, Han, V, Jiawan, VC, Douma, G, van Harten, B, et al. Psychiatric comorbidity is highly prevalent in idiopathic cervical dystonia and significantly influences health-related quality of life: results of a controlled study. Parkinsonism Relat Disord (2016) 30:7–12. doi:10.1016/j.parkreldis.2016.06.004
15. Stamelou, M, Edwards, MJ, Hallett, M, and Bhatia, KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain (2012) 135:1668–81. doi:10.1093/brain/awr224
16. Gundel, H, Busch, R, Ceballos-Baumann, A, and Seifert, E. Psychiatric comorbidity in patients with spasmodic dysphonia: a controlled study. J Neurol Neurosurg Psychiatry (2007) 78:1398–400. doi:10.1136/jnnp.2007.121699
17. Berman, BD, Junker, J, Shelton, E, Sillau, SH, Jinnah, HA, Perlmutter, JS, et al. Psychiatric associations of adult-onset focal dystonia phenotypes. J Neurol Neurosurg Psychiatry (2017) 88:595–602. doi:10.1136/jnnp-2016-315461
18. Novaretti, N, Cunha, A, Bezerra, TC, Pena, PM, de Oliveira, DS, Macruz, BM, et al. The prevalence and correlation of non-motor symptoms in adult patients with idiopathic focal or segmental dystonia. Tremor Other Hyperkinet Mov (N Y) (2019) 9:596. doi:10.7916/fhnv-v355
19. Heiman, GA, Ottman, R, Saunders-Pullman, RJ, Ozelius, LJ, Risch, NJ, and Bressman, SB. Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology (2004) 63:631–7. doi:10.1212/01.wnl.0000137113.39225.fa
20. Moraru, E, Schnider, P, Wimmer, A, Wenzel, T, Birner, P, Griengl, H, et al. Relation between depression and anxiety in dystonic patients: implications for clinical management. Depress Anxiety (2002) 16:100–3. doi:10.1002/da.10039
21. Loiacono, B, Sunnquist, M, Nicholson, L, and Jason, LA. Activity measurement in pediatric chronic fatigue syndrome. Chronic Illn (2022) 18(2):268–76. doi:10.1177/1742395320949613
22. Gagua, T, Tkeshelashvili, B, Gagua, D, and McHedlishvili, N. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. J Pediatr Adolesc Gynecol (2013) 26(6):350–4. doi:10.1016/j.jpag.2013.06.018
23. Pike, NA, Roy, B, Gupta, R, Singh, S, Woo, MA, Halnon, NJ, et al. Brain abnormalities in cognition, anxiety, and depression regulatory regions in adolescents with single ventricle heart disease. J Neurosci Res (2018) 96(6):1104–18. doi:10.1002/jnr.24215
24. Zafar, AB, Ness, J, Dowdy, S, Avis, K, and Bashir, K. Examining sleep, fatigue, and daytime sleepiness in pediatric multiple sclerosis patients. Mult Scler (2012) 18(4):481–8. doi:10.1177/1352458511424307
Keywords: dystonia, age, non-motor symptoms, motor severity, pediatric-onset
Citation: Zhou Y, Wang L, Li H and Wu Y (2024) Non-motor symptoms in patients with isolated dystonia: comparison between the age of onset. Dystonia 3:11468. doi: 10.3389/dyst.2024.11468
Received: 12 April 2023; Accepted: 31 January 2024;
Published: 09 February 2024.
Edited by:
Aparna Wagle Shukla, University of Florida, United StatesCopyright © 2024 Zhou, Wang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiwen Wu, d3l3MTEzODBAcmpoLmNvbS5jbg==
†These authors have contributed equally to this work
 Yifan Zhou
Yifan Zhou Lingbing Wang†
Lingbing Wang† Hongxia Li
Hongxia Li