- 1Department of Dermatology, Kido Hospital, Niigata, Japan
- 2Division of Dermatology, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan
- 3Department of Surgery, Kido Hospital, Niigata, Japan
Dear Editors,
Pyoderma gangrenosum (PG) is an ulcerative neutrophilic dermatosis often associated with systemic diseases, such as rheumatoid arthritis, inflammatory bowel diseases, and hematological neoplasms. PG can occur in postoperative patients due to the pathergy phenomenon, which is the development of PG lesions at sites of trauma. The term “postoperative PG” (or “postsurgical PG”) is used for the pathergic development of PG at surgical sites [1]. Cancer immunotherapy with immune checkpoint inhibitors (ICIs) may induce various immune-related adverse events, including neutrophilic dermatoses. We report a case of postoperative PG in a patient undergoing long-term therapy with nivolumab, an anti-PD-1 ICI.
An 84-year-old man presented with a purulent lesion at a recent surgical site. Ten years prior, he underwent resection of gastric cancer, which recurred as peritoneal dissemination 3 years after the resection and was resistant to multiple lines of chemotherapy. Remission was achieved with nivolumab therapy and maintained for 5 years and 8 months with administration of nivolumab every 2–3 weeks without immune-related adverse events. Left inguinal hernia repair surgery was performed 19 days after the last infusion of nivolumab. The remission of gastric cancer was confirmed by imaging studies 4 weeks before the surgery. The diagnosis of inguinal hernia was also confirmed by the preoperative imaging studies and intraoperative findings. The surgery was completed successfully without any complications in the perioperative period. On postoperative day 14, he developed a fever of 39.7°C and a painful necrotic ulcer with violaceous borders, surrounding erythema, and subcutaneous abscess at the incision site (Figure 1A). His neutrophil count was elevated to 23.2 × 109/L, and the C-reactive protein was elevated to 16.2 mg/dL. Although antibiotic drug therapy was initiated, bacterial cultures of the purulent exudates, necrotic tissues, and blood were negative. A skin biopsy specimen taken from a non-ulcerated margin demonstrated edema, dense diffuse infiltrate of neutrophils with nuclear dust, lymphocytes, and histiocytes throughout the dermis to the subcutaneous tissue, and hemorrhage without fibrin deposition (Figures 1B, C). Bacteria, mycobacteria, or fungi were not detected in serial sections. He did not have arthritis or bowel symptoms, and no hematological abnormality other than the increased neutrophil count was present. The diagnosis of postoperative PG was made, and intravenous prednisolone 60 mg (1 mg/kg) daily was started 3 days after presentation. As the ulcer continued to expand despite the prednisolone (and antibiotic drug) therapy for 1 week, we administered adalimumab twice at an interval of 2 weeks (160 mg and 80 mg). The PG lesion gradually improved, and prednisolone was tapered to 20 mg daily. Nivolumab was not restarted, as imaging studies 1 week after PG onset (6 weeks after the last infusion of nivolumab) showed no recurrence of gastric cancer, and nivolumab therapy might have a potential risk of PG exacerbation. However, he developed disseminated intravascular coagulation caused by the recurrence of peritoneal dissemination and lung metastasis 2 months after the cessation of nivolumab and died 2 months later.
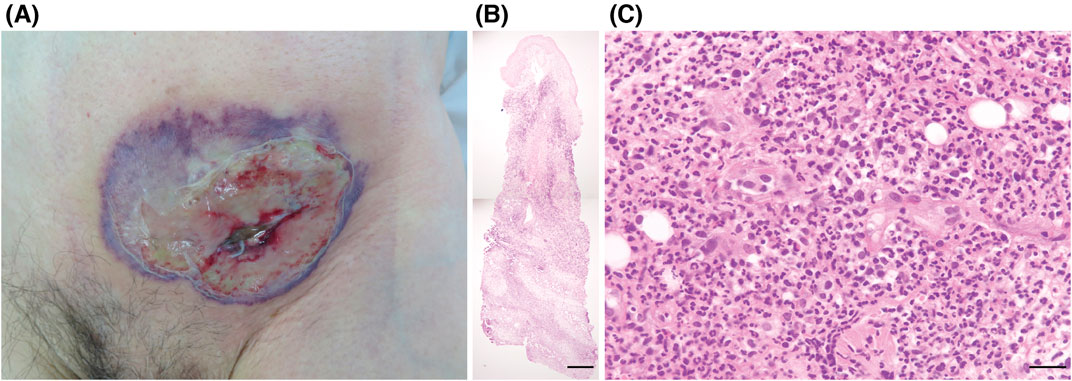
Figure 1. (A) Clinical presentation. A necrotic ulcer with violaceous borders, surrounding erythema, and subcutaneous abscess at the incision site in the left lower abdomen. (B, C) Histopathological findings from the non-ulcerated margin (hematoxylin-eosin). Edema, dense diffuse infiltrate of neutrophils with nuclear dust, lymphocytes, and histiocytes throughout the dermis to the subcutaneous tissue, and hemorrhage without fibrin deposition. Scale bars: (B) 500 μm, (C) 25 μm.
To our knowledge, four cases of ICI-associated PG have been reported previously, none of which were triggered by surgery (Supplementary Table S1) [2–5]. Alterations in T-cell immune tolerance induced by ICIs have been proposed to lead to T-cell dysregulation, such as abnormal IL-17 production, which may contribute to the development of PG [4, 5]. ICI was discontinued in all cases. One case was associated with myelodysplastic neoplasm [3], but like in our case, the other reported cases did not have systemic diseases traditionally associated with PG [2, 4, 5]. In these cases, however, paraneoplastic association of PG with the primary underlying solid organ malignancies cannot be excluded. In our case, PG may also have developed in association with an occult recurrence of gastric cancer, although postoperative PG has less association with systemic disease [1]. Compared with the reported cases of ICI-associated PG, which typically developed within 6 months after the initiation of ICIs [2–5], our patient developed PG following surgery after prolonged nivolumab therapy. Therefore, it is possible that PG in our case developed independently of nivolumab. However, the unusual features of this case, namely, pyrexia and initial resistance to high-dose systemic corticosteroid therapy, both uncommon in postoperative PG [1], suggest a potential role for nivolumab-induced dysregulated inflammation following surgery in the development or exacerbation of postoperative PG.
While a direct causal link between PG and nivolumab remains uncertain in this case, our findings suggest a potential risk of postoperative PG in patients undergoing ICI therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because our institution does not require ethical approval for reporting individual cases or case series. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.13751/full#supplementary-material
References
1. Tolkachjov, SN, Fahy, AS, Wetter, DA, Brough, KR, Bridges, AG, Davis, MD, et al. Postoperative pyoderma gangrenosum (PG): the Mayo Clinic experience of 20 years from 1994 through 2014. J Am Acad Dermatol (2015) 73(4):615–22. doi:10.1016/j.jaad.2015.06.054
2. Rudolph, BM, Staib, F, Von Stebut, E, Hainz, M, Grabbe, S, and Loquai, C. Neutrophilic disease of the skin and intestines after ipilimumab treatment for malignant melanoma: simultaneous occurrence of pyoderma gangrenosum and colitis. Eur J Dermatol (2014) 24(2):268–9. doi:10.1684/ejd.2014.2297
3. Welborn, ME, Kubicki, SL, and Patel, AB. Pyoderma gangrenosum following initiation of immune chekpoint inhibitor therapy. J Immunother Precis Oncol (2018) 1(2):82–4. doi:10.4103/JIPO.JIPO_11_18
4. Tsibris, H, Lian, C, and Ho, A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell carcinoma. Dermatol Online J (2021) 27(4). doi:10.5070/D3274053158
Keywords: immune checkpoint inhibitor, immune-related adverse event, neutrophilic dermatosis, paraneoplastic pyoderma gangrenosum, postsurgical pyoderma gangrenosum
Citation: Sato M, Kawai K and Yamada A (2024) Postoperative pyoderma gangrenosum in a patient undergoing long-term nivolumab therapy. J. Cutan. Immunol. Allergy 7:13751. doi: 10.3389/jcia.2024.13751
Received: 05 September 2024; Accepted: 15 November 2024;
Published: 22 November 2024.
Copyright © 2024 Sato, Kawai and Yamada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhiro Kawai, a2F6a2F3YWlAbTIua3VmbS5rYWdvc2hpbWEtdS5hYy5qcA==
 Miki Sato
Miki Sato Kazuhiro Kawai
Kazuhiro Kawai Akira Yamada3
Akira Yamada3