- 1Department of Dermatology, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
- 2Department of Dermatology, JA Hiroshima General Hospital, Hiroshima, Japan
- 3Department of Dermatology, Kawasaki Medical School, Kurashiki, Japan
- 4Division of Dermatology, Department of Internal Related, Kobe University Graduate School of Medicine, Kobe, Japan
- 5Department of Dermatology, Division of Medicine for Function and Morphology of Sensory Organs, Faculty of Medicine, Osaka Medical and Pharmaceutical University, Takatsuki, Japan
- 6Department of Dermatology, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan
- 7Department of Dermatology, Hiroshima City Hiroshima Citizens Hospital, Hiroshima, Japan
Background: Exposure to sweating stimuli triggers transient and severe dermal pain in certain individuals. This study aimed to evaluate the analgesic effect of icatibant, a bradykinin B2 receptor antagonist, for the dermal pain induced by sweating stimuli and explored the role of bradykinin in its pathomechanism.
Methods: This single-blind, randomized, crossover study enrolled adult patients who had experienced severe sweating-induced dermal pain. Participants randomly received icatibant (30 mg) or placebo on Day 1 and vice versa on Day 2. Before and after drug administration, dermal pain induced by an artificial thermal loading was assessed using the visual analog scale (VAS).
Results: Four patients were enrolled, three of whom completed the full procedure with the termination in the other. Among the three patients, the pain-VAS score decreased more with icatibant than with saline in one patient, whereas it decreased more with saline than with icatibant in the other two patients. The pain duration decreased more with icatibant than with saline in two patients, whereas it decreased equally with both treatments in the other. Icatibant did not affect the changes in whole blood and plasma histamine, and serum angiotensin-converting enzyme levels after the induction of the dermal pain. A safety profile showed injection site pain associated with icatibant.
Conclusion: Blockade of bradykinin B2 receptor signaling by icatibant contributed to the reduction of dermal pain during sweating stimuli in one patient but not in two others. The role of bradykinin in the dermal pain varies among patients.
Clinical trial registration: https://jrct.niph.go.jp/, identifier jRCTs061210021.
Introduction
Exposure to sweating stimuli such as bathing, exercise, or mental stress triggers transient and severe dermal pain in certain populations of individuals [1–3]. The sweating-induced dermal pain may occur alone or, more commonly, be accompanied by transient eruptions and/or generalized hypohidrosis [1], referred to as cholinergic urticaria [2] or acquired idiopathic generalized anhidrosis (AIGA) [3]. Considering that the pain is short-lived but severe and significantly disrupts patients’ daily lives [1], the underlying molecular mechanisms need to be elucidated to develop effective treatments. The short duration of the pain suggests the involvement of endogenous pain-producing substances with short lifetimes, such as histamine and bradykinin, in its pathogenesis.
This study aimed to evaluate the analgesic effect of icatibant, a bradykinin B2 receptor antagonist, in patients experiencing the sweating-induced dermal pain and demonstrated the role of bradykinin in the pathomechanism of the dermal pain. Thus, we performed a single-blind, randomized, crossover study on the efficacy of subcutaneous injections of icatibant for the dermal pain induced by an artificial thermal load.
Methods
Participant enrollment
Study participants included adult patients who had experienced severe dermal pain induced by thermal sweating stimuli (e.g., bathing), regardless of the presence or absence of cholinergic urticaria and hypohidrosis, for more than 3 months. After obtaining consent from study participants at Hiroshima University Hospital, eligibility was confirmed based on the inclusion and exclusion criteria (Supplementary Table S1). Due to the slow enrollment with a limited number of patients eligible to participate in the study, the VAS requirement in the inclusion criteria was relaxed from 60 mm to 40 mm to promote patient enrollment as of 21 June 2023. The procedures for the four patients presented in this report had been completed prior to the amendment, which did not affect the results of these patients.
Study design
The study design is as described in a previous article [4] (Supplementary Figure S1). Briefly, upon enrollment, participants were assigned to one of two groups [Icatibant-placebo group (Group A) or Placebo-icatibant group (Group B)]. Participants in Group A received icatibant (30 mg/3 mL) on Day 1 and placebo (saline, 3 mL) on Day 2, while those in Group B did placebo on Day 1 and icatibant on Day 2. Before and after administration of drugs, dermal pain induced by thermal load was assessed using the visual analog scale (VAS). The change of VAS was calculated to evaluate the efficacy of drugs. Thermal loading was performed by placing the lower legs of the participants in warm water (approximately 43°C) for 30 min to induce dermal pain.
Specifically, on Day 1, after baseline VAS assessment on the dermal pain during the first thermal loading, icatibant or saline was subcutaneously injected at least 90 min after the first thermal load or 30 min after the skin symptoms resolved, whichever occurred later. The second thermal load started 45 ± 15 min after the drug administration, followed by the second pain-VAS assessment. On Day 2, the same procedure as on Day 1 was followed to evaluate the efficacy of the other drug on the dermal pain. Saline was subcutaneously administered to patients who receive icatibant on Day 1, while icatibant to those who received placebo on Day 1. A washout period of ≥9 h was allowed between drug administration on Day 1 and 2. In addition, to explore related mediators, blood and plasma histamine levels and serum angiotensin-converting enzyme (ACE) levels were measured before and after every thermal load.
Ethical approval and informed consent
The study was conducted in compliance with the principles of the Declaration of Helsinki (1996), Clinical Trial Act, and all applicable regulatory requirements. Ethical approval was obtained from the Hiroshima University Certified Review Board (reference approval number: CRB6180006). Written informed consent was obtained from all participants before any study procedure. The protocol and trial progress were registered in the Japan Registry of Clinical Trials (jRCTs061210021) on 8 July 2022.
Results
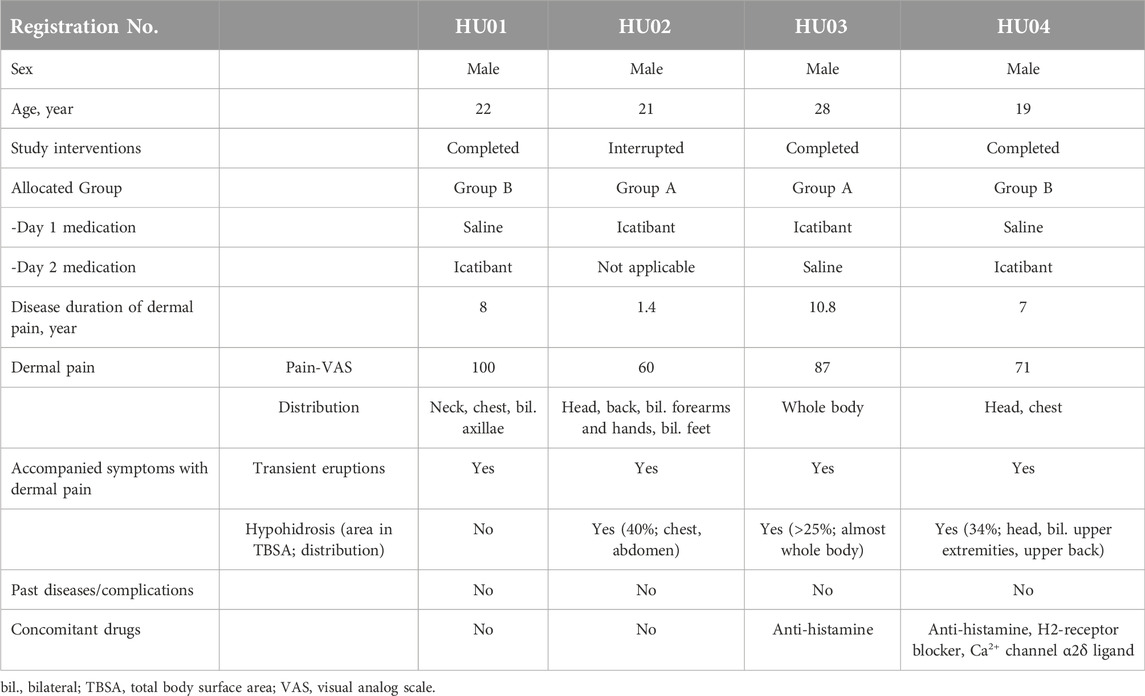
Four patients [male: female = 4:0; age (median, range): 21.5, 19–28] were enrolled in this study (Table 1). Ten patients were initially scheduled to participate; however, the study was terminated upon completion of the procedure in four patients owing to the lack of apparent efficacy of icatibant. Three patients (HU01, 03, and 04) completed the full crossover interventions and assessments. The other patient (HU02) was unable to induce baseline pain in the saline arm (Day 2); hence, the procedure was terminated. Two of the four patients were allocated to Group A, and the others to Group B. Sweating-induced dermal pain was associated with transient eruptions in all of the four patients, and generalized (>25%) hypohidrosis in HU02, HU03, and HU04. None of the patients had any pre-existing medical conditions or complications.
The primary endpoint of the study was to compare the changes in pain VAS scores before and after treatments with icatibant and saline. Among the three patients (HU-01, 03, and 04) who completed the interventions, the pain VAS score decreased more with icatibant than with saline in one patient, whereas the pain VAS score decreased more with saline than with icatibant in the other two patients (Figure 1A; Supplementary Table S2). As a secondary endpoint, the pain duration decreased more with icatibant than with saline in two patients, whereas it decreased equally with both treatments in the remaining patient (Figure 1B; Supplementary Table S2). Raw data of pain-VAS and duration of pain are shown in Supplementary Table S2. Additionally, changes in whole blood and plasma histamine, and serum ACE levels due to the thermal load-induced dermal pain were evaluated before and after treatments in two and three patients, respectively (Figure 2; Supplementary Table S3). No significant changes in whole blood and plasma histamine, or serum ACE levels were observed after the induction of the dermal pain in any of the patients tested. Icatibant treatment did not alter these changes. Adverse events were assessed in four patients including the patient (HU02) with the terminated procedure. Injection site pain was associated with icatibant in four patients, and with saline in one patient. Insomnia was observed in one patient but was not considered to be related to drug administration.
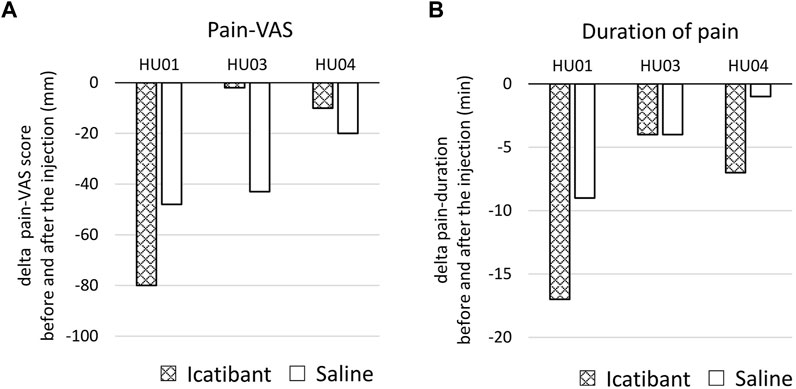
Figure 1. Effect of icatibant on the sweating-induced dermal pain. (A) The changes in pain-VAS scores before and after the injection of icatibant or saline. (B) The changes in duration of pain before and after the injection of icatibant or saline. Data at each time point are shown in Supplementary Table S2. VAS, visual analog scale.
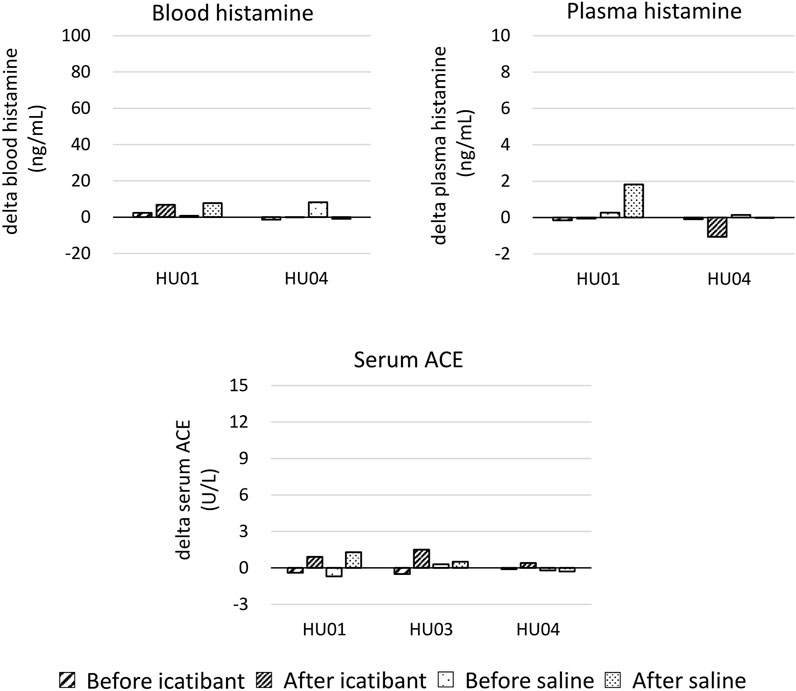
Figure 2. The changes in whole blood and plasma histamine, and serum angiotensin-converting enzyme (ACE) due to the thermal load-induced dermal pain were evaluated before and after the injection of icatibant or saline. The changes in blood histamine, plasma histamine, and/or serum ACE data in HU02 and HU03 could not be calculated due to missing data. Data at each time point are shown in Supplementary Table S3.
Discussion
Bradykinin is an endogenous pain mediator [5], which may play a role in the dermal pain during sweating stimuli because the predominant kinin-producing activity is detected in the skin during thermal sweating [6–9]. Icatibant, a bradykinin B2 receptor antagonist, is approved for the treatment of acute attacks of hereditary angioedema but is off-label for dermal pain associated with sweating stimuli. In this study, the remarkable efficacy of icatibant was not observed, with a better response in one patient but a poorer response in two patients, in contrast to saline. Patients were not allowed to change their baseline concomitant treatments that may alleviate symptoms, including antihistamines, at least for 2 weeks during this trial. Concomitant antihistamines probably had no impact on the results in HU03 and HU04. Moreover, blood histamine and ACE levels were measured as biomarkers. ACE is an enzyme involved in the kallikrein/kinin system that degrades and inactivates bradykinin [5]. Hence, no significant relationship was found between the occurrence of dermal pain, circulating histamine levels, and the kallikrein-kinin system.
A limitation of this study is that it was terminated after completing the procedure in only four patients owing to the apparent lack of efficacy of icatibant. Statistical analyses were not possible because of the small number of patients. Given that no clear benefit from icatibant was expected, and the physical burden of injection site pain and thermal loading on the patients was deemed significant, we concluded that continuing the study was not ethically justified. In addition, repeated sweating stimulations with a short interval may reduce the sweating-induced dermal pain as observed in a patient (HU02) in this study. In the future, a randomized control trial with painless oral medication to block bradykinin such as deucrictibant [10] in patients’ real daily lives would overcome these limitations.
Overall, this study showed that the blockade of bradykinin B2 receptor signaling by icatibant contributed to the reduction of the dermal pain during sweating stimuli in one patient but not in two others. This indicates that the role of bradykinin in the dermal pain varies among patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Hiroshima University Certified Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ST, MH, YA, AF, and HM designed the study. ST, DM, EM, YA, SI, YO, and MH contributed to data collection. ST, DM, EM, YA, SI, AF, YO, HM, and MH made an interpretation of the results. ST and MH wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is conducted with the free supply of the research drug, Firazyr® (subcutaneous injection 30 mg syringe), from Takeda Pharmaceuticals International AG, a subsidiary of Takeda Pharmaceutical Company Limited under Grant Number IIR-JP-002565 (ST and MH). Personnel in Takeda Pharmaceuticals International AG and Takeda Pharmaceutical Company Limited is not involved in the data management, statistical analyses, or auditing of the study. This study is also supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP19ek0410059 (ST and MH).
Acknowledgments
We extend special thanks to all the patients and staff involved in this study. A part of this manuscript overlaps with a previous publication [4], which described the protocol of this study.
Conflict of interest
MH has received honoraria for speaker/advisor and gift of a medication (icatibant) for this clinical study from Takeda Pharmaceutical. AF has received honorarium as a speaker/advisor from CSL Behring, Takeda Pharmaceutical and Torii Pharmaceutical Company Ltd. ST has received honorarium and gift of a medication (icatibant) for this clinical study from Takeda Pharmaceutical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2025.14276/full#supplementary-material
Abbreviations
AIGA, acquired idiopathic generalized anhidrosis; jRCT, Japan Registry of Clinical Trials; VAS, visual analogue scale; ACE, angiotensin converting enzyme.
References
1. Takahagi, S, Okamoto, M, Ishii, K, Tanaka, A, Mizuno, H, Harada, N, et al. Clinical and histological characterization of transient dermal pain triggered by sweating stimuli. Allergol Int (2022) 71:362–72. doi:10.1016/j.alit.2022.01.003
2. Mizuno, M, Fukunaga, A, Washio, K, Imamura, S, Oda, Y, and Nishigori, C. A visual analogue scale for itch and pain in 23 cases of cholinergic urticaria. J Eur Acad Dermatol Venereol (2020) 34:e493–5. doi:10.1111/jdv.16410
3. Munetsugu, T, Fujimoto, T, Oshima, Y, Sano, K, Murota, H, Satoh, T, et al. Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J Dermatol (2017) 44:394–400. doi:10.1111/1346-8138.13649
4. Takahagi, S, Hide, M, Aoyama, Y, Fukunaga, A, and Murota, H. A single-blind, randomized, crossover study on the efficacy of icatibant for sweating-induced dermal pain (icatibant for sweating-induced dermal pain). Medicine (2023) 102:e33971. doi:10.1097/MD.0000000000033971
5. Brusco, I, Fialho, MFP, Becker, G, Brum, ES, Favarin, A, Marquezin, LP, et al. Kinins and their B(1) and B(2) receptors as potential therapeutic targets for pain relief. Life Sci (2023) 314:121302. doi:10.1016/j.lfs.2022.121302
6. Poblete, MT, Reynolds, NJ, Figueroa, CD, Burton, JL, Muller-Esterl, W, and Bhoola, KD. Tissue kallikrein and kininogen in human sweat glands and psoriatic skin. Br J Dermatol (1991) 124:236–41. doi:10.1111/j.1365-2133.1991.tb00567.x
7. Fox, RH, and Hilton, SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol (1958) 142:219–32. doi:10.1113/jphysiol.1958.sp006011
8. Hibino, T, Takemura, T, and Sato, K. Human exocrine sweat contains tissue kallikrein and kininase II. J Invest Dermatol (1994) 102:214–20. doi:10.1111/1523-1747.ep12371765
9. Matus, CE, Ehrenfeld, P, and Figueroa, CD. The family of kallikrein-related peptidases and kinin peptides as modulators of epidermal homeostasis. Am J Physiol Cell Physiol (2022) 323:C1070–C1087. doi:10.1152/ajpcell.00012.2022
Keywords: dermal pain, sweating, icatibant, bradykinin, histamine
Citation: Takahagi S, Matsubara D, Murakami E, Aoyama Y, Imamura S, Fukunaga A, Oda Y, Murota H and Hide M (2025) Effect of icatibant on sweating-induced dermal pain. J. Cutan. Immunol. Allergy 8:14276. doi: 10.3389/jcia.2025.14276
Received: 30 December 2024; Accepted: 29 January 2025;
Published: 10 February 2025.
Copyright © 2025 Takahagi, Matsubara, Murakami, Aoyama, Imamura, Fukunaga, Oda, Murota and Hide. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunsuke Takahagi, dGFrc2h1bnNAZ21haWwuY29t; Michihiro Hide, ZWQxaC13MWRlLXJvYWRAaGlyb3NoaW1hLXUuYWMuanA=
 Shunsuke Takahagi
Shunsuke Takahagi Daiki Matsubara1
Daiki Matsubara1 Emi Murakami
Emi Murakami Atsushi Fukunaga
Atsushi Fukunaga Hiroyuki Murota
Hiroyuki Murota Michihiro Hide
Michihiro Hide