- 1Department of Neurology, University of New Mexico Health Sciences Center and New Mexico VA Health Care System, Albuquerque, NM, United States
- 2Department of Neurology, Virginia Commonwealth University, Richmond, VA, United States
- 3Dystonia Medical Research Foundation, Chicago, IL, United States
- 4Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, United States
- 5Computational Neurobiology Laboratory, Salk Institute for Biological Studies, and Institute for Neural Computation, University of California, San Diego (UCSD), La Jolla, CA, United States
- 6Department of Neurology, Emory University School of Medicine, Atlanta, GA, United States
- 7Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States
- 8Parkinson’s Disease Center and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine, Houston, TX, United States
- 9Department of Neurology, Mayo Clinic, Scottsdale, AZ, United States
- 10Expertise Centre Movement Disorders, Groningen, Department of Neurology, University of Groningen, University Medical Centre Groningen (UMCG), Groningen, Netherlands
- 11Department of Physical Therapy, Massachusetts General Hospital (MGH) Institute of Health Professions, Boston, MA, United States
- 12Dystonia Europe, Brussels, Belgium
- 13Parkinson School of Health Sciences and Public Health, Loyola University, Chicago, IL, United States
Introduction: To establish clinical trial readiness for dystonia, a crucial step is to develop a Patient-Centered Outcome (PCO) measure to capture therapeutic response in focal dystonia such as in cervical dystonia (CD). Botulinum neurotoxin (BoNT) is the gold standard treatment for focal dystonia and yields improvement; yet the therapy may not meet all patient expectations as there is a high rate of discontinuation. A PCO that can measure therapeutic response, including the waxing and waning benefit of BoNT, across multiple domains and is easy to use on a frequent basis in the home environment is critical.
Methods: A modified iterative Delphi process based on FDA (Food and Drug Administration) guidelines was used to develop and select items to document patient symptoms and response to treatment. Potential items then were improved using patient focus groups, validated for content with specialist panels, and confirmed items based on a patient survey. Using data from 200 CD patients in the Dystonia Coalition Natural History Database, initial PCO items were identified. Utilizing Random Forests, prospective items were analyzed for their contribution to the overall severity scores on the clinical and patient-centered outcome scales. Items that were repetitive were merged. Iterative meetings with a specialist panel consisting of neurologists, physical therapists, and Patient Advocacy Group (PAG) representatives as well as virtual focus groups of CD patients were held. An online survey was conducted with over 600 CD patients participating. Finally, specialist panel members provided input for a content validity ratio (CVR) with iterations until there was good agreement as to the relevance and clarity of the items.
Results: PCO measures tailored for CD were successfully developed. The PCO consists of 16 items covering three domains (motor, disability, and psychosocial) and reflects the input of international specialist panels, more than 800 CD patients, and PAGs (patient advocacy groups) following FDA guidance. The PCO is simple enough to be used in an app-based format compatible with smartphones and tablets.
Conclusion: This comprehensive CD PCO measure was developed through the combination of using robust existing patient centered data (from previous Dystonia Coalition Projects); active engagement with PAGs to provide the patient voice; and use of virtual focus groups and online surveys. This PCO will be used in a prospective study to characterize the therapeutic response to BoNT over time. This will provide peak effect size as well as capturing the “yo-yo” effect during BoNT treatment; and will prepare for a future trials.
Introduction
Cervical dystonia (CD) is defined by involuntary contractions of neck muscles leading to abnormal postures and movements of the head and neck [1–3]. It is frequently associated with neck pain [4, 5], poor quality of life [6], impaired activities of daily living and reduced ability to work, as well as psychosocial difficulties [7, 8].
The standard of care treatment for CD involves injections with botulinum neurotoxin (BoNT) [9, 10]. Because CD is a chronic and lifelong condition, individuals may be treated for many decades. BoNT injections have been shown to improve motor symptoms, pain, and quality of life, yet longitudinal studies have suggested that approximately one-third of individuals discontinue treatment [11, 12]. There are many potential reasons for discontinuation including insufficient efficacy and low satisfaction, frequent or unpredictable adverse effects, logistical issues such as travel or cost, co-existing psychiatric concerns or unrealistic expectations, and others [13–20].
An important factor that has emerged from studies of satisfaction during treatment of CD with BoNT involves the waxing and waning of symptoms associated with typical treatment cycles. There are improvements within a few weeks following injections, a plateau period of good symptom control, and then a wearing-off period where symptoms return. Treatments are typically provided at approximately 3 months intervals, and the waxing and waning of symptoms with repeated treatment is associated with a cyclical pattern that has been called the yo-yo effect [21] or the roller-coaster effect (Figure 1) [19]. Not surprisingly, satisfaction with treatment varies according to the treatment cycle. There also is evidence that the temporal profile of benefit varies considerably among individuals, with some preferring injection intervals less than 2 months and others waiting for 5–6 months between injections [22–24]. Although cycles of waxing and waning symptoms are widely appreciated, there are virtually no data regarding the magnitude of changes in symptoms or the temporal pattern of these changes among individual cases on a frequent basis (i.e., weekly or daily) [25].
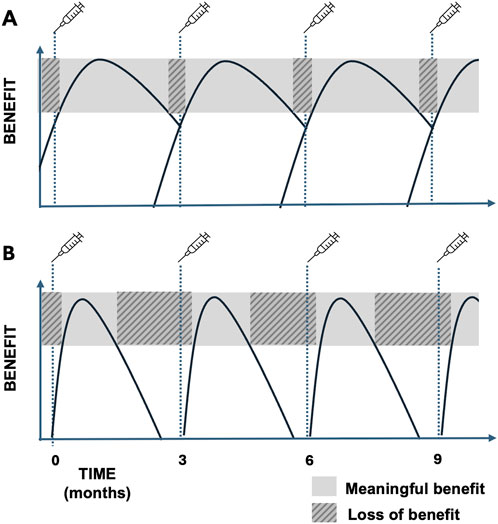
Figure 1. (A) Ideal BoNT cycle with most of the cycle spent with meaningful benefit from the therapy and minimal wearing off. (B) BoNT cycle showing loss of benefit for much of the typical 3-month cycle due to loss of efficacy prior to next scheduled BoNT treatment.
There are many potential strategies for mitigating the waxing-waning temporal profile of symptom control associated with BoNT injections. They include using shorter injection intervals, higher doses, development of novel BoNT preparations with longer durations of action or less prominent fluctuations in symptom control, and adjunctive oral medications or physiotherapy programs that reduce wearing off symptoms. Indeed, recent clinical trials have attempted to address the efficacy of adjunctive oral medications [26] or physiotherapy [27] for individuals with CD being treated with BoNT. The assessment of these novel treatment strategies is hampered by the lack of information regarding the temporal dynamics of waxing and waning, both within and across individuals. Novel BoNT preparations with potentially longer durations of action have also been reported recently [28], although strategies for measuring the duration of responses in a clinically meaningful way are not currently available.
Efficient tools to precisely measure the temporal dynamics of BoNT responses are needed to optimize existing BoNT therapies and to test potential new therapies. These tools should be simple and efficient, applicable on a weekly or even daily basis, and they should be sensitive to change over time periods expected from treatment interventions. Although this manuscript focuses on CD, other focal dystonias, such as blepharospasm and laryngeal dystonia, also would benefit from a simple, efficient and robust tool for capturing change with therapy. Efforts for blepharospasm and laryngeal dystonia require different questions and specialist input, so need to be developed and described separately. Clinician-rated scales such as the Toronto-Western Torticollis Rating Scale (TWSTRS) [29] are not feasible for frequent repeated application, because they require traveling to centers for assessment by trained clinicians. Additionally, individuals with CD tend to rate their outcomes less favorably than their physicians [30, 31], with sometimes poor correlations regarding improvements between clinician ratings and patient impressions [32]. It is possible to use patient-centered outcome (PCO) tools such as the Cervical Dystonia Impact Profile -58 (CDIP-58) or the Craniocervical dystonia questionnaire (CDQ-24) [29, 33]. However, the CDIP-58 has a large number of questions (N = 58) making it impractical for frequent repeated use, many items are redundant, and it contains several questions that are not sensitive to short-term changes in severity, such as relinquishing a license to drive or retiring from work. The CDQ-24 comprehensively addresses quality of life but does not address motor symptoms, limiting its scope. The goal of this study was to develop a simple and efficient PCO for CD that is sensitive to changes in symptoms and could be easily administered through a digital platform such as a smartphone or tablet.
Materials and methods
Study design
We received institutional approval from an ethical standards committee on human experimentation. All participants (patients) in the study provided written informed consent. The overall design for the patient centered outcome (PCO) followed US Food and Drug Administration guidelines for developing patient-centric measurement tools, which involve incorporating input from all relevant stakeholders.1 The plan was therefore guided by a conceptually driven, iterative content development process that integrated input from expert clinicians, patient advocacy groups, and affected patients. It also included a step recommended to assess the minimal amount of improvement affected individuals desired for specific symptoms (Figure 2).
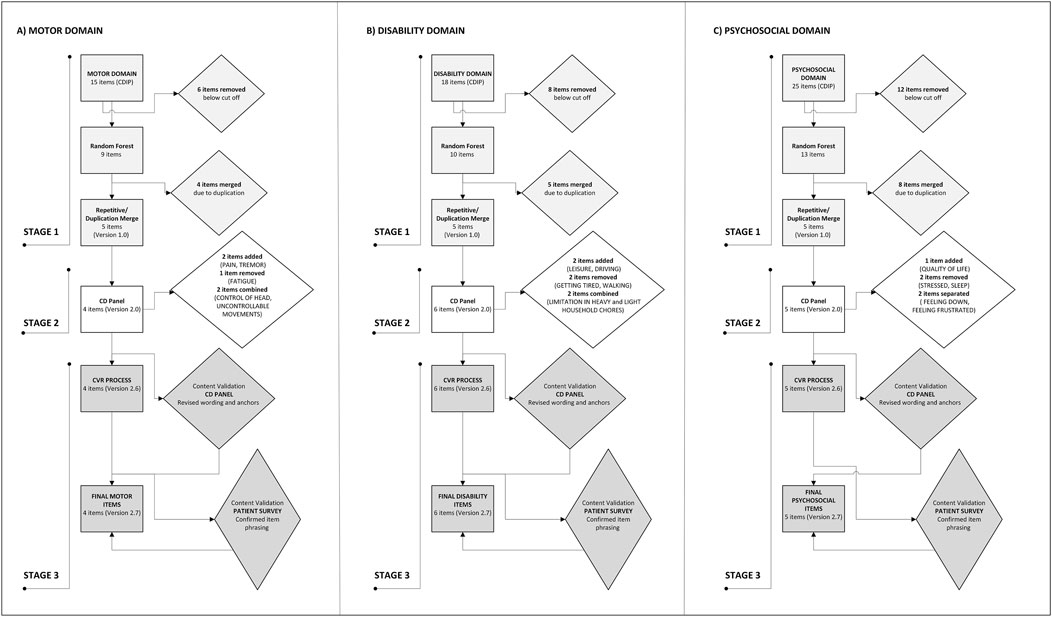
Figure 2. Flow diagram of item selection, revision, and finalization through the three stages of the process for the (A) MOTOR, (B) DISABILITY, and (C) PSYCHOSOCIAL domains.
Stage 1. Content development and item generation
The first step involved content development and item generation. Existing items were collected from previously validated symptom severity assessment tools for CD including the CDIP-58, the CDQ-24, and the TWSTRS (motor, disability, and pain subscales). Actual data for these scales were obtained from a prior study of 200 individuals with CD [34]. Items that were similar or overlapping were merged, and items not appropriate for frequent repeated use were eliminated or modified to generate a smaller list of relevant items falling into three domains (MOTOR, DISABILITY, and PSYCHOSOCIAL).
A pivotal aspect of the methodology involved rigorous statistical analysis to identify variables that significantly impact a Total Score across the three domains chosen. The process employed Random Forests, an ensemble learning technique known for its robust handling of high-dimensional data. This method constructs multiple decision trees during training and outputs the mean prediction of these trees for regression tasks, thereby elucidating the relative importance of each variable. The significance of variables within the Random Forest regression models was assessed by their impact on the out-of-bag mean square error, a reliable measure of prediction error for data excluded from each bootstrap sample. Variables that resulted in a larger value were ranked as more important, signifying their pivotal role in accurately predicting the Total Score. This facilitated the prioritization of variables for inclusion, ensuring effectiveness in capturing the nuances of CD symptom severity and impact.
Stage 2. Item improvement and revision of items
The second step involved item revision and improvement. This step was accomplished through an iterative process with a panel of specialists (both neurologists and physical therapists) who had extensive clinical expertise and prior research contributions in CD. The panel also included patient advocacy group representatives from the Dystonia Medical Research Foundation2 and Dystonia Europe.3 The whole panel participated in assessment of candidate items to determine if there were missing items and then ranked all items according to importance. Ranking was done using a 9-point scale ranging from not important (1) to critically important (9). Finally, the candidate list was reduced to the most important items.
Refinement of candidate items selected by the specialist panel was done via teleconferences with three stakeholder focus groups of ∼30 individuals with CD held 1 week apart to identify deficiencies and to rank the resulting list from the perspective of affected individuals. Research staff provided written transcription of the focus group proceedings. Qualitative analyses followed the principles of thematic narrative analysis, with the goal of identifying themes associated with participant feedback [35]. The qualitative data were quantified by counting thematic categories of coded responses for each item such as “understood the question as intended” and “would recommend alternate wording.” The chair of the CD specialist panel (SPR) incorporated the feedback into the candidate items. The candidate items with the inclusion of feedback from focus groups then moved to the content validity stage.
Stage 3. Content validity
Content validity was accomplished through a Quantification of Content Validity Ratings process [36]. Each candidate item was distributed to all members of the specialist panel. Rating consisted of two aspects: RELEVANCY and CLARITY. For RELEVANCY, the scale included 1 [not relevant], 2 [item needs some revision], 3 [relevant but needs minor revision], 4 [very relevant]. For CLARITY, the scale consisted of 1 [not clear], 2 [item needs some revision], 3 [clear but needs minor revision], 4 [very clear]. Each specialist rated each item, and all results were compiled. Any items with CVR < 0.62 were revised and re-circulated until the item reached consensus [36].
Finally, an online survey was used to obtain further feedback from a larger community of individuals with CD. Links to the survey were advertised by the patient advocacy groups, and any affected individual could participate. The screening question for each survey asked participants to self-identify a diagnosis (i.e., “Have you received a diagnosis of cervical dystonia or spasmodic torticollis?”) If the answer was yes, they were asked to read the instructions and then answer the questions. Each participant was asked three questions about each item: 1) does this item reflect your experience with CD; 2) would a treatment that can improve this symptom be meaningful to you; and, 3) what minimal amount of improvement would be meaningful to you with anchors ranging between 0% (no improvement) to 100% (full improvement).
Stage 4. Testing performance of PCO
To assess real-life performance of the PCO for capturing changes in the severity of individual items at repeated intervals over time, items were programmed into a platform (SymptomSnap, developed by TekSynap, all rights reserved) that could be used on a device such as a smartphone, tablet, or computer. Each item was rated using a slider scale from 0 to 10, with reminders for subjects to enter ratings every week. A pilot group of eight individuals with CD were provided the tool and asked to provide symptom ratings over a 12-week period. Following these ratings, they were asked three questions to assess the tool: 1) how easy was this tool to use? 2) Do you think it is useful to document changes in the severity of your condition? and, 3) would you recommend this tool to others?
Results
Following stage 1 (content development and item generation) and stage 2 (item improvement and revision), a starting total of 58 items was reduced to 16 total items divided into three domains including four MOTOR items, six DISABILITY items, and six PSYCHOSOCIAL items (Figure 2; Table 1). The last domain included one question about overall quality of life and one question about overall severity. All items incorporated language to emphasize the relevance of the question to CD, to avoid responses that may reflect other comorbidities. For some items such as ability to drive, there was an “opt-out” option of a not applicable check box in the event a patient did not drive at baseline.
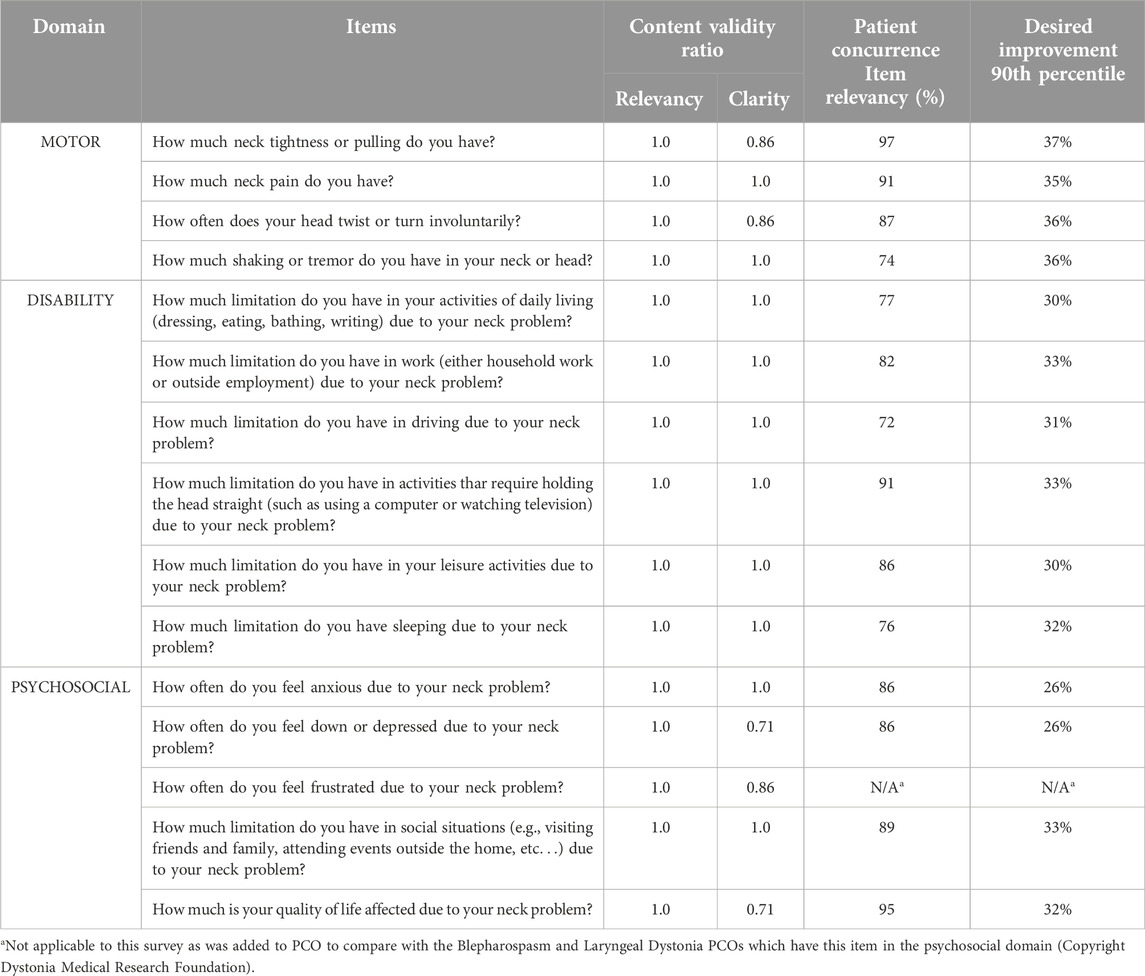
Table 1. PCO items shown by domain, by content validity scores from specialist panels, by patient concurrence rates and by amount of desired improvement.
Each item was anchored by consistent wording depending on whether the question asked about severity or about duration. For instance, “How much neck tightness or pulling do you have?” was anchored by “NONE” and “EXTREME”. The anchors were connected by a sliding bar with values from 0 to 10. If a question asked about duration such as “How often does your head twist or turn involuntarily?”, the anchors were “NEVER” and “ALWAYS.” For the quality-of-life question “How much is your quality of life affected due to your neck problem?”, the anchors were “NOT AFFECTED” and “VERY MUCH AFFECTED” (Figure 3). The highest scores possible are 40, 60, and 60 for MOTOR, DISABILITY, and PSYCHOSOCIAL domains, respectively.
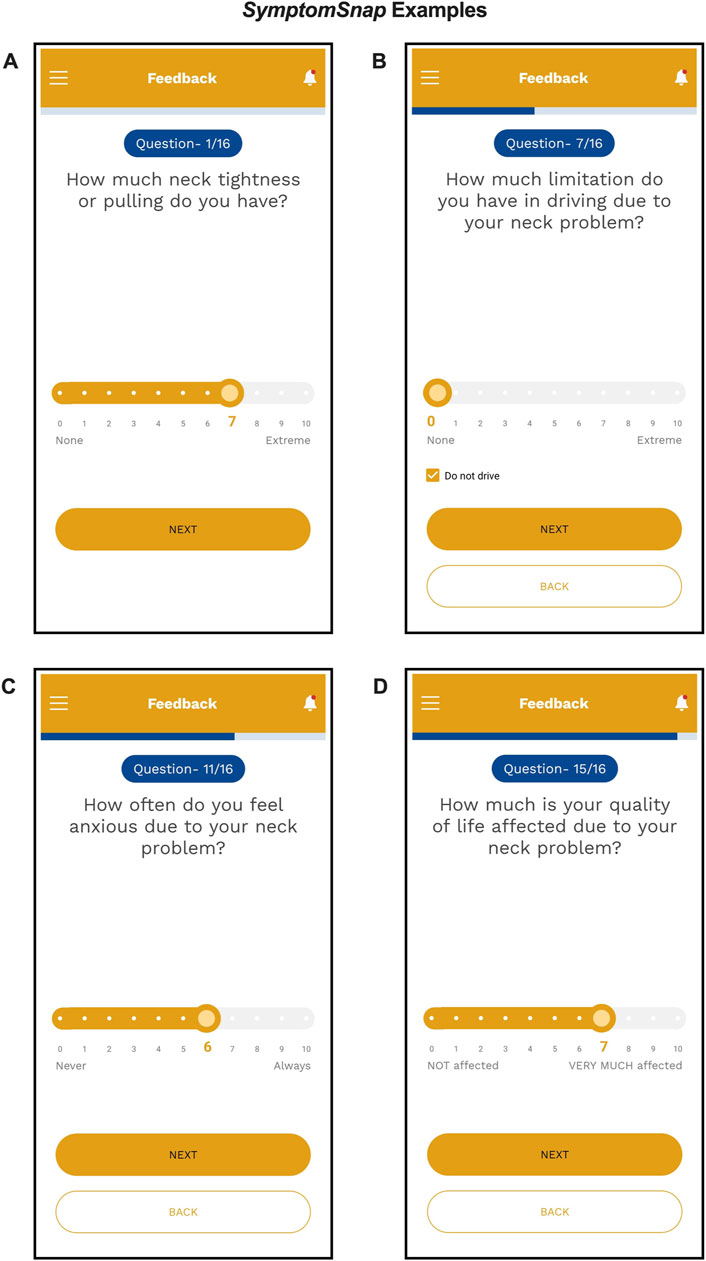
Figure 3. Screenshots of example PCO questions and anchors from the SymptomSnap app demonstrating how a participant would enter their response for: (A) MOTOR Question 1 with anchors “None to Extreme”; (B) DISABILITY Question 7 with ability to mark “Do not drive”; (C) PSYCHOSOCIAL Question 11 with anchors “Never to Always”; and, (D) PSYCHOSOCIAL Question 15 with anchors “Not affected to Very much affected.”
For stage 3 (assessment by the broader CD community), the survey for the relevance of the candidate items was open for 4 weeks, with 590 responses. All PCO items had a concurrence rate for relevancy higher than 70%. The item with highest concurrence (97%) was “neck tightness or pulling.” The item with the lowest concurrence (72%) was “ability to drive” (Table 1).
The degree of desired improvement varied across the items. The lowest degree of improvement was expected for anxiety/depression and the highest degree of improvement was expected for twisting or turning of the neck (Table 1). At least 95% of subjects reported that a change of 25% (SD 1.8%) is the minimal change that would be meaningful (small effect size). At least 90% of subjects reported a change of 33% (SD 2.8%) is the minimal change that would be meaningful (medium effect size). And finally, at least 75% of subjects reported a change of 50% (SD 0.3%) is the minimal change that would be meaningful (large effect size).
The pilot performance of the PCO for 8 individuals with CD over a single BoNT injection cycle revealed good adherence to weekly assessment with an average missing data rate of 2.6% over 12 weeks (range 0.0%–5.0%) (Figure 4). There were only three missing data points in the sample, which came from three different individuals indicating that the majority of participants were able to provide a full dataset. Example cases for the two of the eight individuals for the total MOTOR score, total PSYCHOSOCIAL score and total DISBAILITY score show change over the course of a typical BoNT injection cycle of 12 weeks (Figure 5). All users described the tool as easy to use, quick to enter (less than 10 min), appropriately documented their symptoms and would recommend its use to others. A larger cohort is currently being followed to assess the performance of the PCO over multiple BoNT cycles.
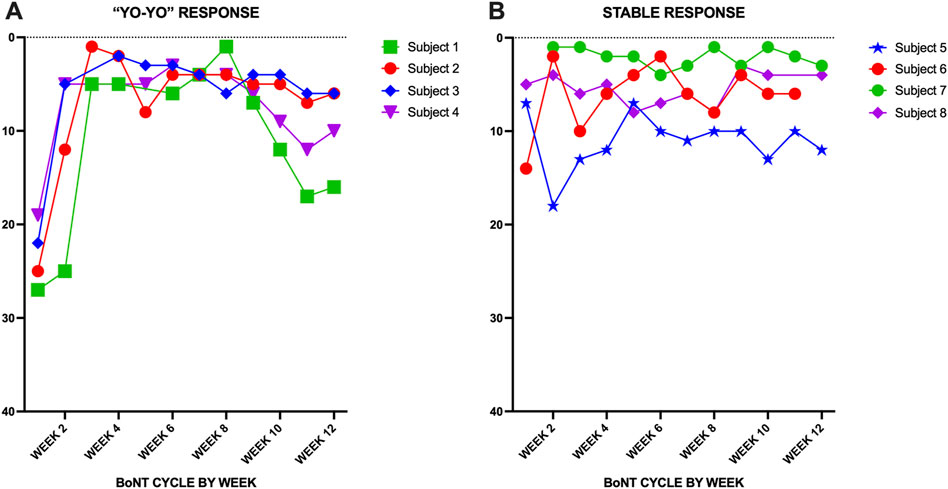
Figure 4. (A) PCO total motor scores shown over one BoNT 12-week treatment cycle for four CD patients showing the typical “yo-yo” response. (B) Relatively stable response during a BoNT cycle in four patients.
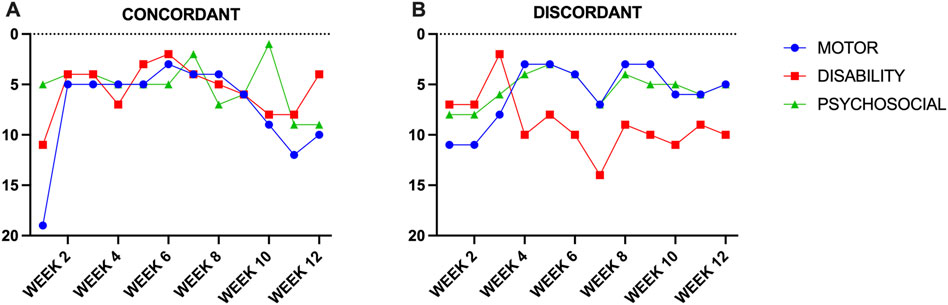
Figure 5. (A) Concordant improvement in motor, disability and psychosocial scores during a BoNT cycle. (B) Discordant response with improvement in motor and psychosocial scores and worsening of disability scores.
Discussion
This study provides evidence that a simple digital tool can be used to collect information regarding changes in the most meaningful symptoms in CD at a frequency sufficient to chart the temporal profile of responses to a treatment intervention. One of the key advantages of using this approach is that it makes collection of real-time data in the real-world environment of CD subjects viable. This technique of data sampling is called ecological momentary assessment [37]. It can generate a large and varied number of observations per subject allowing for a deeper understanding of different contributions to particular problem. The current study aimed to collect data at weekly intervals, but other intervals (daily or monthly) are also feasible.
One of the interesting findings to emerge from this study was information regarding the minimum amount of improvement that would be considered significant to individuals with CD. Prior clinical trials have been based on clinician-rated scales such as the TWSTRS. A 25% improvement in the TWSTRS score is often used as a criterion for success, but what is not known is whether 25% improvement is sufficient for a patient to want to continue therapy. Even if the target TWSTRS improvement was increased to an arbitrary goal of 50%, the outcome could yield a statistically positive result yet remain an unsatisfactory outcome from the patient perspective. The current study provides information on what patients hope to achieve. Another interesting observation was that the degree of desired improvement was item-dependent. For instance, for 90% of respondents with CD, the minimal meaningful improvement was 37% for neck tightness whereas a 26% improvement would be acceptable for depression/anxiety.
This tool may have immediate practical value for individuals with CD and their clinicians as a medical symptom diary, similar to diaries presently in use for headaches or seizures. Currently, treatment changes in the clinic for CD are driven mostly by patient recollection of outcomes of prior treatments, which is sometimes challenging. This quantifiable real-time information provided by the PCO tool is likely to provide more reliable information. The tool can provide information on the peak response and duration of a single BoNT treatment, as well as the waxing and waning responses observed with repeated treatment. Information from one treatment cycle can be stored and compared with subsequent treatment cycles when changes are introduced. It could also be used to monitor the potential benefit of add-on therapies such as adjunctive oral medications or physiotherapy, and to measure the duration of benefit in a clinically meaningful way.
Data availability statement
The datasets presented in this article are not readily available because the study describes the development of a patient-centered outcome and does not have an available dataset. Any data that was used in the generation of individual items that came from the Dystonia Coalition is publicly available through a data request. Requests to access the datasets should be directed to https://dc.rarediseasesnetwork.org/resources-researchers-and-clinicians.
Ethics statement
The studies involving humans were approved by the Washington University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SPR, JP, HJ, and FQ contributed to the conception and design of the study. SPR, BB, JH, CC, DP, GK-B, LW, SP, JJ, CA, MT, TK, MB, JP, HJ, and PR contributed to the data collection, organization, and quality management. FQ performed the statistical analysis. SPR and HJ wrote the first draft of the manuscript. SPR, BB, JH, CC, DP, GK-B, LW, SP, JJ, CA, MT, TK, MB, JP, HJ, and PR edited and revised sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. U54 NS116025/NS/NINDS NIH HHS/United States.
Conflict of interest
MT reports grants from the Netherlands Organisation for Health Research and Development Domain: NWO-TTW (2022-9), ZonMW Topsubsidie (91218013) and ZonMW Program Translational Research (40-44600-98-323). She also received two European Fund for Regional Development from the European Union [01492947 & DIMATIO (EFRO-0059)] and an European Joint Programme on Rare Diseases (EJP RD) Networking Support Scheme. A grant from the Health Holland and the PPP allowance program (PPP-2023-00). Furthermore, from the province of Friesland, the Stichting Wetenschapsfonds Dystonie and unrestricted grants from Ipsen, Actelion and Merz, STIL, Abbvie and TEVA. BB has received research grant support from the Dystonia Coalition (receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke), the Parkinson’s Foundation, the VCU School of Medicine, the Administration for Community Living, and the Dystonia Medical Research Foundation. He has received honoraria from the International Parkinson and Movement Disorder Society and serves on the Medical and Scientific Advisory Council of the Dystonia Medical Research Foundation as well as the Medical Advisory Board of the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
2https://dystonia-foundation.org
References
1. Albanese, A, Bhatia, KP, Cardoso, F, Comella, C, Defazio, G, Fung, VSC, et al. Isolated cervical dystonia: diagnosis and classification. Mov Disord (2023) 38(8):1367–78. doi:10.1002/mds.29387
2. Defazio, G, Belvisi, D, Comella, C, Hallett, M, Jinnah, HA, Cimino, P, et al. Validation of a guideline to reduce variability in diagnosing cervical dystonia. J Neurol (2023) 270(5):2606–12. doi:10.1007/s00415-023-11585-6
3. Kilic-Berkmen, G, Pirio Richardson, S, Perlmutter, JS, Hallett, M, Klein, C, Wagle-Shukla, A, et al. Current guidelines for classifying and diagnosing cervical dystonia: empirical evidence and recommendations. Mov Disord Clin Pract (2022) 9(2):183–90. doi:10.1002/mdc3.13376
4. Rosales, RL, Cuffe, L, Regnault, B, and Trosch, RM. Pain in cervical dystonia: mechanisms, assessment and treatment. Expert Rev Neurother (2021) 21:1125–34. doi:10.1080/14737175.2021.1984230
5. Albanese, A, Wissel, J, Jost, WH, Castagna, A, Althaus, M, Comes, G, et al. Pain reduction in cervical dystonia following treatment with incobotulinumtoxinA: a pooled analysis. Toxins (Basel). (2023) 15:333. doi:10.3390/toxins15050333
6. Girach, A, Vinagre Aragon, A, and Zis, P. Quality of life in idiopathic dystonia: a systematic review. J Neurol (2019) 266:2897–906. doi:10.1007/s00415-018-9119-x
7. Medina Escobar, A, Pringsheim, T, Goodarzi, Z, and Martino, D. The prevalence of depression in adult onset idiopathic dystonia: systematic review and metaanalysis. Neurosci Biobehav Rev (2021) 125:221–30. doi:10.1016/j.neubiorev.2021.02.036
8. Medina Escobar, A, Martino, D, and Goodarzi, Z. The prevalence of anxiety in adult-onset isolated dystonia: a systematic review and meta-analysis. Eur J Neurol (2021) 28:4238–50. doi:10.1111/ene.15050
9. Castelao, M, Marques, RE, Duarte, GS, Rodrigues, FB, Ferreira, J, Sampaio, C, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev (2017) 12:CD003633. doi:10.1002/14651858.CD003633.pub3
10. Simpson, DM, Hallett, M, Ashman, EJ, Comella, CL, Green, MW, Gronseth, GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology (2016) 86:1818–26. doi:10.1212/WNL.0000000000002560
11. Jinnah, HA, Comella, CL, Perlmutter, J, Lungu, C, and Hallett, M, Dystonia Coalition Investigators. Longitudinal studies of botulinum toxin in cervical dystonia: why do patients discontinue therapy? Toxicon (2018) 147:89–95. doi:10.1016/j.toxicon.2017.09.004
12. Lee, JI, Jansen, A, Samadzadeh, S, Kahlen, U, Moll, M, Ringelstein, M, et al. Long-term adherence and response to botulinum toxin in different indications. Ann Clin Transl Neurol (2021) 8:15–28. doi:10.1002/acn3.51225
13. Comella, C, and Bhatia, K. An international survey of patients with cervical dystonia. J Neurol (2015) 262:837–48. doi:10.1007/s00415-014-7586-2
14. Dressler, D, Tacik, P, and Saberi, FA. Botulinum toxin therapy of cervical dystonia: duration of therapeutic effects. J Neural Transm (2015) 122:297–300. doi:10.1007/s00702-014-1253-8
15. Jinnah, HA, Goodmann, E, Rosen, AR, Evatt, M, Freeman, A, and Factor, S. Botulinum toxin treatment failures in cervical dystonia: causes, management, and outcomes. J Neurol (2016) 263:1188–94. doi:10.1007/s00415-016-8136-x
16. Leplow, B, Eggebrecht, A, and Pohl, J. Treatment satisfaction with botulinum toxin: a comparison between blepharospasm and cervical dystonia. Patient Prefer Adherence (2017) 11:1555–63. doi:10.2147/PPA.S141060
17. Marciniec, M, Szczepanska-Szerej, A, and Rejdak, K. Cervical dystonia: factors deteriorating patient satisfaction of long-term treatment with botulinum toxin. Neurol Res (2020) 42:987–91. doi:10.1080/01616412.2020.1796430
18. Tyslerowicz, M, Kiedrzynska, W, Adamkiewicz, B, Jost, WH, and Slawek, J. Cervical dystonia - improving the effectiveness of botulinum toxin therapy. Neurol Neurochir Pol (2020) 54:232–42. doi:10.5603/PJNNS.a2020.0021
19. Comella, C, Ferreira, JJ, Pain, E, Azoulai, M, and Om, S. Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia. J Neurol (2021) 268:903–12. doi:10.1007/s00415-020-10217-7
20. Erro, R, Picillo, M, Pellecchia, MT, and Barone, P. Improving the efficacy of botulinum toxin for cervical dystonia: a scoping review. Toxins (2023) 15:391. doi:10.3390/toxins15060391
21. Pirio Richardson, S, and Jinnah, HA. New approaches to discovering drugs that treat dystonia. Expert Opin Drug Discov (2019) 10:893–900. doi:10.1080/17460441.2019.1623785
22. Sethi, KD, Rodriguez, R, and Olayinka, B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ (2012) 15:419–23. doi:10.3111/13696998.2011.653726
23. Ojo, OO, and Fernandez, HH. Is it time for flexibility in botulinum inter-injection intervals? Toxicon (2015) 107:72–6. doi:10.1016/j.toxicon.2015.09.037
24. Colosimo, C, Charles, D, Misra, VP, Maisonobe, P, and Om, S, INTEREST IN CD2 study group. How satisfied are cervical dystonia patients after 3 years of botulinum toxin type A treatment? Results from a prospective, long-term observational study. J Neurol (2019) 266:3038–46. doi:10.1007/s00415-019-09527-2
25. Wollner, J, Weise, D, and Leplow, B. Subjective versus objective symptom intensities ratings in cervical dystonia and hemifacial spasm across a botulinum neurotoxin cycle. Brain Behav (2021) 11(3):e02023. doi:10.1002/brb3.2023
26. Fox, SH, Swan, M, Jinnah, HA, de Freitas, MET, de Oliveira, LM, Al-Shorafat, D, et al. An open-label phase 2a study to evaluate the safety and tolerability of perampanel in cervical dystonia. Mov Disord Clin Pract (2021) 8:743–9. doi:10.1002/mdc3.13229
27. Loudovici-Krug, D, Derlien, S, Best, N, and Gunther, A. Physiotherapy for cervical dystonia: a systematic review of randomised controlled trials. Toxins (2022) 14:784. doi:10.3390/toxins14110784
28. Comella, CL, Jankovic, J, Hauser, RA, Patel, AT, Banach, MD, Ehler, E, et al. Efficacy and safety of daxibotulinumtoxinA for injection in cervical dystonia: ASPEN-1 phase 3 randomized controlled trial. Neurology (2024) 102:e208091. doi:10.1212/WNL.0000000000208091
29. Albanese, A, Sorbo, FD, Comella, C, Jinnah, HA, Mink, JW, Post, B, et al. Dystonia rating scales: critique and recommendations. Mov Disord (2013) 28:874–83. doi:10.1002/mds.25579
30. Skogseid, IM, and Kerty, E. The course of cervical dystonia and patient satisfaction with long-term botulinum toxin A treatment. Eur J Neurol (2005) 12:163–70. doi:10.1111/j.1468-1331.2004.01053.x
31. Trosch, RM, Espay, AJ, Truong, D, Gil, R, Singer, C, LeWitt, PA, et al. Multicenter observational study of abobotulinumtoxinA neurotoxin in cervical dystonia: the ANCHOR-CD registry. J Neurol Sci (2017) 376:84–90. doi:10.1016/j.jns.2017.02.042
32. Cotton, AC, Scorr, L, McDonald, W, Comella, C, Perlmutter, JS, Goetz, CG, et al. Assessing the severity of cervical dystonia: ask the doctor or ask the patient. Movement Disord Clin Pract (2023) 10:1399–403. doi:10.1002/mdc3.13827
33. Muller, J, Wissel, J, Kemmler, G, Voller, B, Bodner, T, Schneider, A, et al. Craniocervical dystonia questionnaire (CDQ-24): development and validation of a disease-specific quality of life instrument. J Neurol Neurosurg Psychiatry (2004) 75(5):749–53. doi:10.1136/jnnp.2003.013441
34. Comella, CL, Perlmutter, JS, Jinnah, HA, Waliczek, TA, Rosen, AR, Galpern, WR, et al. Clinimetric testing of the comprehensive cervical dystonia rating scale. Mov Disord (2016) 31:563–9. doi:10.1002/mds.26534
35. Dixon-Woods, M, Agarwal, S, Jones, D, Young, B, and Sutton, A. Synthesising qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Pol (2005) 10(1):45–53. doi:10.1177/135581960501000110
36. O'Keefe-McCarthy, S, McGillion, M, Nelson, S, Clarke, S, McFetridge-Durdle, J, and Watt-Watson, J. Content validity of the Toronto pain management inventory-acute coronary syndrome version. Can J Cardiovasc Nurs (2014) 24:11–8.
Keywords: efficacy, botulinum toxin, dystonia, outcomes, patient-centered
Citation: Pirio Richardson S, Berman BD, Hieshetter J, Comella C, Peterson DA, Kilic-Berkmen G, Wright L, Pentecost S, Reyes P, Jankovic J, Adler CH, Tijssen MAJ, Kimberley TJ, Benson M, Perlmutter JS, Qeadan F and Jinnah HA (2024) A digital patient-centered outcome tool for cervical dystonia. Dystonia 3:13478. doi: 10.3389/dyst.2024.13478
Received: 02 July 2024; Accepted: 11 September 2024;
Published: 20 September 2024.
Edited by:
Aasef Shaikh, Case Western Reserve University, United StatesCopyright © 2024 Pirio Richardson, Berman, Hieshetter, Comella, Peterson, Kilic-Berkmen, Wright, Pentecost, Reyes, Jankovic, Adler, Tijssen, Kimberley, Benson, Perlmutter, Qeadan and Jinnah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Pirio Richardson, c3BpcmlvcmljaGFyZHNvbkBzYWx1ZC51bm0uZWR1
 Sarah Pirio Richardson
Sarah Pirio Richardson Brian D. Berman2
Brian D. Berman2 David A. Peterson
David A. Peterson Joseph Jankovic
Joseph Jankovic Marina A. J. Tijssen
Marina A. J. Tijssen H. A. Jinnah
H. A. Jinnah