Abstract
Background: Tapinarof is a nonsteroidal, topical, aryl hydrocarbon receptor agonist. Tapinarof has been shown to be efficacious and have acceptable safety profile in the treatment of atopic dermatitis (AD).
Objective: We sought to evaluate the improvement effect of tapinarof on skin barrier function in patients with AD.
Methods: This was an open-label, uncontrolled, single-center study. Japanese patients aged ≥20 years with AD (N = 30) were included in this study. Patients applied tapinarof cream 1% once daily to the target areas on the volar forearm for 8 weeks. The primary endpoints were changes from baseline in stratum corneum hydration (SCH) and transepidermal water loss (TEWL) at the target affected area at week 8.
Results: The mean SCH value at the target affected area was 13.656 AU at baseline, 16.904 AU at week 4, and 16.423 AU at week 8. The SCH at the target affected area significantly increased from baseline to week 8, with a mean change of 2.826 AU (p = 0.0433). The mean TEWL value at the target affected area was 17.35 g/m2/hr at baseline, 10.01 g/m2/hr at week 4, and 9.52 g/m2/hr at week 8. The TEWL at the target affected area significantly decreased from baseline to week 8, with a mean change of −8.03 g/m2/hr (p < 0.0001). Clinical signs of AD at the target affected area were improved over time. No serious, severe, or treatment-related AEs were reported.
Conclusion: Treatment with tapinarof led to an increase in SCH and a decrease in TEWL in patients with AD, indicating the potential improvement effect of tapinarof on skin barrier function.
Introduction
Atopic dermatitis (AD) is a common, chronic, inflammatory skin disease [1]. The lifetime prevalence of AD is estimated to be >15% in many countries [2]. Quality of life of patients with AD is markedly affected by visible skin lesions and sleep disturbance due to persistent intense itching [3, 4]. The pathogenesis of AD is multifactorial, involving abnormal immune responses, genetic and environmental factors, and skin barrier dysfunction [5–7]. Type 2 helper T (Th2) cytokines, such as interleukin (IL)-4 and IL-13, play an important role in the development of AD [2]. Skin barrier dysfunction in AD is associated with loss-of-function mutations in the gene of filaggrin, a critical skin barrier-related protein in the stratum corneum [8, 9]. The expression of skin barrier-related proteins can be downregulated by Th2 cytokines, which are overexpressed in AD skin lesions, leading to further skin barrier dysfunction [8, 9]. Compared to healthy skin, a decrease in stratum corneum hydration (SCH) and an increase in transepidermal water loss (TEWL) are noted at AD skin lesions [10], which reflects less hydrated skin resulting from the barrier dysfunction in patients with AD.
The primary goal of treatment of AD is to maintain a long-term remission in which signs and symptoms of AD are absent or minimal without disturbance of daily activities [1]. Topical corticosteroids are the mainstay of current treatment of AD; however, long-term use of topical corticosteroids is associated with specific adverse reactions such as skin atrophy and may cause skin barrier dysfunction [11]. Although nonsteroidal topical drugs become available, there remains a need for efficacious topical drugs that can be used long-term and improve skin barrier function.
Tapinarof is a non-steroidal, topical aryl hydrocarbon receptor (AhR) agonist [12]. The AhR is a cytosolic ligand-dependent transcription factor. By activating AhR, tapinarof upregulates the expression of skin barrier-related proteins, such as filaggrin, hornerin, and involucrin [12]. The pharmacological action of tapinarof also includes downregulation of the proinflammatory cytokine expression and upregulation of the antioxidative enzyme expression through activation of the nuclear factor erythroid 2 related factor 2 pathway [12, 13]. Thus, tapinarof can be a novel topical drug for AD with characteristic pharmacological actions including the potential for improvement of skin barrier function.
To date, several clinical studies to evaluate the efficacy and safety of tapinarof have been conducted in patients with AD [14–17]. These study results revealed that tapinarof was efficacious and had acceptable safety profile. In the present study, we sought to evaluate the improvement effect of tapinarof on skin barrier function in patients with AD.
Methods
Study design
This was an open-label, uncontrolled, single-center (Hosui General Medical Clinic, Hokkaido, Japan) study in which Japanese patients with AD (N = 30) received tapinarof cream 1% once daily for 8 weeks (Figure 1). Patients visited the study site at 2, 4, and 8 weeks after initiation of treatment. This study was approved by an institutional review board (Sapporo Skin Clinic Institutional Review Board) and was conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. This study is registered in Japan Registry of Clinical Trials (jRCT, https://jrct.niph.go.jp/), with the registration number of jRCT2011210062.
FIGURE 1
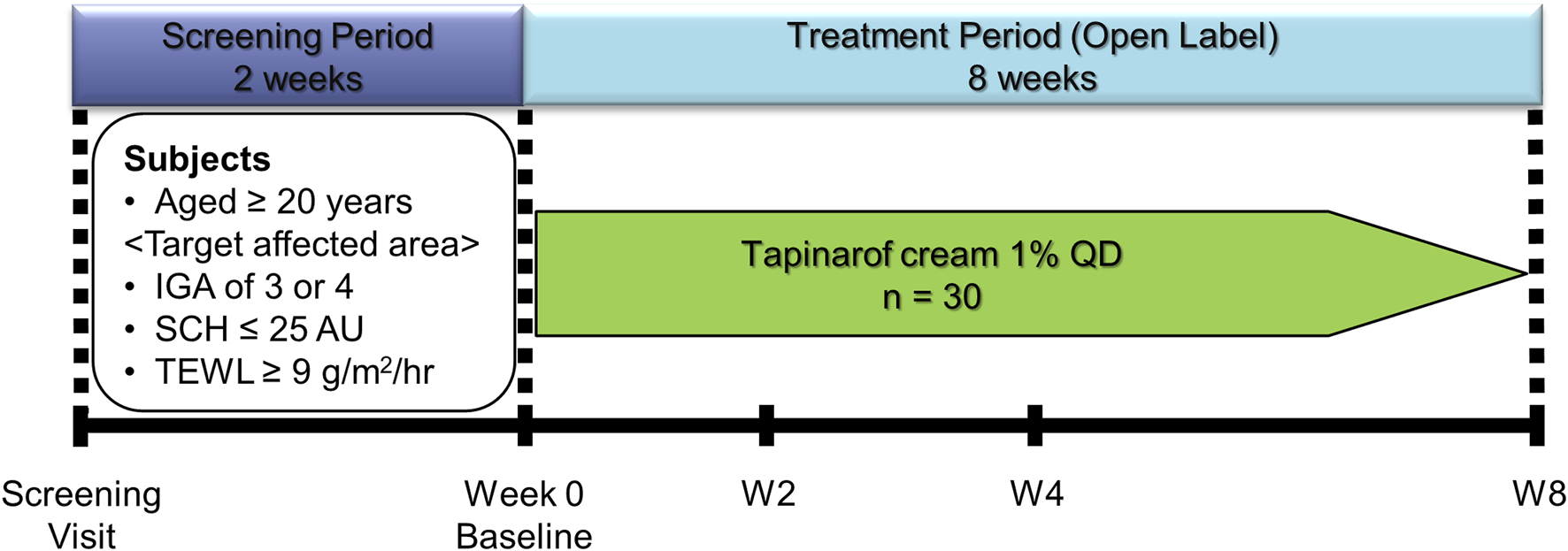
Study Design. AD, atopic dermatitis; AU, arbitrary unit; IGA, Investigator’s Global Assessment; QD, once daily; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Study population
This study enrolled Japanese patients aged ≥20 years with a clinical diagnosis of AD according to the criteria of the Japanese Dermatological Association [1]. Eligible patients had a target affected area of approximately ≥25 cm2 on the left or right volar forearm, an Investigator’s Global Assessment (IGA, Supplementary Table S1) score of 3 (moderate) or 4 (severe) at the target affected area, a target unaffected area of approximately ≥25 cm2 on the same volar forearm as for the target affected area, and a SCH value of ≤25 (in arbitrary unit, AU) and a TEWL value of ≥9 g/m2/hr at the target affected area. Exclusion criteria were summarized in the Supplementary Material. Healthy volunteers aged ≥20 years (N = 10) were also enrolled in this study to obtain reference data of SCH and TEWL values and did not receive study treatment. A target area on the left or right volar forearm in each healthy volunteer was determined by the investigator. All patients and healthy volunteers provided written informed consent to participate in this study.
Study treatment
Patients were instructed to apply a thin layer of study treatment once daily to the target areas (target affected area and target unaffected area) throughout the 8-week treatment period. Therapies that may have interfered with study assessments were prohibited during the study (e.g., biologic agents, phototherapy at the target areas, and topical agents at the target areas; Supplementary Material for details).
Study assessments
The primary endpoints were changes from baseline in SCH and TEWL at the target affected area at week 8. The measurement of SCH at the target areas was performed with Corneometer® CM825 (Courage+Khazaka electronic GmbH, Cologne, Germany). The measurement of TEWL at the target areas were performed with Tewameter® TM300 (Courage+Khazaka electronic GmbH, Cologne, Germany). The patients and healthy volunteers were kept at rest for at least 20 min in a constant temperature and humidity room before the measurement of SCH and TEWL. The severity of AD at the target affected area was assessed with IGA and severity scores. The IGA score was a static 5-point morphological assessment of overall disease severity from 0 (clear) to 4 (severe) (Supplementary Table S1). The severity score corresponded to the clinical sign score in Eczema Area and Severity Index [18], rating the severity of each of four clinical signs (erythema, infiltration/papulation, excoriation, and lichenification) on a 6-point scale (0 = none, 1 = mild, 1.5 = mild to moderate, 2 = moderate, 2.5 = moderate to severe, 3 = severe), and the overall score ranged from 0 to 12. Safety assessments included the incidence and severity of adverse events (AEs).
Statistical analyses
The sample size calculation was based on the data from a study of delgocitinib ointment, where SCH and TEWL values were evaluated in patients with AD [19]. A sample size of 30 was determined to have sufficient power for analysis of the primary endpoints with a 2-sided t-test at the 5% significance level, with at least 99.9% power for SCH and 88.8% power for TEWL. The primary analyses of efficacy (SCH, TEWL, IGA, and severity score) used the full analysis set (FAS), which included all patients who underwent the assessment of efficacy at least once after initiation of study treatment. Mean changes from baseline in SCH and TEWL were calculated by study visit, and the t-test was performed at week 8 for the mean changes from baseline at the target affected area. No adjustment was performed for multiple tests. The frequency distribution of IGA scores was generated by study visit. The mean percent change from baseline in severity score was calculated by study visit. The safety analyses used the safety analysis population, which included all patients who underwent the assessment of safety at least once after initiation of study treatment.
Results
Study population
A total of 30 patients with AD received study treatment, and 29 patients completed the 8-week treatment. One patient was discontinued from the study because of an AE. In addition, 10 healthy volunteers were enrolled in the study. The mean age and sex distribution in patients with AD were similar to those in healthy volunteers. Patients with AD had less hydrated skin than healthy volunteers, as indicated by baseline SCH and TEWL values. Approximately 90% of patients with AD had a baseline IGA score of 3 (moderate) at the target affected area (Table 1).
TABLE 1
| Patients with AD (N = 30) | Healthy volunteers (N = 10) | |
|---|---|---|
| Age (years) | 36.3 (10.6) | 36.7 (13.6) |
| Sex [n (%)] | ||
| Male | 12 (40.0) | 5 (50.0) |
| Female | 18 (60.0) | 5 (50.0) |
| Duration of AD (years) | 29.2 (9.7) | — |
| SCH (AU) | 13.656 (4.811)a | 30.590 (8.934) |
| TEWL (g/m2/hr) | 17.35 (7.92)a | 5.97 (1.84) |
| IGA scorea [n (%)] | ||
| 3 (moderate) | 28 (93.3) | — |
| 4 (severe) | 2 (6.7) | — |
| Severity scorea | 8.03 (1.58) | — |
Demographics and baseline characteristics.
Data are displayed as mean (SD) unless otherwise indicated. Healthy volunteers were enrolled in this study to obtain reference data of SCH and TEWL values and did not receive study treatment.
AD, atopic dermatitis; AU, arbitrary unit; IGA, Investigator’s Global Assessment; SCH, stratum corneum hydration; SD, standard deviation; TEWL, transepidermal water loss.
These were assessed at the target affected area in patients with AD.
Efficacy
The mean SCH value at the target affected area was 13.656 AU at baseline, 16.904 AU at week 4, and 16.423 AU at week 8 (Figure 2A; Supplementary Table S2). The SCH at the target affected area significantly increased from baseline to week 8, with a mean change of 2.826 AU (95% confidence interval [CI], 0.091 to 5.561 AU; p = 0.0433) (Figure 2B; Supplementary Table S2). The mean TEWL value at the target affected area was 17.35 g/m2/hr at baseline, 10.01 g/m2/hr at week 4, and 9.52 g/m2/hr at week 8 (Figure 2C; Supplementary Table S2). The TEWL at the target affected area significantly decreased from baseline to week 8, with a mean change of −8.03 g/m2/hr (95% CI, −11.23 to −4.83 g/m2/hr; p < 0.0001) (Figure 2D; Supplementary Table S2).
FIGURE 2
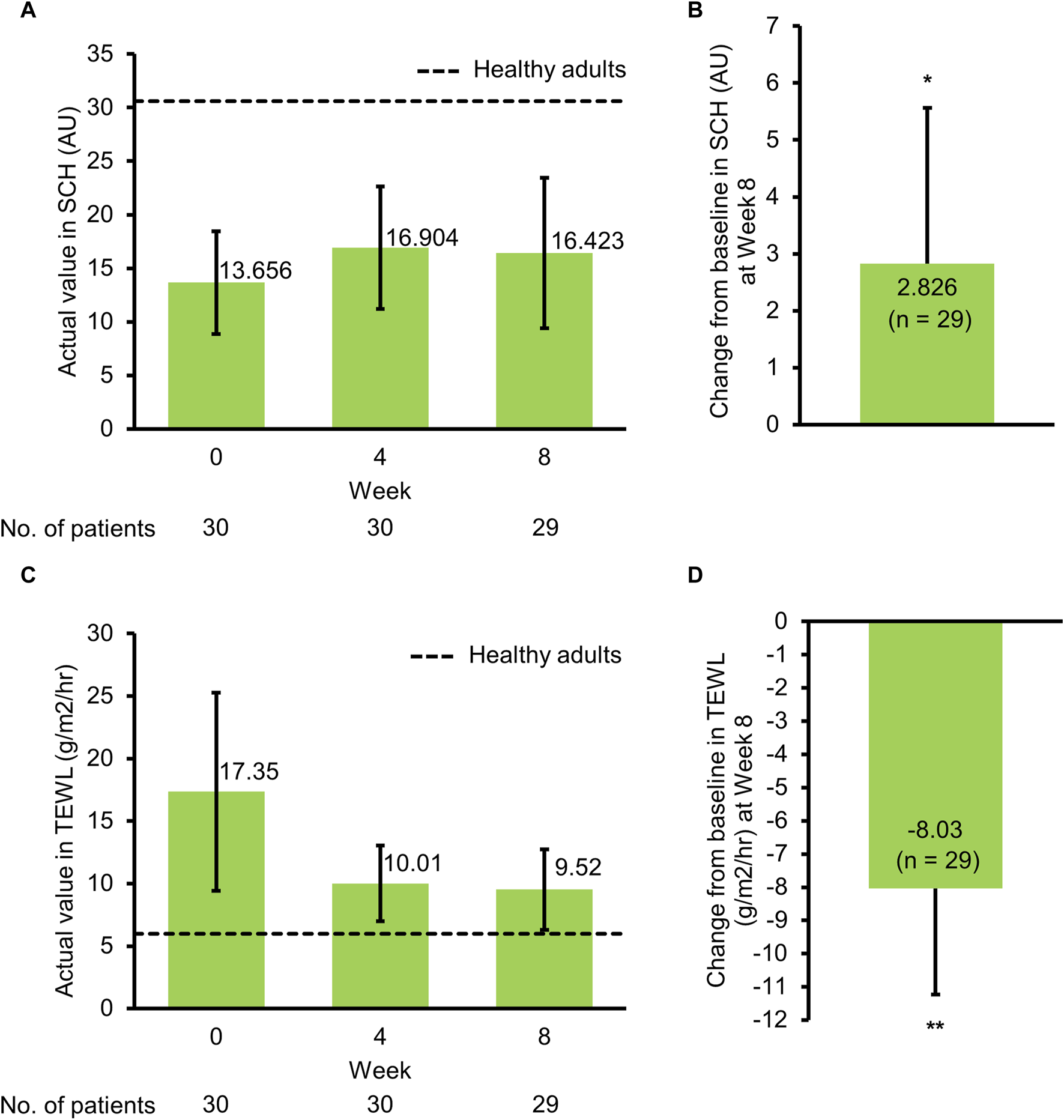
Effects on skin barrier functions in patients with atopic dermatitis. (A) Actual value (mean ± SD) in SCH by study visit, (B) change from baseline (mean + 95% CI) in SCH at Week 8, (C) actual value (mean ± SD) in TEWL by study visit, (D) change from baseline (mean − 95% CI) in TEWL at Week 8. AU, arbitrary unit; CI, confidence interval; SCH, stratum corneum hydration; SD, standard deviation; TEWL, transepidermal water loss. *p = 0.043, **p < 0.0001 (t-test).
The IGA score at the target affected area was improved over time. Approximately 60% of patients achieved an IGA score of 0 (clear) or 1 (almost clear) at week 8 (Figure 3). The severity score at the target affected area decreased over time. The mean percent change from baseline in severity score was −57.19% at week 4 and −67.18% at week 8 (Figure 4; Supplementary Table S3). Representative clinical images of the target affected area are presented in Figure 5.
FIGURE 3
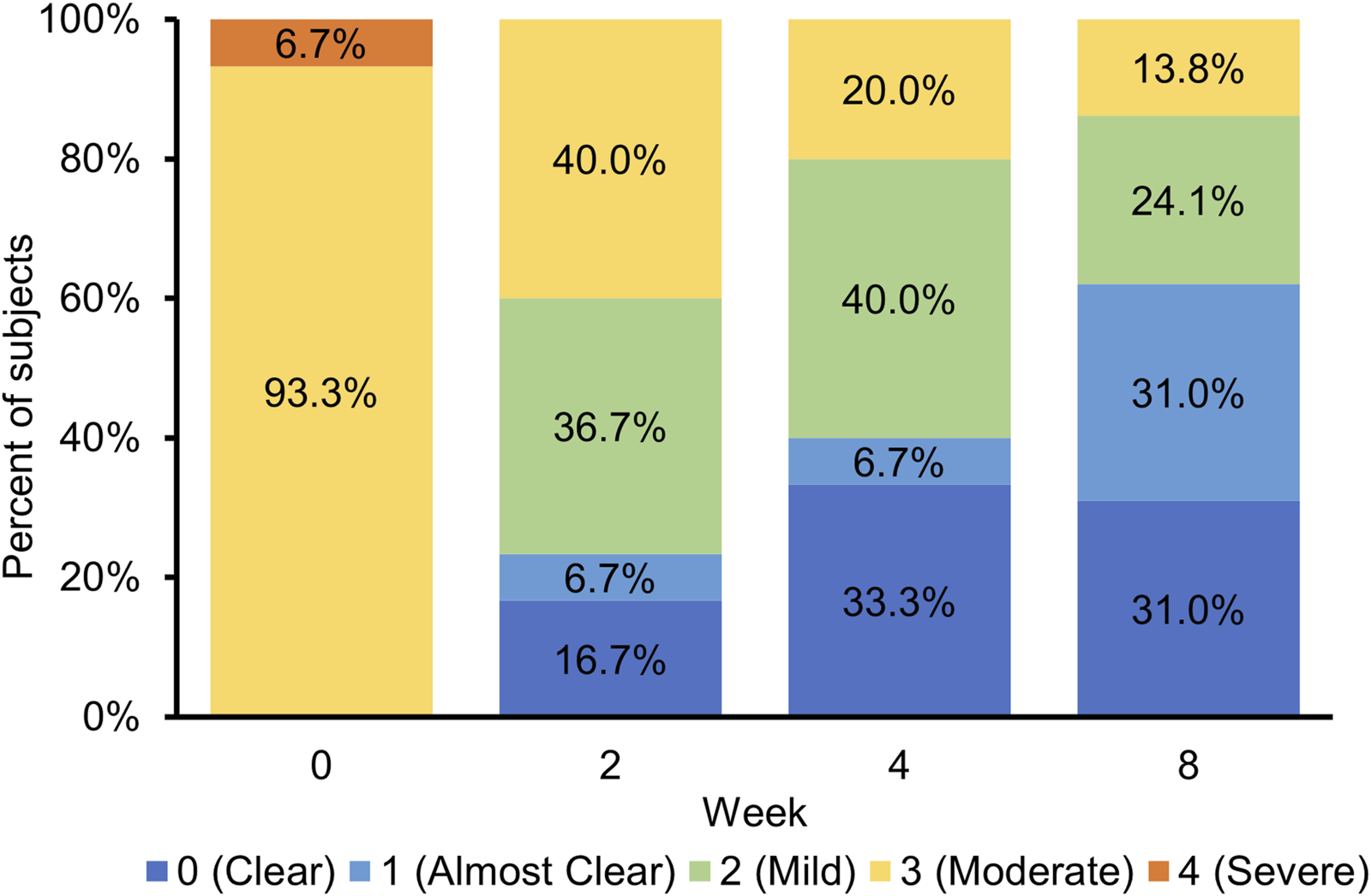
Frequency distribution of investigator’s global assessment (IGA) scores by study visit.
FIGURE 4
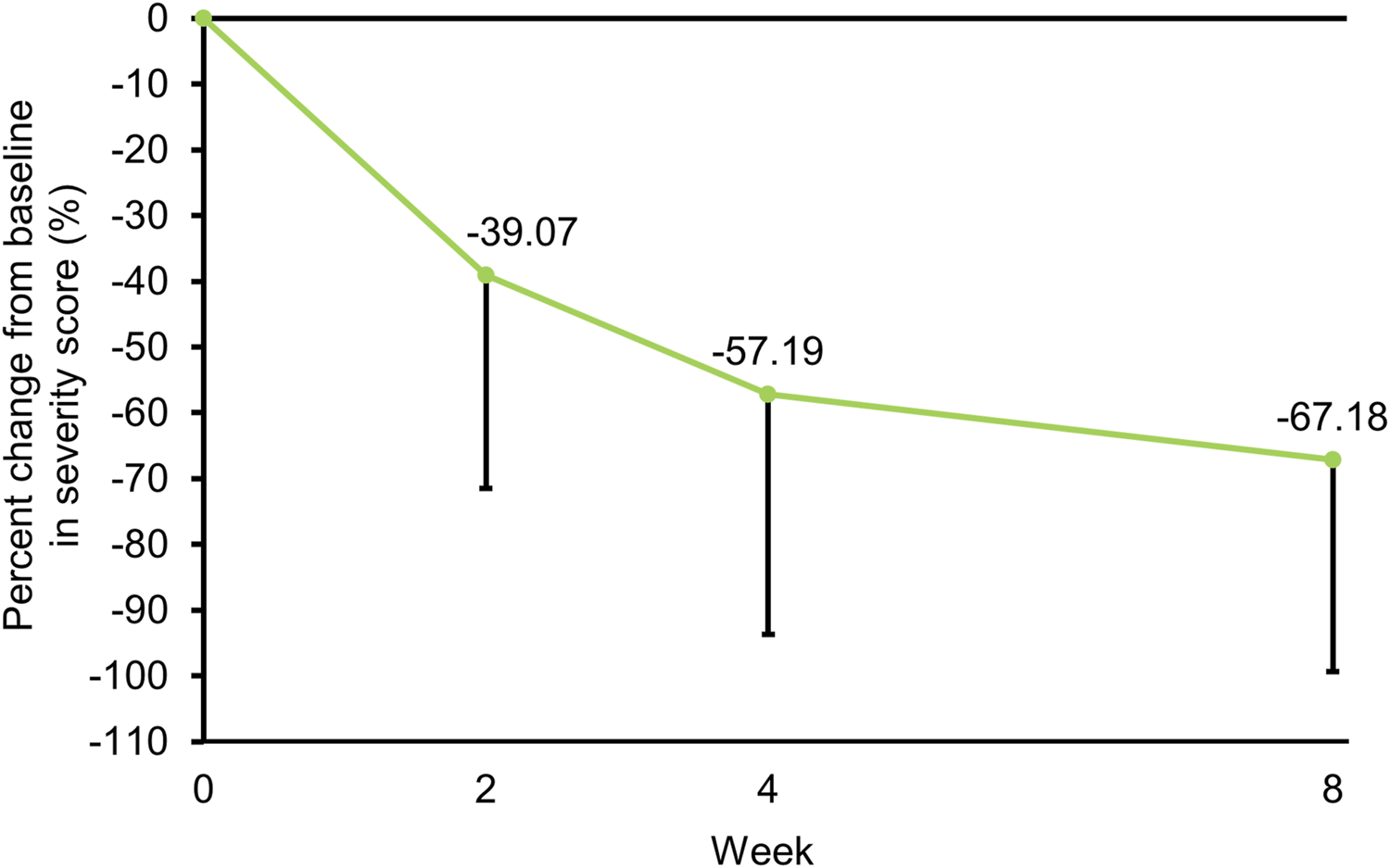
Percent change (mean − SD) from baseline in severity score over time. SD, standard deviation.
FIGURE 5
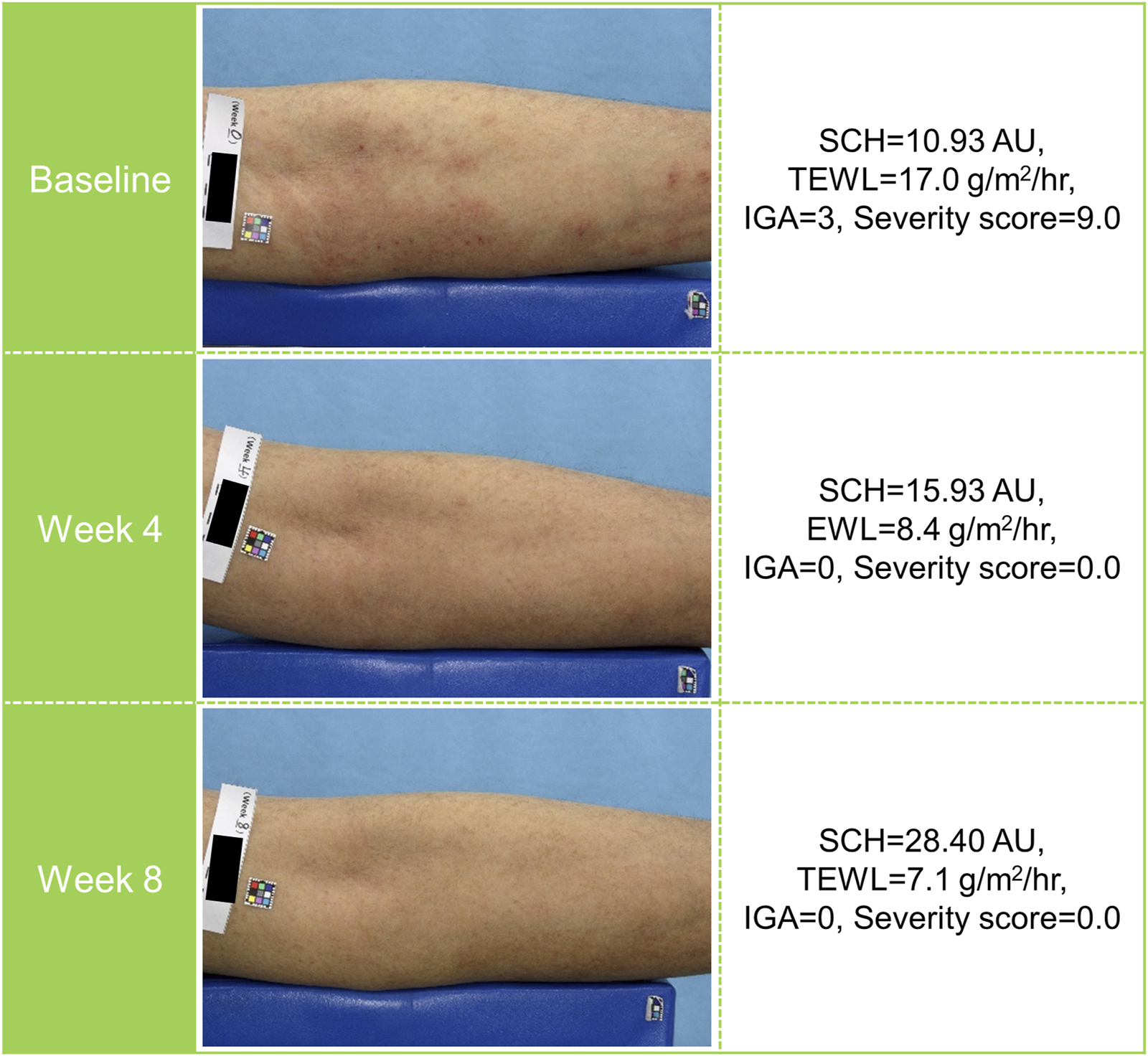
Representative clinical images of a patient receiving tapinarof. AU, arbitrary unit; IGA, Investigator’s Global Assessment; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Safety
Adverse events were reported in 5 of 30 (16.7%) patients (Table 2). No serious, severe, or treatment-related AEs were reported. One patient was discontinued from the study because of a moderate AE of toxic skin eruption.
TABLE 2
| Patients with AD (N = 30) | |
|---|---|
| AEs | 5 (16.7) |
| COVID-19 | 1 (3.3) |
| Folliculitis | 1 (3.3) |
| Hordeolum | 1 (3.3) |
| Lymphadenitis | 1 (3.3) |
| Toxic skin eruptiona | 1 (3.3) |
| Serious AEs | 0 |
| Severe AEs | 0 |
| Treatment-related AEs | 0 |
| AEs leading to study discontinuation | 1 (3.3) |
Adverse events.
Data are displayed as number of subjects (%). The AE terms reported by the investigator were coded using MedDRA V.24.1.
AD, atopic dermatitis; AE, adverse event; COVID-19, coronavirus disease 2019; MedDRA, medical dictionary for regulatory activities terminology.
The toxic skin eruption was a skin reaction observed not only at the application site but also at non-application sites. The event led to study discontinuation, but the investigator considered that it was not related to the study treatment.
Discussion
This study was conducted to evaluate the improvement effect of tapinarof applied once daily for 8 weeks on skin barrier function in patients with moderate or severe AD. At baseline, patients with AD had lower SCH and higher TEWL values than healthy volunteers (Table 1), which was in agreement with results of recent clinical studies [10, 19]. There appeared to be a difference in SCH and TEWL between baseline and week 4, although no statistical tests were performed. At week 8, SCH and TEWL at the target affected area were significantly improved from baseline (Figures 2B, D), indicating the potential improvement effect of tapinarof on skin barrier function. No apparent differences in SCH and TEWL values were found between week 4 and week 8 (Figures 2A, C), whereas AD clinical signs, as assessed with IGA and severity scores, were improved from baseline through week 8 (Figures 3, 4). Given that the clinical efficacy of tapinarof tended to increase over 52 weeks of treatment [20], treatment with tapinarof beyond 8 weeks can further improve skin barrier function in patients with AD.
Non-steroidal topical drugs have become available for the treatment of AD. In contrast to topical corticosteroids, topical calcineurin inhibitors do not cause skin atrophy [1]. Topical calcineurin inhibitors have been reported to have a different effect on skin barrier function from that of topical corticosteroids [21–24]. Recently approved topical drugs such as Janus kinase inhibitors and phosphodiesterase 4 inhibitors have been shown to upregulate the expression of skin barrier proteins, including filaggrin and loricrin [25, 26]. Tapinarof also upregulates these skin barrier proteins by a different mechanism of action via AhR activation [12]. The upregulation effect of tapinarof on skin barrier proteins was enhanced in combination with a Janus kinase inhibitor [27]. Combination treatment with tapinarof and other topical drugs may provide a better improvement effect on skin barrier function in patients with AD.
This study was in an open-label manner without any treatment control group, which limited the interpretation of data. It is uncertain whether there are any differences in improving skin barrier function between tapinarof and other topical drugs. Treatment with tapinarof in this study was 8-week short duration, whereas patients with AD generally require long-term treatment. Longer treatment duration should be considered to evaluate the durability of improvement effect on skin barrier function, which can contribute to long-term maintenance of remission in AD. Additionally, this study included only Japanese patients; therefore, the improvement effect of tapinarof on skin barrier function is uncertain in non-Japanese patients, who potentially have different phenotypes of AD [28, 29]. In clinical studies including non-Japanese patients, however, tapinarof demonstrated the clinical efficacy in the treatment of AD [14, 15, 17], indicating that tapinarof is most likely to show the improvement effect on skin barrier function in non-Japanese patients.
In summary, 8-week treatment with tapinarof led to an increase in SCH and a decrease in TEWL in patients with AD. Clinical signs of AD were improved over time. No new safety concerns emerged. The potential improvement effect of tapinarof on skin barrier function can be beneficial in the long-term maintenance of remission in AD.
Statements
Data availability statement
The datasets presented in this article are not readily available because the participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available. Requests to access the datasets should be directed to RM, ryusei.murata@jt.com.
Ethics statement
The studies involving humans were approved by Sapporo Skin Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the study concept and design, the conduct of the study, and data interpretation. RM and SF drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. The present study was funded by Japan Tobacco Inc.
Acknowledgments
We thank all the patients and their families, as well as investigators and site staff involved in the conduct of present study.
Conflict of interest
AI received advisory board honoraria, consulting fees or speaker honoraria from AbbVie, Eli Lilly Japan, Japan Tobacco, Maruho, Novartis, Sanofi, LEO pharma, and Torii Pharmaceutical, and received research grants from AbbVie, Eli Lilly Japan, Japan Tobacco, Novartis, Otsuka Pharmaceutical, Amgen, and Sanofi. GT received a research grant and consulting fee from Japan Tobacco. RM, SF, and SY are employees of Japan Tobacco Inc.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.13418/full#supplementary-material
References
1.
Saeki H Ohya Y Furuta J Arakawa H Ichiyama S Katsunuma T et al English version of clinical Practice guidelines for the management of atopic dermatitis 2021. J Dermatol (2022) 49(10):e315–e375. 10.1111/1346-8138.16527
2.
Weidinger S Beck LA Bieber T Kabashima K Irvine AD . Atopic dermatitis. Nat Rev Dis Primers (2018) 4(1):1. 10.1038/s41572-018-0001-z
3.
Carroll CL Balkrishnan R Feldman SR Fleischer AB Jr Manuel JC . The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol (2005) 22(3):192–9. 10.1111/j.1525-1470.2005.22303.x
4.
Lewis-Jones S . Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract (2006) 60(8):984–92. 10.1111/j.1742-1241.2006.01047.x
5.
Werfel T Allam JP Biedermann T Eyerich K Gilles S Guttman-Yassky E et al Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol (2016) 138(2):336–49. 10.1016/j.jaci.2016.06.010
6.
Egawa G Kabashima K . Multifactorial skin barrier deficiency and atopic dermatitis: essential topics to prevent the atopic march. J Allergy Clin Immunol (2016) 138(2):350–8. 10.1016/j.jaci.2016.06.002
7.
Otsuka A Nomura T Rerknimitr P Seidel JA Honda T Kabashima K . The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev (2017) 278(1):246–62. 10.1111/imr.12545
8.
Cabanillas B Novak N . Atopic dermatitis and filaggrin. Curr Opin Immunol (2016) 42:1–8. 10.1016/j.coi.2016.05.002
9.
Kim BE Leung DY . Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol Res (2012) 4(1):12–6. 10.4168/aair.2012.4.1.12
10.
Montero-Vilchez T Segura-Fernández-Nogueras M-V Pérez-Rodríguez I Soler-Gongora M Martinez-Lopez A Fernández-González A et al Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J Clin Med (2021) 10:359. 10.3390/jcm10020359
11.
Hengge UR Ruzicka T Schwartz RA Cork MJ . Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol (2006) 54(1):1–15. 10.1016/j.jaad.2005.01.010
12.
Smith SH Jayawickreme C Rickard DJ Nicodeme E Bui T Simmons C et al Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol (2017) 137(10):2110–9. 10.1016/j.jid.2017.05.004
13.
Furue M Hashimoto-Hachiya A Tsuji G . Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci (2019) 20(21):5424. 10.3390/ijms20215424
14.
Peppers J Paller AS Maeda-Chubachi T Wu S Robbins K Gallagher K et al A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol (2019) 80(1):89–98. 10.1016/j.jaad.2018.06.047
15.
Paller AS Stein Gold L Soung J Tallman AM Rubenstein DS Gooderham M . Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol (2021) 84(3):632–8. 10.1016/j.jaad.2020.05.135
16.
Japan Tobacco Inc. JT files new drug application of JTE-061 cream, aryl hydrocarbon receptor (AhR) agonist, for the treatment of atopic dermatitis and plaque psoriasis in Japan (2023). Available at: https://www.jt.com/media/news/2023/pdf/20230915_E01.pdf (Accessed April 23, 2024).
17.
Dermavant Sciences. Dermavant submits supplemental new drug application (sNDA) to FDA for VTAMA® (tapinarof) cream, 1% for the treatment of atopic dermatitis in adults and children 2 Years of age and older (2024). Available at: https://www.dermavant.com/dermavant-submits-supplemental-new-drug-application-snda-to-fda-for-vtama/ (Accessed April 23, 2024).
18.
Hanifin JM Thurston M Omoto M Cherill R Tofte SJ Graeber M . The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group Exp Dermatol (2001) 10(1):11–8. 10.1034/j.1600-0625.2001.100102.x
19.
Abe M Iizuka H Nemoto-Hasebe I Nemoto O Toyama H Ohashi-Doi K et al Clinical effect of delgocitinib 0.5% ointment on atopic dermatitis eczema intensity and skin barrier function. J Cutan Immunol Allergy (2022) 5:38–46. 10.1002/cia2.12213
20.
JRCT. Phase 3 clinical study of JTE-061 cream – 52-week long-term study in patients with atopic dermatitis – [jRCT2031210256] (2024). Available at: https://jrct.niph.go.jp/en-latest-detail/jRCT2031210256 (Accessed May 30, 2024).
21.
Jensen JM Pfeiffer S Witt M Bräutigam M Neumann C Weichenthal M et al Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol (2009) 123(5):1124–33. 10.1016/j.jaci.2009.03.032
22.
Jensen JM Weppner M Dähnhardt-Pfeiffer S Neumann C Bräutigam M Schwarz T et al Effects of pimecrolimus compared with triamcinolone acetonide cream on skin barrier structure in atopic dermatitis: a randomized, double-blind, right-left arm trial. Acta Derm Venereol (2013) 93(5):515–9. 10.2340/00015555-1533
23.
Danby SG Chittock J Brown K Albenali LH Cork MJ . The effect of tacrolimus compared with betamethasone valerate on the skin barrier in volunteers with quiescent atopic dermatitis. Br J Dermatol (2014) 170(4):914–21. 10.1111/bjd.12778
24.
Dähnhardt-Pfeiffer S Dähnhardt D Buchner M Walter K Proksch E Fölster-Holst R . Comparison of effects of tacrolimus ointment and mometasone furoate cream on the epidermal barrier of patients with atopic dermatitis. J Dtsch Dermatol Ges (2013) 11(5):437–43. 10.1111/ddg.12074
25.
Amano W Nakajima S Kunugi H Numata Y Kitoh A Egawa G et al The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol (2015) 136(3):667–77. 10.1016/j.jaci.2015.03.051
26.
Tsuji G Hashimoto-Hachiya A Yumine A Takemura M Kido-Nakahara M Ito T et al PDE4 inhibition by difamilast regulates filaggrin and loricrin expression via keratinocyte proline-rich protein in human keratinocytes. J Dermatol Sci (2023) 110(2):61–8. 10.1016/j.jdermsci.2023.04.007
27.
Vu YH Hashimoto-Hachiya A Takemura M Yumine A Mitamura Y Nakahara T et al IL-24 negatively regulates keratinocyte differentiation induced by tapinarof, an aryl hydrocarbon receptor modulator: implication in the treatment of atopic dermatitis. Int J Mol Sci (2020) 21(24):9412. 10.3390/ijms21249412
28.
Nomura T Honda T Kabashima K . Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol (2018) 30(9):419–28. 10.1093/intimm/dxy015
29.
Czarnowicki T He H Krueger JG Guttman-Yassky E . Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol (2019) 143(1):1–11. 10.1016/j.jaci.2018.10.032
Summary
Keywords
aryl hydrocarbon receptor (AhR), atopic dermatitis, clinical trial, stratum corneum hydration (SCH), skin barrier, tapinarof, transepidermal water loss (TEWL)
Citation
Igarashi A, Tsuji G, Murata R, Fukasawa S and Yamane S (2024) Improvement effects of tapinarof on the skin barrier function in Japanese patients with atopic dermatitis. J. Cutan. Immunol. Allergy 7:13418. doi: 10.3389/jcia.2024.13418
Received
20 June 2024
Accepted
23 July 2024
Published
06 August 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Igarashi, Tsuji, Murata, Fukasawa and Yamane.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryusei Murata, ryusei.murata@jt.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.