- Department of Dermatology, Graduate School of Medical and Dental Sciences, Institute of Science Tokyo (IST), Tokyo, Japan
Steroid pulse therapy is considered effective in the treatment of acquired idiopathic generalized anhidrosis (AIGA). However, nearly half of patients with AIGA do not respond to treatment, or relapse, suggesting the need to stratify patients on the basis of biomarkers to optimize the treatment plan. Although several factors associated with response to treatment, such as the duration since disease onset and serum carcinoembryonic antigen (CEA) levels, have been reported, the relationships between these factors and their effect on the relapse rate have not been evaluated. This retrospective cohort study of 210 patients showed a poor prognosis in patients with high serum total immunoglobulin E (IgE) levels because of a high relapse rate. Serum total IgE levels tended to be inversely related to serum CEA levels, but a multivariate analysis showed that high serum total IgE levels were an independent risk factor for relapse. However, low serum CEA levels were an independent risk factor for non-response to steroid pulse therapy, even after adjustment for factors such as the duration since disease onset, which showed an inverse trend with serum CEA levels. These results suggest that, in addition to the typical subtype of AIGA with high serum CEA levels in the active phase of the disease, there may be an allergic type of the disease with high serum total IgE levels and a poor prognosis with steroids. Therefore, stratifying patients by measuring serum total IgE and CEA levels may aid in predicting the effect of steroid pulse therapy.
Introduction
Acquired idiopathic generalized anhidrosis (AIGA) is a rare and intractable disease characterized by the sudden loss of sweating [1–4]. In addition to thermoregulatory dysfunction, 70% of patients have cholinergic urticaria and/or prickling pain, which greatly reduces patients’ quality of life [5, 6]. Although the pathogenesis of the disease remains unknown, immunological mechanisms may be involved, as patients often show a response to steroid therapy [1, 6–11].
Serum carcinoembryonic antigen (CEA) and total IgE levels are reportedly elevated in some patients with AIGA [12–15]. Serum CEA is well known as a tumor marker [16, 17], and is expressed in several kinds of glandular epithelia such as the intestinal mucosa and sweat glands. It is reportedly overexpressed in the sweat glands of patients with AIGA, and levels of serum CEA can be a clinical marker that reflects the activity of the disease [14, 18]. Serum total immunoglobulin E (IgE) levels are often elevated in patients with allergic diseases such as atopic dermatitis, and in some patients with AIGA [1, 12]. However, the clinical characteristics of patients with elevation of these serum markers have not been fully investigated.
Although steroid pulse therapy, is the most reported treatment for AIGA, approximately half of patients show no response to this therapy, or they relapse within 1 year [6, 8]. Steroid therapy may cause considerable side effects. Therefore, if serum markers could predict the effectiveness of the therapy, this would be helpful for treatment planning. Several factors have been reported to be associated with a response to pulse steroid therapy, such as the time since disease onset, serum CEA levels, and sex [6, 18]. However, little is known about predictors of relapse after remission.
To investigate the clinical implications and predictive utility of serum CEA and IgE levels, we analyzed the relationship between clinical characteristics and the levels of these serum markers. We also compared the response and relapse rates after therapy between patients with or without elevated serum markers.
Materials and methods
Ethics
We conducted this study in accordance with the principles of the Declaration of Helsinki with the approval of the ethics committee of the Institute of Science Tokyo (M2020-058). The committee approved an opt-out method for obtaining consent.
Patients
To investigate the clinical characteristics of patients with AIGA and their responses to steroid therapy, we retrospectively collected data from all patients with AIGA who were diagnosed at the Institute of Science Tokyo (Tokyo Medical and Dental University) Hospital from April 2008 to March 2021. A diagnosis of AIGA was made in accordance with the Japanese guidelines [3].
Diagnosis of cholinergic urticaria
In patients with urticaria, cholinergic urticaria can be diagnosed if a provocation test with sweating stimuli is positive [19, 20]. For the provocation tests, patients were exposed to heat stress in a sauna at 60°C for 15 min, and patients who had wheals induced were considered to have cholinergic urticaria. However, there were a relatively large number of patients who had episodes of exercise- or heat-induced wheals and/or skin pain even though the provocation test was negative. Therefore, we divided the patients into three groups: those with “urticaria and/or pain episodes” and a positive challenge test, those with “urticaria and/or pain episodes” and a negative challenge test, and those without such episodes (Tables 1–4).
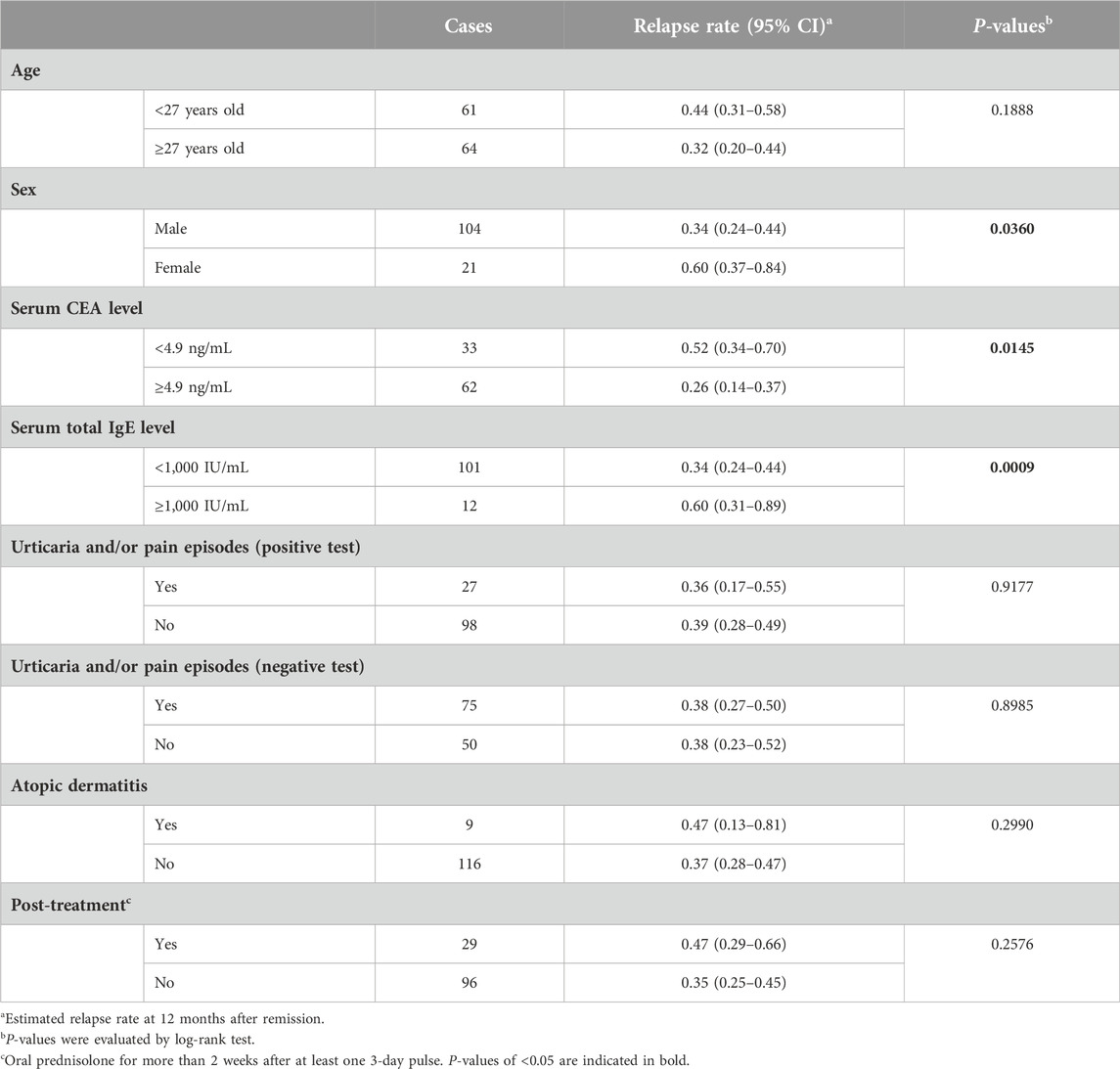
Table 4. Univariate analysis (log-rank test) for relapse rate after remission with steroid pulse therapy.
Assessment of response to steroid pulse therapy
We determined a patient’s response to steroid pulse therapy using the methods we reported in the past [6]. Changes in sweat area on the body surface at 1 month after steroid pulse therapy were assessed by a detailed interview (confirmed in some cases by a subsequent starch–iodine test). With appropriate interviewing, it is possible to estimate changes in sweat area with reasonable accuracy (93% accuracy compared with a subsequent starch–iodine test [6]). Patients were considered responders if they had an increase in sweat area of ≥25% of their body surface area. If the patient received multiple steroid pulse therapies, those who met the criteria after at least one were considered responders.
Analysis of relapse after remission
The determination of relapse after remission with steroid pulse therapy also followed the method we previously reported [6]. Patients who responded to steroid pulse therapy were evaluated for changes in sweat area at each outpatient follow-up visit by a detailed interview (confirmed in some cases by a subsequent starch–iodine test). Patients with a reduction in sweat area of ≥25% were considered to have relapsed. The date of relapse was determined as the date on which the patient noticed a decrease in sweating or, if this was not clear, the date of the outpatient follow-up or starch–iodine test. For patients with multiple relapses, only the first relapse was included in the analysis.
Determination of cutoff values
To evaluate the associations between serum CEA or total IgE levels and the treatment response, receiver operating characteristic analysis was used to determine cutoff values for serum CEA and serum total IgE levels. Although there are hospital reference values for serum CEA and serum total IgE levels, optimal cutoff values vary by the disease type, disease activity, and study [21, 22]. Regarding serum CEA levels, we calculated the cutoff values for predicting the response to steroid pulse therapy and for predicting relapse after remission. Regarding serum total IgE levels, we calculated the optimal cutoff for predicting relapse after remission. The optimal cutoffs were calculated using the “perfcurve” function [23] of the “Statistics and Machine Learning Toolbox” in MATLAB (version 2024a; MathWorks, Natick, MA, United States). For simplicity, we used a cutoff value of 1,000 IU/mL of serum total IgE, which has the same sensitivity and specificity as the calculated value of 1,083 IU/mL. The cutoff values for age and “delay” (elapsed time since onset) were the same as those in our previous report [6]. In the Cox regression analysis, the proportional hazards assumption was assessed with Kaplan–Meier curves for the two groups classified according to the defined cutoffs (Supplementary Figure S1).
Statistical analysis
We performed statistical analyses using the Mann–Whitney U test (Tables 1, 2), Kruskal–Wallis test (Tables 1, 2), chi-square test (Table 3), logistic regression analysis (Supplementary Table S1), log-rank test (Table 4), and Cox regression analysis (Supplementary Table S2). Bonferroni correction was used for multiple comparisons (Table 1). MATLAB was used for data processing, including P-value calculation and visualization.
Results
Clinical characteristics
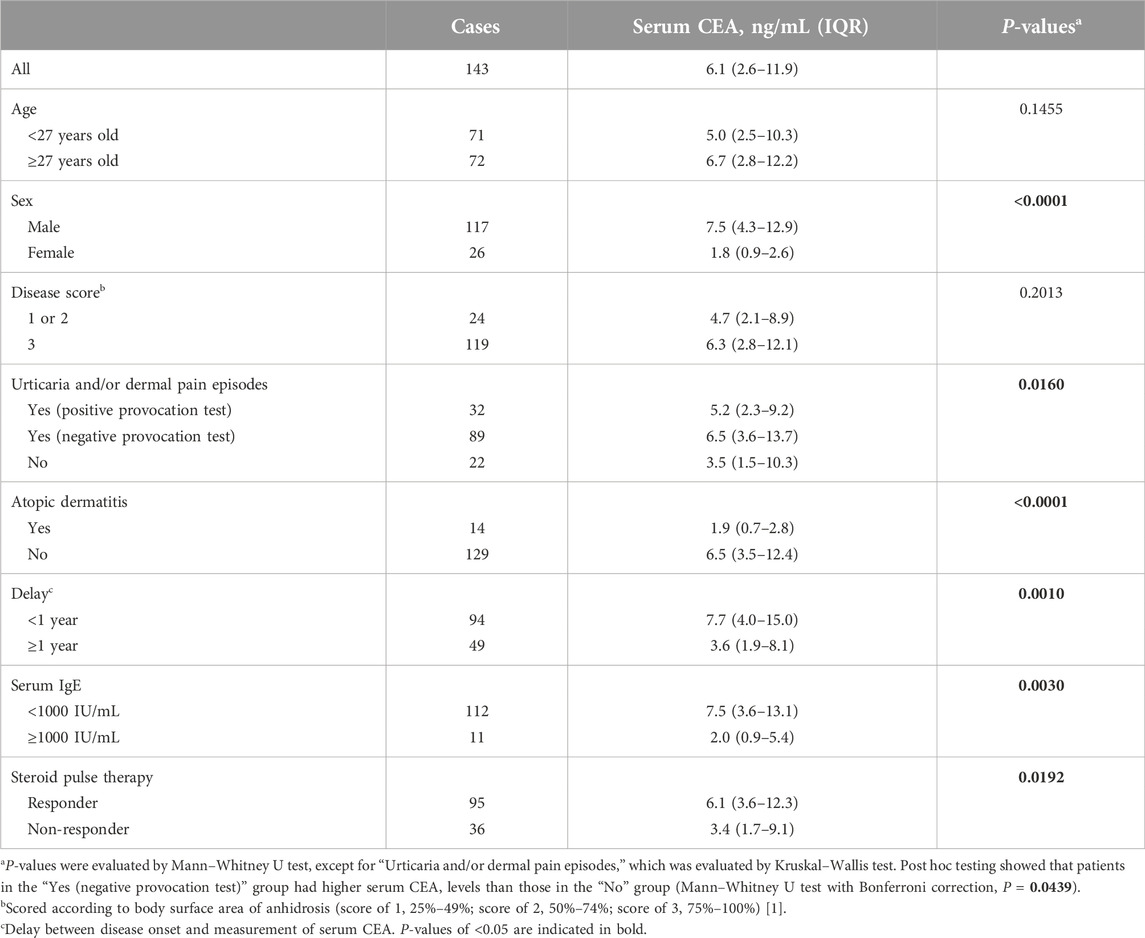
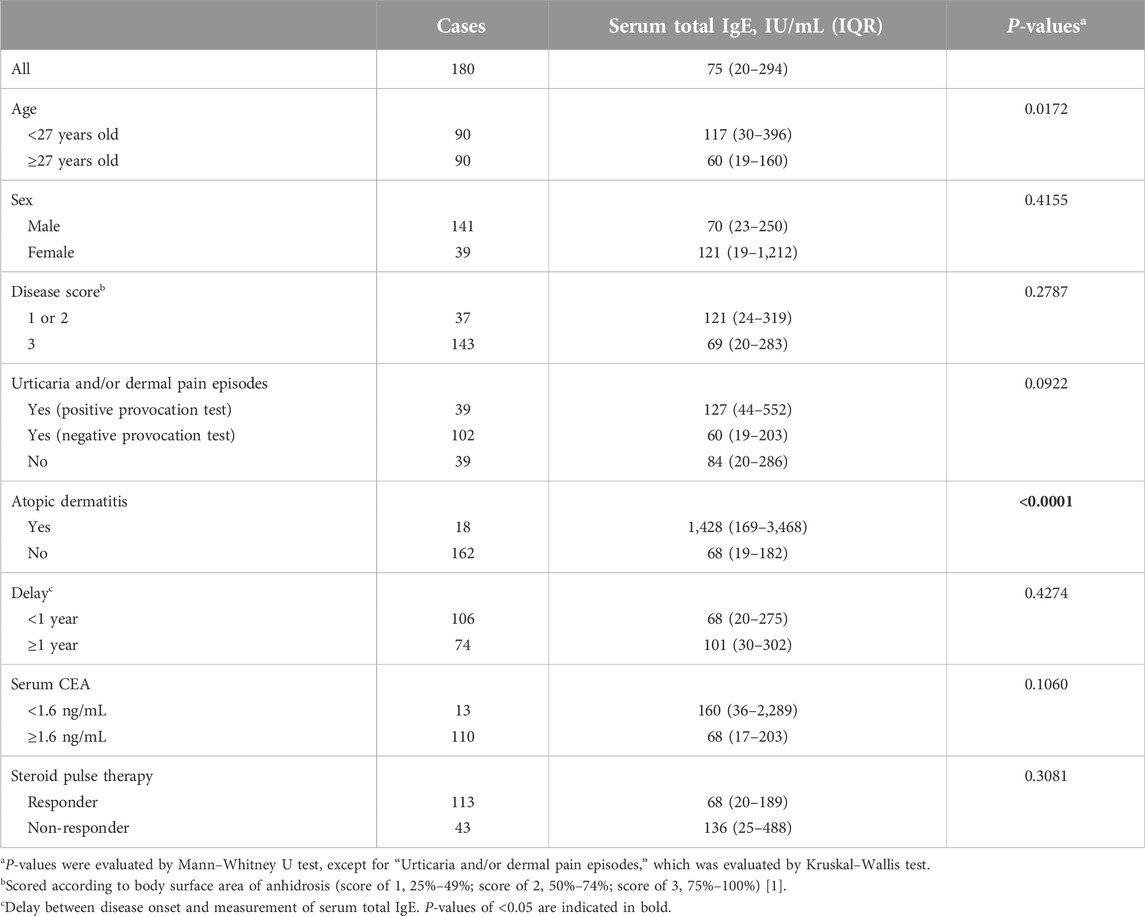
To investigate the proportion of patients with high serum CEA and/or total IgE levels, we collected data from 210 patients with AIGA. The male/female ratio of the patients was 3.2, and the median age was 27 years [interquartile range (IQR), 19–40], consistent with previous reports [1, 3, 12]. Of the 210 patients, 143 and 180 patients were assessed for serum CEA and total IgE levels, respectively, before steroid pulse therapy. The median serum CEA level was 6.1 ng/mL (IQR, 2.6–11.9 ng/mL). No patients were found to have malignant tumors. The median total IgE level was 75 IU/mL (IQR, 20–294 IU/mL).
We observed an opposite trend between serum CEA and total IgE levels (P = 0.0030, Table 1). When these markers were examined in relation to clinical characteristics, higher serum CEA levels were found in male patients than in female patients and in patients who were examined within 1 year after onset than in those who were examined at ≥ 1 year after onset (P < 0.0001 and P = 0.0010, respectively). Serum CEA levels tended to be higher in patients who had episodes of urticaria and/or dermal pain induced by sweating stimuli but had a negative provocation test than those who did not have episodes of urticaria and/or dermal pain (P = 0.0439). However, higher serum total IgE levels were found in younger patients than in older patients and in patients with coexisting atopic dermatitis than in those without coexisting atopic dermatitis (P = 0.0172 and P < 0.0001, respectively, Table 2).
Effectiveness of steroid pulse therapy
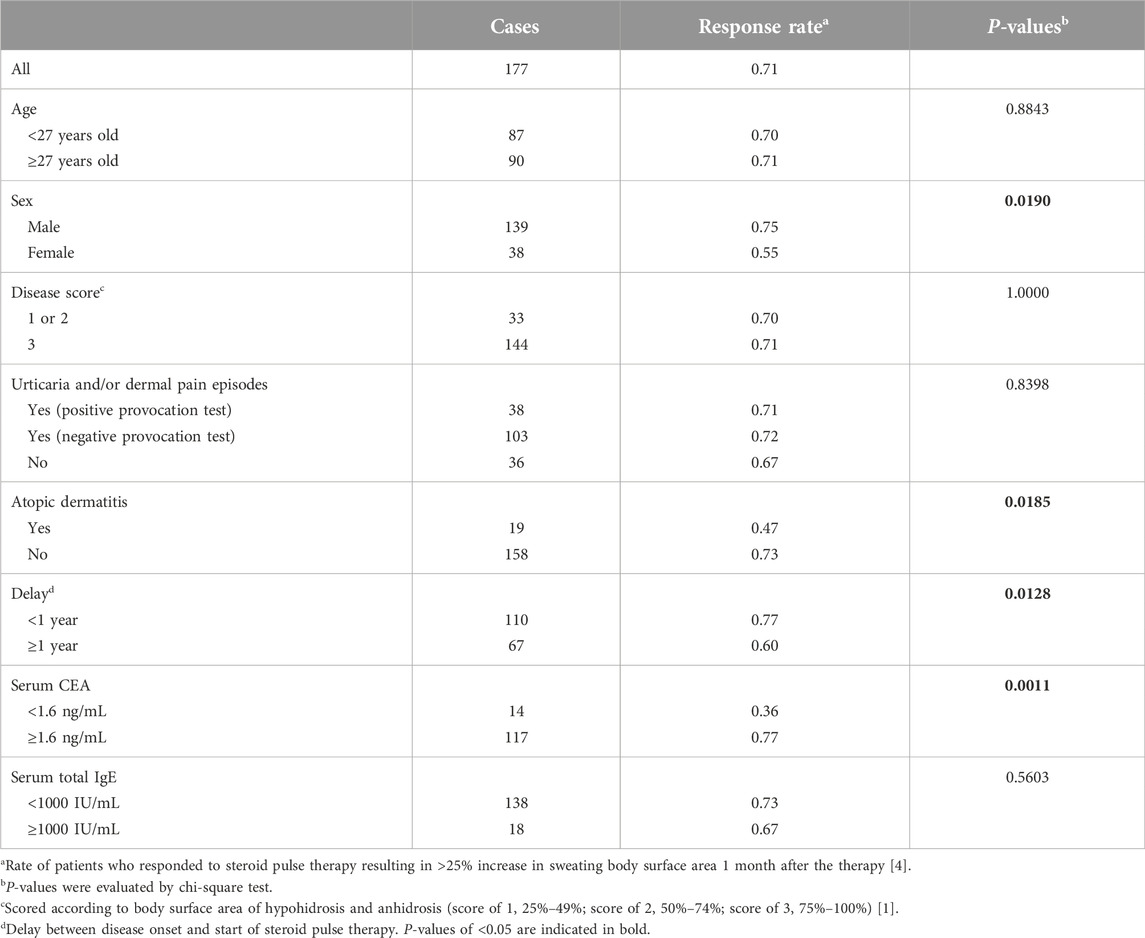
We examined whether the serum markers had relationships with responsiveness to steroid pulse therapy (Supplementary Figure S2). The response rate to steroid pulse therapy among our patients [125 out of 177 (71%)] was comparable to those in previous reports [1, 6]. We found that patients who responded to the therapy showed higher CEA levels than non-responders (P = 0.0192, Table 1). The optimal serum CEA cutoff level for predicting a treatment response was 1.6 ng/mL (P = 0.0011, Table 3). Other factors, namely, male sex, non-concomitant atopic dermatitis, and <1 year from disease onset to treatment (P = 0.0190, P = 0.0185, and P = 0.0128, respectively), were also associated with a good treatment response (Table 3). The odds ratio for high serum CEA levels after adjustment for these factors was 4.23 (P = 0.0421, Supplementary Table S1).
Next, we explored the correlation between the serum markers and relapse rate after remission achieved by steroid pulse therapy (Supplementary Figure S2). We conducted a time-to-event analysis involving the 125 patients who responded to pulse therapy. The traces were started on the last day of steroid pulse therapy and ended at relapse [44 of 125 (35%)] or were censored if the primary doctor decided to end follow-up [24 of 125(19%)], the patient did not attend the return visit [24 of 125 (19%)], or the end of the study (13 August 2022) was reached [33 of 125 (26%)]. Follow-up to relapse or for at least 12 months was completed for 74% (93 of 125) of patients, and the median follow-up duration for patients with no relapse was 13.5 months. Most relapses were seen within 12 months of remission, with an estimated relapse rate of 38% at month 12 (Supplementary Figure S3).
To examine the effect of serum CEA and total IgE levels on relapse using the time-to-event data, we estimated optimal cutoff values (4.9 ng/mL and 1,000 IU/mL, respectively). In univariate analysis, there was a trend toward more frequent recurrence in the low serum CEA and high serum IgE groups (P = 0.0145 and P = 0.0009, respectively; Table 4; Supplementary Figure S1). We then calculated the hazard ratio incorporating the two serum markers and sex because female patients showed more frequent recurrence than male patients (P = 0.0360). The adjusted hazard ratio was 2.41 for the low CEA group (P = 0.0337) and 4.56 for the high IgE group (P = 0.0083, Supplementary Table S2).
The disease activity of AIGA is affected by the seasons, which may affect the analysis. We previously reported that steroid pulse therapy was effective from March to September [6]. Therefore, in the current study, we compared the number of courses of steroid pulse therapy administered during this period between the low (<1.6 ng/mL) and high (≥1.6 ng/mL) CEA groups, but found no significant difference between the groups (median of 1.5 and 1.0 courses, respectively; Mann–Whitney U test, P = 0.7543). We also reported that relapses were more frequent in the autumn (September to November) than in other seasons, which may bias the relapse rate depending on whether the observation period includes the autumn [6]. Therefore, we reanalyzed the data by excluding cases in which the follow-up period after remission did not include autumn. However, we still found that the relapse rate was higher in the high (≥1000 IU/mL) IgE group than the low (<1000 IU/mL) IgE group (log rank test, P = 0.0010). Therefore, we believe that our results of the analysis of the relationship between serum markers and the effectiveness of steroid pulse therapy were not affected by the seasons.
Finally, we examined whether there was an association between serum markers and the effect of steroid pulse therapy for urticaria and/or dermal pain. Of the 141 patients with urticaria and/or dermal pain, 78 (55%) reported that their symptoms had improved at 1 month after steroid pulse therapy. The low serum CEA (<1.6 ng/mL) and high serum IgE (≥1000 IU/mL) groups of patients had a slightly lower rate of improvement of urticaria and/or dermal pain than the mean rate [4/9 (44%) and 5/13 (38%); chi-square test, P = 0.2908 and P = 0.1536, respectively], but this was not significant. We also examined the rate of re-exacerbation of urticaria and/or dermal pain after steroid pulse therapy. Approximately half of the patients whose symptoms improved experienced re-exacerbation within 12 months (Supplementary Figure S4A). Regarding serum CEA levels, there was no significant difference in the rate of re-exacerbation between the low (<4.9 ng/mL) and high (≥4.9 ng/mL) CEA groups (Supplementary Figure S4B). Regarding serum total IgE levels, only five patients with high serum total IgE levels (≥1000 IU/mL) experienced an improvement of urticaria and/or dermal pain after steroid pulse therapy, which was insufficient to evaluate the rate of re-exacerbation. Overall, the relationship between serum markers and the effectiveness of steroid pulse therapy on urticaria and/or dermal pain was not as clear as that between serum markers and the effectiveness of steroid pulse therapy on sweating.
Discussion
We investigated the clinical implications of serum CEA and total IgE levels for the efficacy of steroid pulse therapy in patients with AIGA. We observed an inverse trend between serum CEA and total IgE levels. Higher serum CEA levels were found in male patients and in patients examined within 1 year of disease onset. The coexistence of atopic dermatitis was correlated not only with high serum total IgE levels but also with low serum CEA levels. The response to steroid pulse therapy was better in patients with high serum CEA levels, men, those with no coexisting atopic dermatitis, and within 1 year of disease onset. The multivariate analysis showed that high serum CEA levels were an independent factor for a good response. However, the relapse rate after steroid pulse therapy was higher in patients with high serum total IgE levels, low serum CEA levels, and in women. The multivariate analysis showed that high serum total IgE levels were an independent risk factor for relapse. These results suggest that there are at least two subtypes of AIGA: a typical subtype that shows high serum CEA levels, especially in the early or active phase of the disease, and an allergic subtype with high serum total IgE levels and a poor prognosis with steroids (Supplementary Figure S5).
Serum CEA can be used not only as a clinical marker for disease activity but also as a predictor of responsiveness to steroid pulse therapy for patients with AIGA [14, 18]. This may be partly due to the observation that high serum CEA levels were associated with a short disease duration (Table 1), and a short disease duration was related to good responsiveness to the therapy, as we reported previously [6]. However, serum CEA levels were also associated with factors, such as sex, urticaria, and atopic dermatitis, which were not considered relevant to the disease duration (Table 1). This finding suggests that there are different underlying pathologies in patients with AIGA and high or low serum CEA levels, which may influence the response to steroids.
Conversely, serum total IgE can be used as a predictor of recurrence. The patients with high serum total IgE levels had a high relapse rate after remission achieved by steroid pulse therapy. High serum total IgE is frequently related to patients’ allergic condition. Furthermore, some patients with AIGA were reported to have a sweat allergy [10]. The high recurrence rate may reflect the persistence of allergic inflammation against antigens related to sweat.
AIGA is often complicated by cholinergic urticaria, as well as dermal pain triggered by sweating stimuli [24, 25]. Urticaria could not be induced in most patients, at least under our experimental conditions (60°C sauna for 15 min). Furthermore, among patients with episodes of urticaria and/or dermal pain, those with a negative provocation test tended to have higher serum CEA levels, and those with a positive test tended to have slightly higher serum total IgE levels, suggesting the involvement of different mechanisms [25].
The relationship between serum CEA levels and the effectiveness of steroid pulse therapy on urticaria and/or dermal pain was not as clear as that between serum CEA levels and the effectiveness of steroid pulse therapy on sweating. The reason for this finding may be because of the small number of patients with urticaria and/or dermal pain, and because the effects of steroid pulse therapy on urticaria and/or dermal pain were less pronounced than those on sweating. In addition, this finding may reflect the difference in the direct cause of anhidrosis and urticaria in patients with AIGA. Although the pathogenesis of AIGA is not yet understood, the direct cause of anhidrosis is believed to mainly be sweat gland dysfunction, and the direct cause of urticaria is mast cell activation, although they are indirectly related [1, 11, 20]. In this study, the effect of steroid pulse therapy on urticaria and/or dermal pain was correlated with its effect on anhidrosis (chi-square test, P = 0.0074), but the concordance rate was limited to 62% (Cohen’s kappa = 0.21). This finding suggests that the effects of steroid pulse therapy on sweat gland dysfunction and mast cell activation are different. CEA is a molecule expressed in luminal cells in sweat glands [13, 14]. Therefore, serum CEA levels should reflect the state of sweat glands more directly than mast cells. Consequently, serum CEA levels may be correlated more strongly with the effect of steroid pulse therapy on sweating than with its effect on urticaria and/or dermal pain.
One of the limitations of this study is the estimation of cutoff values for serum biomarkers. The optimal cutoff of serum CEA levels for predicting a treatment response was not the same as the cutoff for recurrence (1.6 ng/mL and 4.9 ng/mL, respectively). This finding may have been due to an insufficient number of cases included, which likely reduced the precision of the estimate. However, patients with AIGA may not be a population that can be divided into two subgroups on the basis of a single cutoff, and a certain percentage of patients may have been in an intermediate category (i.e., patients with the mixed condition of steroid-responsive caused by sweating impairment and steroid-resistant caused by sweating impairment [26–28]). Serum CEA levels tend to fluctuate with disease activity [14] and decrease with time since the disease onset (Table 1). Therefore, a certain number of patients may have intermediate serum CEA levels depending on the time of measurement. In addition, serum CEA levels are affected by other organs, such as the gastrointestinal tract and liver [16, 17]. To enable accurate prediction of a treatment response, not only improving the estimation of cutoffs by accumulating cases, but also constructing a prediction model that combines multiple factors, such as serum biomarkers and histological findings, are necessary [28].
New treatment methods should be developed for non-responders to steroid therapy with low CEA levels and/or high IgE levels. In cases of low CEA levels after a long period of disease, inflammation may cause sweat gland degeneration [26, 29, 30]. Such cases would require a treatment that encourages the regeneration of sweat glands. However, in cases of high IgE levels with a strong allergic predisposition, long-term immune control should be considered to prevent relapse. Specifically, molecularly targeted drugs that selectively suppress inflammation specific to the disease, with minimal side effects, would be desirable.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Institute of Science Tokyo. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the committee approved an opt-out method for obtaining consent because of little or no risk to participants. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MF and TI: Conceptualization, Methodology, Data curation and Writing–Original draft; CU, HT, and TF: Data curation; MI, TN, and HY: Project administration and Data curation; NO: Conceptualization, Supervision and Writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by AMED under Grant Number JP20ek0109435 and MHLW Research on rare and intractable diseases Program Grant Number JPMH22FC1015.
Acknowledgments
This work was supported by AMED under Grant Number JP20ek0109435 and MHLW Research on Rare and Intractable Diseases Program Grant Number JP22FC1015. We thank Amanda Holland, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2025.14190/full#supplementary-material
References
1. Nakazato, Y, Tamura, N, Ohkuma, A, Yoshimaru, K, and Shimazu, K. Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology (2004) 63(8):1476–80. doi:10.1212/01.WNL.0000142036.54112.57
2. Cao, R, and Tey, HL. Prognosis of acquired idiopathic generalized anhidrosis. J Dtsch Dermatol Ges (2017) 15(9):942–5. doi:10.1111/ddg.13296
3. Munetsugu, T, Fujimoto, T, Oshima, Y, Sano, K, Murota, H, Satoh, T, et al. Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J Dermatol (2017) 44(4):394–400. doi:10.1111/1346-8138.13649
4. Wang, Y, Scheffel, J, Vera, CA, Liu, W, Günzel, D, Terhorst-Molawi, D, et al. Impaired sweating in patients with cholinergic urticaria is linked to low expression of acetylcholine receptor CHRM3 and acetylcholine esterase in sweat glands. Front Immunol (2022) 13(July):955161–11. doi:10.3389/fimmu.2022.955161
5. Munetsugu, T, Fujimoto, T, Satoh, T, Nakazato, Y, Ohshima, Y, Asahina, M, et al. Evaluation of the correlation between severity of acquired idiopathic generalized anhidrosis and quality of life scores. J Dermatol (2017) 44(7):747–52. doi:10.1111/1346-8138.13785
6. Iida, T, Nakamura, M, Inazawa, M, Munetsugu, T, Nishida, M, Fujimoto, T, et al. Prognosis after steroid pulse therapy and seasonal effect in acquired idiopathic generalized anhidrosis. J Dermatol (2021) 48(3):271–8. doi:10.1111/1346-8138.15666
7. Ando, Y, Fujii, S, Sakashita, N, Uchino, M, and Ando, M. Acquired idiopathic generalized anhidrosis: clinical manifestations and histochemical studies. J Neurol Sci (1995) 132(1):80–3. doi:10.1016/0022-510X(95)00125-L
8. Ohshima, Y, Yanagishita, T, Ito, K, Tamada, Y, Nishimura, N, Inukai, Y, et al. Treatment of patients with acquired idiopathic generalized anhidrosis. Br J Dermatol (2013) 168(2):430–2. doi:10.1111/j.1365-2133.2012.11112.x
9. Sawada, Y, Nakamura, M, Bito, T, Sakabe, JI, Kabashima-Kubo, R, Hino, R, et al. Decreased expression of acetylcholine esterase in cholinergic urticaria with hypohidrosis or anhidrosis. J Invest Dermatol (2014) 134(1):276–9. doi:10.1038/jid.2013.244
10. Fukunaga, A, Hatakeyama, M, Tsujimoto, M, Oda, Y, Washio, K, and Nishigori, C. Steroid treatment can improve the impaired quality of life of patients with acquired idiopathic generalized anhidrosis. Br J Dermatol (2015) 172(2):537–8. doi:10.1111/bjd.13285
11. Kageyama, R, Honda, T, and Tokura, Y. Acquired idiopathic generalized anhidrosis (AIGA) and its complications: implications for AIGA as an autoimmune disease. Int J Mol Sci. (2021) 22(16):8389. doi:10.3390/ijms22168389
12. Murakami, K, Sobue, G, Terao, S, and Mitsuma, T. Acquired idiopathic generalized anhidrosis: a distinctive clinical syndrome. J Neurol (1988) 235(7):428–31. doi:10.1007/BF00314488
13. Honma, M, Iinuma, S, Kanno, K, Komatsu, S, Minami-Hori, M, and Ishida-Yamamoto, A. Correlation of disease activity and serum level of carcinoembryonic antigen in acquired idiopathic generalized anhidrosis: a case report. J Dermatol. (2015) 42(9):900–2. doi:10.1111/1346-8138.12926
14. Honma, M, Iinuma, S, Kanno, K, Komatsu, S, Minami-Hori, M, Iizuka, H, et al. Serum carcinoembryonic antigen (CEA) as a clinical marker in acquired idiopathic generalized anhidrosis: a close correlation between serum CEA level and disease activity. J Eur Acad Dermatol Venereol (2016) 30(8):1379–83. doi:10.1111/jdv.13390
15. Nakazato, Y, Tamura, N, Ikeda, K, Yamamoto, T, and Tokura, Y. A case of idiopathic pure sudomotor failure associated with prolonged high levels of serum carcinoembryonic antigen. Clin Auton Res (2016) 26(6):451–3. doi:10.1007/s10286-016-0376-4
16. Hammarström, S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol (1999) 9(2):67–81. doi:10.1006/scbi.1998.0119
17. Kuespert, K, Pils, S, and Hauck, CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol (2006) 18(5):565–71. doi:10.1016/j.ceb.2006.08.008
18. Sakiyama, T, Endo, Y, Nakamizo, S, and Kabashima, K. Elevation of serum carcinoembryonic antigen as a possible predictor of response to pulse methylprednisolone in acquired idiopathic generalized anhidrosis. Clin Exp Dermatol (2024) 50:125–9. doi:10.1093/ced/llae363
19. Magerl, M, Altrichter, S, Borzova, E, Giménez-Arnau, A, Grattan, CEH, Lawlor, F, et al. The definition, diagnostic testing, and management of chronic inducible urticarias - the EAACI/GA2LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy (2016) 71(6):780–802. doi:10.1111/all.12884
20. Fukunaga, A, Oda, Y, Imamura, S, Mizuno, M, Fukumoto, T, and Washio, K. Cholinergic urticaria: subtype classification and clinical approach. Am J Clin Dermatol (2023) 24(1):41–54. doi:10.1007/s40257-022-00728-6
21. Yakabe, T, Nakafusa, Y, Sumi, K, Miyoshi, A, Kitajima, Y, Sato, S, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol (2010) 17(9):2349–56. doi:10.1245/s10434-010-1004-5
22. Agarwal, R, Aggarwal, AN, Garg, M, Saikia, B, and Chakrabarti, A. Cut-off values of serum IgE (total and A. fumigatus -specific) and eosinophil count in differentiating allergic bronchopulmonary aspergillosis from asthma. Mycoses (2014) 57(11):659–63. doi:10.1111/myc.12214
23. Provost, F, and Fawcett, T. Robust classification for imprecise environments. Mach Learn (2001) 42(3):203–31. doi:10.1023/A:1007601015854
24. Mizuno, M, Fukunaga, A, Washio, K, Imamura, S, Oda, Y, and Nishigori, C. A visual analogue scale for itch and pain in 23 cases of cholinergic urticaria. J Eur Acad Dermatol Venereol (2020) 34(9):e493–5. doi:10.1111/jdv.16410
25. Takahagi, S, Okamoto, M, Ishii, K, Tanaka, A, Mizuno, H, Harada, N, et al. Clinical and histological characterization of transient dermal pain triggered by sweating stimuli. Allergol Int (2022) 71(3):362–72. doi:10.1016/j.alit.2022.01.003
26. Miyazoe, S, Matsuo, H, Ohnishi, A, Tajima, F, Fujishita, S, Ichinose, K, et al. Acquired idiopathic generalized anhidrosis with isolated sudomotor neuropathy. Ann Neurol (1998) 44(3):378–81. doi:10.1002/ana.410440314
27. Ogino, J, Saga, K, Kagaya, M, Kamada, A, Kaneko, R, and Jimbow, K. Idiopathic acquired generalized anhidrosis due to occlusion of proximal coiled ducts. Br J Dermatol. (2004) 150(3):589–93. doi:10.1111/j.1365-2133.2004.05872.x
28. Shimoda-Komatsu, Y, Yamazaki, Y, Kimishima, M, Mizukawa, Y, and Ohyama, M. Clinicopathological digital image analyses before and after thermal stimulation subdivide acquired idiopathic generalized anhidrosis into inflammatory and non-inflammatory type. J Dermatol Sci (2022) 108(1):12–21. doi:10.1016/j.jdermsci.2022.10.001
29. Sano, K, Asahina, M, Uehara, T, Matsumoto, K, Araki, N, and Okuyama, R. Degranulation and shrinkage of dark cells in eccrine glands and elevated serum carcinoembryonic antigen in patients with acquired idiopathic generalized anhidrosis. J Eur Acad Dermatol Venereol (2017) 31(12):2097–103. doi:10.1111/jdv.14443
30. Sano, K, Asahina, M, Uehara, T, Araki, N, Yamanaka, Y, Matsumoto, K, et al. Clear cell injury associated with reduced expression of carbonic anhydrase II in eccrine glands consistently occurs in patients with acquired idiopathic generalized anhidrosis. J Dermatol (2021) 48(4):439–46. doi:10.1111/1346-8138.15722
Keywords: IgE, carcinoembryonic antigen, anhidrosis, steroid pulse therapy, cohort study
Citation: Fujita M, Iida T, Uchida C, Inazawa M, Takeshita H, Fujimoto T, Namiki T, Yokozeki H and Okiyama N (2025) An allergic subtype of acquired idiopathic generalized anhidrosis resistant to steroid pulse therapy – a retrospective, single-center, cohort study. J. Cutan. Immunol. Allergy 8:14190. doi: 10.3389/jcia.2025.14190
Received: 10 December 2024; Accepted: 18 March 2025;
Published: 26 March 2025.
Copyright © 2025 Fujita, Iida, Uchida, Inazawa, Takeshita, Fujimoto, Namiki, Yokozeki and Okiyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadatsune Iida, aWlkYS5kZXJtQHRtZC5hYy5qcA==
 Maiko Fujita
Maiko Fujita Tadatsune Iida
Tadatsune Iida Naoko Okiyama
Naoko Okiyama