- 1Department of Biomedical Molecular Sciences, School of Medicine, Fujita Health University, Toyoake, Japan
- 2Department of Pharmacotherapeutics and Informatics, School of Medicine, Fujita Health University, Toyoake, Japan
- 3Department of Nephrology, School of Medicine, Fujita Health University, Toyoake, Japan
Purpose: Coronavirus disease 2019 (COVID-19) mRNA vaccines are used worldwide to prevent severe symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. IgA nephropathy (IgAN) is the most common form of glomerular injury after COVID-19 vaccination; however, because of the low frequency of such events, only a few reports have been published. A large pharmacovigilance database of real-world spontaneous adverse event (AE) reports is essential for evaluating the drug-associated safety signals regarding rare AEs. Herein, we aimed to investigate the frequency of IgAN after the COVID-19 vaccination, using the Japanese Adverse Drug Event Report (JADER) database.
Methods: Data on drug-associated AEs reported between April 2004 and May 2022 were obtained from the JADER database on the Pharmaceuticals and Medical Devices Agency website. To evaluate the safety signals for the targeted AEs, reporting odds ratios (RORs), information components (ICs), and their 95% confidence intervals (CIs) were calculated using two-by-two contingency tables.
Results: A total of 697,885 cases were included in the analysis. Safety signals were detected for IgAN (ROR: 6.49, 95% CI: 4.38–9.61; IC: 2.27, 95% CI: 1.70–2.83). Of 30 cases for IgAN associated with COVID-19 mRNA vaccines, 16 had information available on time to onset. Of the 16 cases, 11 occurred ≤2 days after vaccination, and two occurred >28 days after vaccination.
Conclusion: These results suggest that, compared with other drugs, COVID-19 vaccination is associated with a higher frequency of IgAN. Monitoring of gross hematuria following COVID-19 vaccination should be needed.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused severe health and economic damage. To prevent the symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, COVID-19 mRNA vaccines are being used worldwide. The mRNA is delivered by lipid nanoparticles into the cells and translated to the target protein which promotes a robust immune response [1, 2]. mRNA vaccines are associated with a risk of glomerular nephropathy [3, 4], and relapse of minimal change disease has been reported after COVID-19 vaccination [3, 5, 6].
IgA nephropathy (IgAN) is a type of glomerulonephritis characterized by the deposition of IgA1-containing immune complexes. Previous case reports suggest that COVID-19 vaccination is associated with a relapse of IgAN [7, 8]. These patients presented with gross hematuria within 24 h after receiving the second dose of a COVID-19 mRNA vaccine, and the hematuria resolved within 3–5 days. Although these cases improved without immunosuppressive therapy, a case of new-onset IgAN with severe crescentic glomerulonephritis has been reported after the second vaccination [9]. This case showed mild mesangial expansion, endocapillary hypercellularity, and IgA positivity in granular mesangial cells. Immunosuppressive therapy was initiated after the patient was diagnosed with IgA vasculitis. Kidney function and proteinuria improved after administering pulse intravenous methylprednisolone. Ma and Xu [10] reviewed nine cases of new-onset IgAN after the first vaccination. The median time to onset was 10 days and immunotherapy was needed in seven cases. Thirty-eight cases of IgAN were reported after the second vaccination, including 22 new cases, with a median time to onset of 2 days. IgAN is thought to be the most common glomerular injury after COVID-19 vaccination; however, there are few published reports. In addition, information regarding time to onset (TTO) and outcomes is limited because the frequency of IgAN is lower than that of other adverse events (AEs) following vaccination.
A large pharmacovigilance database of real-world spontaneously reported AEs is essential for evaluating the drug-associated safety signals regarding rare AEs. The Japanese Adverse Drug Event Report (JADER) is a nationwide database of spontaneously reported AEs developed by the Pharmaceuticals and Medical Devices Agency (PMDA), the pharmaceutical regulatory authority in Japan. The calculation of reporting odds ratios (RORs) and information components (ICs) from the JADER database are recognized as indicators for the investigation of drug-associated safety signals [11–14]. Herein, we aimed to investigate the frequency of IgAN after COVID-19 mRNA vaccination by calculating RORs and ICs using the JADER database.
Materials and methods
Data source and data processing
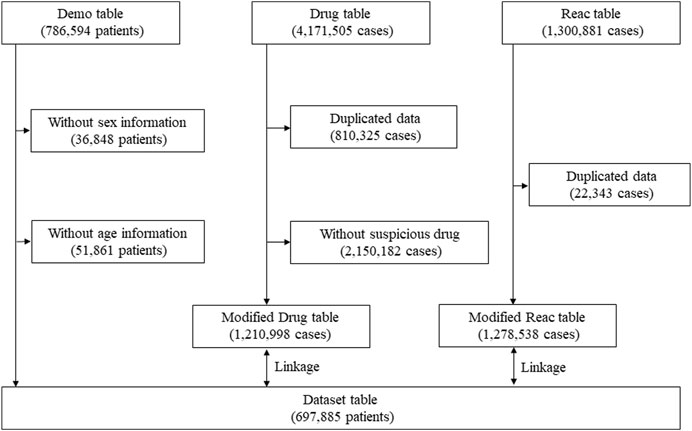
Data extraction was conducted according to previous reports [12, 13, 15]. Briefly, data were obtained from the JADER database between April 2004 and May 2022. Because COVID-19 vaccine was introduced in our country in February 2021 (and in January 2022 for children aged 5–11 years), the database covers the report regarding the abovementioned population. All data in this study were obtained from the PMDA website (https://www.pmda.go.jp/english/index.html). The JADER dataset consists of demographic information “demo,” drug information “drug,” and adverse reaction information “reac” tables. Cases lacking information on sex or age in the “demo” table, and those with duplicate records in the “drug” and “reac” tables were excluded. The medication was classified into three categories: “suspected drug,” “concomitant drug,” and “interaction drug” depending on the contribution to the AE. The cases in the “suspected drug” category were evaluated in this study. To evaluate the safety signals for IgAN, nephritis, and lupus nephritis in patients after COVID-19 mRNA vaccination, COVID-19 mRNA vaccine (recorded as “Coronavirus Modified Uridine RNA Vaccine [SARS-CoV-2]”) was selected from the “drug” table. The “demo” table was linked to the “drug” and “reac” tables using the case identification number (Figure 1).
Definition of IgA nephropathy, nephritis, and lupus nephritis
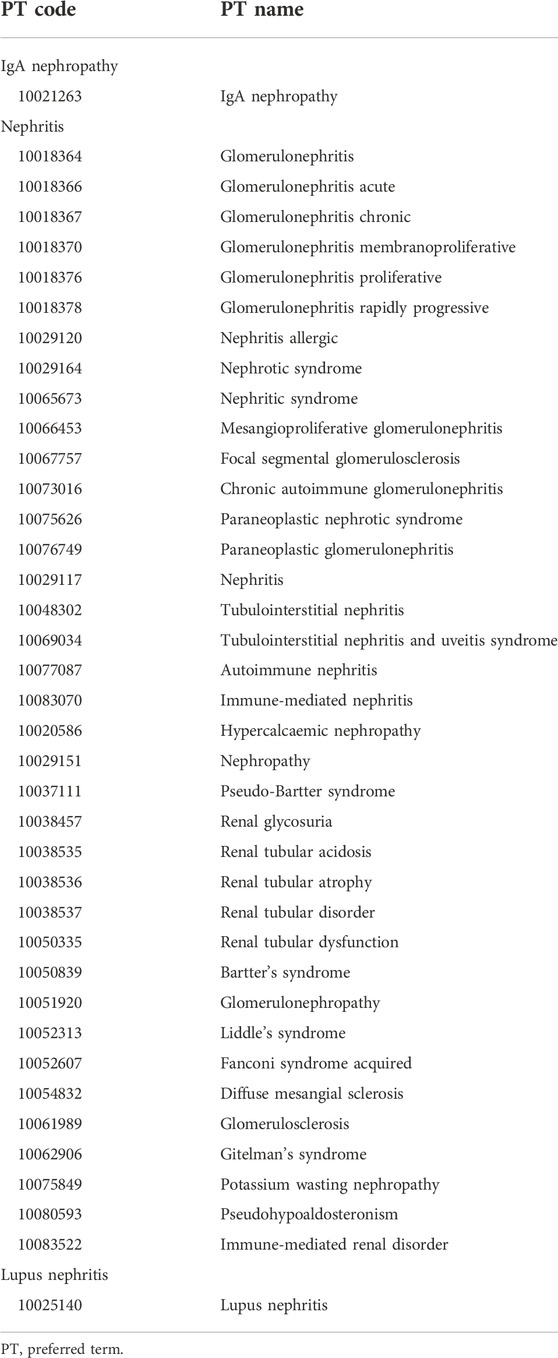
In the JADER database, the medical conditions are defined according to the Medical Dictionary for Regulatory Activities (MedDRA) list of preferred terms (PTs). The AEs in the “reac” table are defined based on the PTs in MedDRA version 25.1. In this study, IgAN, nephritis, and lupus nephritis were selected as the targeted AEs from 37 PTs by two nephrologists (Table 1).
Signal detection
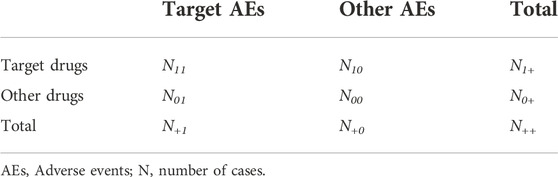
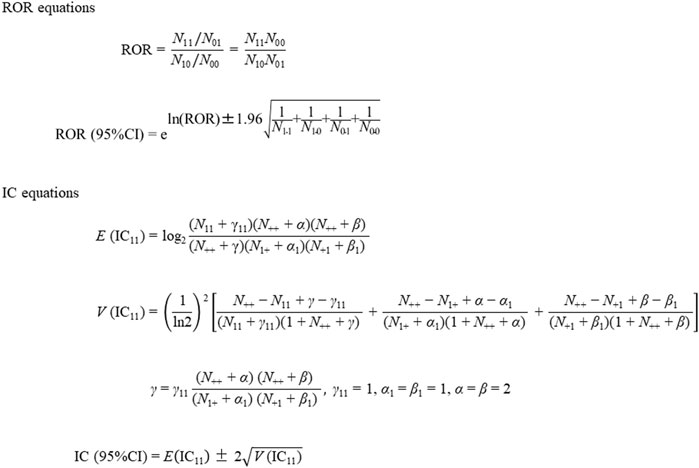
To evaluate the safety signals for targeted AEs, RORs, ICs, and their 95% confidence intervals (CIs) were calculated using two-by-two contingency tables (Table 2) and equations (Figure 2), as described previously [11, 12]. Briefly, RORs and ICs were calculated from a cross-tabulation table based on the number of cases of IgAN associated with COVID-19 mRNA vaccination using the equations shown in Figure 2. We calculated the RORs and ICs using Microsoft 365 Excel (Microsoft Corporation, Redmond, WA, United States). The safety signals for IgAN, nephritis, and lupus nephritis were positive if the lower limit of the 95% CI of the ROR was >1 and that of the IC was >0 [11, 12].
Time to onset assessment
TTO was calculated from the date of the first COVID-19 mRNA vaccination in the “drug” table to the date of the first occurrence of the IgAN recorded in the “reac” table. Patients with missing values or those without data were classified as unknown.
Ethics approval and consent to participate
As this study used an open-access database, ethics approval and consent to participate were not required.
Results
Patient characteristics
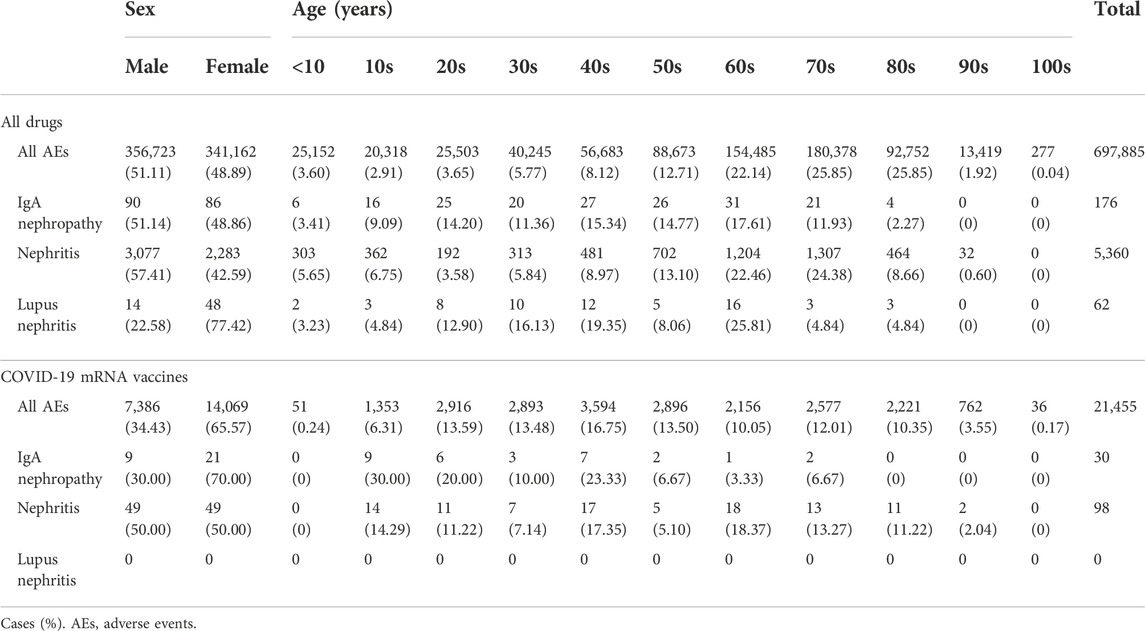
A total of 697,885 cases were included in the analysis. Their sex and age characteristics are shown in Table 3. The number of all AEs associated with COVID-19 mRNA vaccination was 21,455. The frequency of drug-induced nephritis was lower in females than that in males. The frequency of drug-induced IgAN was similar between males and females, while that of drug-induced lupus nephritis was higher in females than that in males. Although the number of AEs reported for all drugs was similar between females and males, the number of reported AEs associated with COVID-19 mRNA vaccine was higher in females than that in males. The number of all AEs reported for the COVID-19 mRNA vaccine was lower in the under 10s than that in the other age groups. The number of cases of IgAN reported as AEs associated with COVID-19 mRNA vaccination was higher in females and younger age groups (10–49 years) than that in males and older age groups (≥50 years), respectively. The number of cases of nephritis and lupus nephritis were 98 and 0, respectively. The occurrence of nephritis reported as AEs associated with COVID-19 mRNA vaccination did not differ by sex or age.
Safety signals of IgA nephropathy and nephritis
The RORs and CIs of IgAN and nephritis reported as AEs of COVID-19 mRNA vaccines are shown in Table 4. Safety signals were detected for IgAN (ROR, 6.49 [95% CI 4.38–9.61] and IC, 2.27 [95% CI 1.70–2.83]), but not for nephritis (ROR, 0.59 [95% CI 0.48–0.72] and IC, −0.74 [95% CI −1.04 to −0.45]).

TABLE 4. Reporting odds ratios and information components of COVID-19 mRNA vaccines for IgA nephropathy and nephritis.
Time to onset and outcomes of IgA nephropathy
In 30 cases of IgAN associated with COVID-19 mRNA vaccine, 16 cases had information available on TTO. Among the 16 cases, 11 cases had a TTO of <2 days and 2 cases had a TTO of >28 days after the vaccination (Table 5). In addition, information on outcomes was obtained on 19 IgAN cases associated with COVID-19 mRNA vaccines. Among the 19 patients, 8 patients were in recovery or remission, and 11 patients were not in recovery (Table 5).
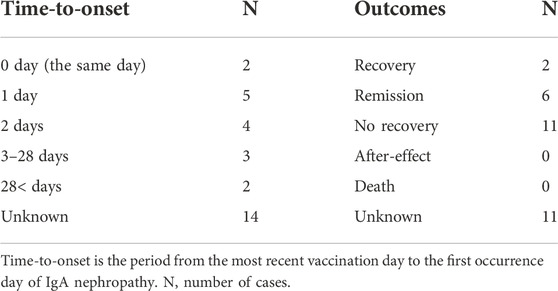
TABLE 5. Time-to-onset and outcomes of occurrence of IgA nephropathy in cases with COVID-19 mRNA vaccines.
Discussion
The results of this study suggest that COVID-19 vaccination increases the frequency of IgAN more than that of other drugs. In addition, females and young individuals show the tendency of an increased frequency of IgAN associated with COVID-19 vaccination compared to that of males and older individuals. Because the frequency of drug-induced nephritis was higher in males than in females, there might be different mechanisms in nephritis associated with COVID-19 vaccination and that associated with other drugs. Most of drug-induced nephritis cases are characterized by tubular necrosis, interstitial nephritis, and tubular obstruction [16]. The causes of this nephritis are administration of antibiotics, anticancer agents, and non-steroidal anti-inflammatory agents, and the frequency is higher in males than in females [17]. Conversely, the impact of sex difference is small in some forms of glomerulonephritis including IgAN [18]. Although the JADER database does not include information regarding renal biopsy, most of nephritis cases associated with COVID-19 vaccination might be glomerulonephritis.
A questionnaire survey conducted in Japan [19] contained questions on the frequency and clinical features of gross hematuria after COVID-19 vaccination. The survey included 27 cases with gross hematuria after COVID-19 vaccination. Most of the patients with gross hematuria were aged 20–29 years, and 81.5% of the cases were in females. Our results are similar to those of the mentioned survey; however, the survey had selection bias because the participants included council members of the Japanese Society of Nephrology (382 facilities), and the participants tended to be from large hospitals. Furthermore, the association between the onset of gross hematuria and COVID-19 vaccination was not evaluated using statistical methods. To avoid selection bias in this study, we used a nationwide database of spontaneous reports of AEs. Although the JADER database might include the same cases included in the survey [19], the database contained 30 reported cases of IgAN. Therefore, our analysis included some cases that were not included in the survey. All patients diagnosed with IgAN developed gross hematuria within 14 days after COVID-19 vaccination in the abovementioned survey, whereas among the cases of IgAN in the JADER database, two occurred more than 28 days after vaccination. Our results suggest that careful monitoring for gross hematuria is needed for 1–2 months after COVID-19 vaccination.
Over 70% of cases with gross hematuria were diagnosed with IgAN in the previous survey [19]. In our study, the number of cases with recovery and remission was 8, whereas 11 patients did not recover. Because we could not ascertain from the data in the JADER database whether the cases of IgAN were relapses or newly diagnosed, the clinical features of the cases are unclear. In most previous case reports, the TTO of IgAN was <7 days [20]; therefore, cases that developed within 7 days might be a relapse of IgAN triggered by COVID-19 vaccination. The frequency of “nephritis” was not increased and no cases of lupus nephritis were detected after COVID-19 vaccination. Because the JADER database is a database of spontaneous reports of AE, many cases are not classified as typical nephritis. However, the frequency of IgAN was increased after COVID-19 vaccination. It is speculated that the mechanism of IgAN after COVID-19 vaccination is a multi-hit process caused by a combination of pre-existing IgA1 bearing galactose-deficient O-glycans and an increase in spike antigen-specific IgA levels after COVID-19 vaccination [10, 21]. Based on this hypothesis, the incidence of IgAN relapse is likely to increase after COVID-19 vaccination.
Mucosal IgA is produced in the early stage and shows more potent neutralizing capacity than that of systemic IgG in immune responses against SARS-CoV-2 infection [22]. Therefore, mucosal immunity is the main target for development of new COVID-19 vaccines [23–25]. Since the development of vaccines targeting mucosal immunity continues, our results could contribute to the detection and monitoring of IgAN following COVID-19 vaccination. The incidence rate of IgAN in Asian countries is higher than that in European countries. The results of this study could influence decision-making regarding COVID-19 vaccination and make clinicians aware of the need to screen patients with gross hematuria for IgAN.
Our study has several limitations. First, a high incidence of systemic AEs has been reported for the COVID-19 mRNA vaccine, and the incidence of AEs is higher with the second dose than that with the first dose [26, 27]; however, in this study, it was unclear whether the cases had an onset after the first or the second dose of the vaccine. Therefore, our study could not clarify whether the vaccine booster relationship and dosing gap affect the frequency of IgAN. Second, the generic drug name “Coronavirus Modified Uridine RNA Vaccine (SARS-CoV-2)” defined as COVID-19 mRNA vaccine does not distinguish between Pfizer-BioNTech and Moderna vaccines. The further studies with data recorded all types of vaccination should be needed to support our results. Third, the association between the IgAN and COVID-19 vaccination was based on the diagnosis of physicians who reported cases to JADER; however spontaneous reporting systems such as JADER are passive reporting systems. There are subject to many biases, including under-reporting, over-reporting, and confounding by comorbidities. To avoid these biases, further studies should compare the incidence rate of IgAN in populations of people who have undergone COVID-19 mRNA vaccination with that of unvaccinated people. In future studies, the analyses should be adjusted for comorbidities and other medications which affect the immune system to minimize the effect of confounding by these factors. In addition, analyses should be stratified by the vaccine type and the number of doses of vaccine received.
Conclusion
The analyses revealed that COVID-19 vaccination is associated with an increased incidence of IgAN. Monitoring of gross hematuria following COVID-19 vaccination is needed. In addition, further studies should be conducted to confirm our findings.
Novelty of the work
This study investigated the frequency of IgAN after the COVID-19 vaccination using the large nationwide Japanese Adverse Drug Event Report (JADER) database. We revealed the association between the onset of IgAN and COVID-19 vaccination by using statistical methods.
Data availability statement
The datasets presented in this article are not readily available because The Pharmaceuticals and Medical Devices Agency, which owns this data, does not permit the direct sharing of the data. JADER is a freely available public database. Requests to access the datasets should be directed to https://www.pmda.go.jp/english/index.html.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HN, TK, KT, and TM designed the study. HN, KK, and TK surveyed the JADER database and performed the statistical analyses. HN, TK, and TM drafted the manuscript. SY, NT, and KT reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Otsuka Pharmaceutical Factory, Inc. for English editing and article publication through scholarship donations. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Editage (https://www.editage.com/) for English language editing.
Abbreviations
AE, adverse event; CI, confidence interval; COVID-19, coronavirus disease 2019; IC, information component; IgAN, IgA nephropathy; JADER, Japanese adverse drug event report; MedDRA, medical dictionary for regulatory activities; PMDA, pharmaceuticals and medical devices agency; PT, preferred term; ROR, reporting odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TTO, time to onset.
References
1. Pardi, N, Hogan, MJ, Naradikian, MS, Parkhouse, K, Cain, DW, Jones, L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med (2018) 215(6):1571–88. doi:10.1084/jem.20171450
2. Jackson, LA, Anderson, EJ, Rouphael, NG, Roberts, PC, Makhene, M, Coler, RN, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med (2020) 383(20):1920–31. doi:10.1056/nejmoa2022483
3. Lebedev, L, Sapojnikov, M, Wechsler, A, Varadi-Levi, R, Zamir, D, Tobar, A, et al. Minimal change disease following the pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis (2021) 78(1):142–5. doi:10.1053/j.ajkd.2021.03.010
4. Negrea, L, and Rovin, BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int (2021) 99(6):1487. doi:10.1016/j.kint.2021.03.002
5. Maas, RJ, Gianotten, S, and van der Meijden, WAG. An additional case of minimal change disease following the pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis (2021) 78(2):312. doi:10.1053/j.ajkd.2021.05.003
6. D'Agati, VD, Kudose, S, Bomback, AS, Adamidis, A, and Tartini, A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int (2021) 100(2):461–3. doi:10.1016/j.kint.2021.04.035
7. Rahim, SEG, Lin, JT, and Wang, JC. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int (2021) 100(1):238. doi:10.1016/j.kint.2021.04.024
8. Perrin, P, Bassand, X, Benotmane, I, and Bouvier, N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int (2021) 100(2):466–8. doi:10.1016/j.kint.2021.05.022
9. Nakatani, S, Mori, K, Morioka, F, Hirata, C, Tsuda, A, Uedono, H, et al. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: Case report. CEN Case Rep (2022) 11(3):358–62. doi:10.1007/s13730-021-00677-9
10. Ma, Y, and Xu, G. New-onset IgA nephropathy following COVID-19 vaccination. QJM (2023) 116(1):26–39. doi:10.1093/qjmed/hcac185
11. Tanaka, H, Ohyama, K, Horikomi, Y, and Ishii, T. Association between anaphylaxis and anti-influenza drug use: An analysis of the Japanese Adverse Drug Event Report database. Drug Discoveries Ther (2021) 15(3):150–5. doi:10.5582/ddt.2021.01053
12. Tanaka, J, Koseki, T, Kondo, M, Ito, Y, and Yamada, S. Analyses of ocular adverse reactions associated with anticancer drugs based on the Japanese pharmacovigilance database. Anticancer Res (2022) 42(9):4439–51. doi:10.21873/anticanres.15944
13. Kato, K, Mizuno, T, Koseki, T, Ito, Y, Takahashi, K, Tsuboi, N, et al. Frequency of immune checkpoint inhibitor-induced vasculitides: An observational study using data from the Japanese adverse drug event report database. Front Pharmacol (2022) 13:803706. doi:10.3389/fphar.2022.803706
14. Sugawara, H, Uchida, M, Suzuki, S, Suga, Y, Uesawa, Y, Nakagawa, T, et al. Analyses of respiratory depression associated with opioids in cancer patients based on the Japanese adverse drug event report database. Biol Pharm Bull (2019) 42(7):1185–91. doi:10.1248/bpb.b19-00105
15. Kato, K, Mizuno, T, Koseki, T, Ito, Y, Hatano, M, Takahashi, K, et al. Concomitant proton pump inhibitors and immune checkpoint inhibitors increase nephritis frequency. In Vivo (2021) 35(5):2831–40. doi:10.21873/invivo.12570
16. Mizokami, F, and Mizuno, T. Acute kidney injury induced by antimicrobial agents in the elderly: Awareness and mitigation strategies. Drugs Aging (2015) 32(1):1–12. doi:10.1007/s40266-014-0232-y
17. Yokoyama, H, Narita, I, Sugiyama, H, Nagata, M, Sato, H, Ueda, Y, et al. Drug-induced kidney disease: A study of the Japan renal biopsy registry from 2007 to 2015. Clin Exp Nephrol (2016) 20(5):720–30. doi:10.1007/s10157-015-1201-4
18. Komatsu, H, Fujimoto, S, Yoshikawa, N, Kitamura, H, Sugiyama, H, and Yokoyama, H. Clinical manifestations of henoch-schönlein purpura nephritis and IgA nephropathy: Comparative analysis of data from the Japan renal biopsy registry (J-RBR). Clin Exp Nephrol (2016) 20(4):552–60. doi:10.1007/s10157-015-1177-0
19. Matsuzaki, K, Aoki, R, Nihei, Y, Suzuki, H, Kihara, M, Yokoo, T, et al. Gross hematuria after SARS-CoV-2 vaccination: Questionnaire survey in Japan. Clin Exp Nephrol (2022) 26(4):316–22. doi:10.1007/s10157-021-02157-x
20. Watanabe, S, Zheng, S, and Rashidi, A. IgA nephropathy relapse following COVID-19 vaccination treated with corticosteroid therapy: Case report. BMC Nephrol (2022) 23(1):135. doi:10.1186/s12882-022-02769-9
21. Abramson, M, Mon-Wei Yu, S, Campbell, KN, Chung, M, and Salem, F. IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med (2021) 3(5):860–3. doi:10.1016/j.xkme.2021.05.002
22. Park, JH, and Lee, HK. Delivery routes for COVID-19 vaccines. Vaccines (Basel) (2021) 9(5):524. doi:10.3390/vaccines9050524
23. Yang, J, Liu, MQ, Liu, L, Li, X, Xu, M, Lin, H, et al. A triple-RBD-based mucosal vaccine provides broad protection against SARS-CoV-2 variants of concern. Cell Mol Immunol (2022) 19(11):1279–89. doi:10.1038/s41423-022-00929-3
24. Havervall, S, Marking, U, Svensson, J, Greilert-Norin, N, Bacchus, P, Nilsson, P, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med (2022) 387(14):1333–6. doi:10.1056/nejmc2209651
25. Lambiase, A, Sacchetti, M, Mallone, F, Tirassa, P, Greco, A, Angeloni, A, et al. Evaluation of the effectiveness of BNT162b2 primary vaccination and booster dose to SARS-CoV-2 in eliciting stable mucosal immunity. Biomedicines (2022) 10(10):2430. doi:10.3390/biomedicines10102430
26. Polack, FP, Thomas, SJ, Kitchin, N, Absalon, J, Gurtman, A, Lockhart, S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2020) 383(27):2603–15. doi:10.1056/nejmoa2034577
Keywords: COVID-19, mRNA vaccine, IgA nephropathy, Japanese adverse drug event report, reporting odds ratio
Citation: Nakao H, Koseki T, Kato K, Yamada S, Tsuboi N, Takahashi K and Mizuno T (2023) COVID-19 mRNA vaccination is associated with IgA nephropathy: an analysis of the Japanese adverse drug event report database. J. Pharm. Pharm. Sci 26:11453. doi: 10.3389/jpps.2023.11453
Received: 07 April 2023; Accepted: 19 June 2023;
Published: 30 June 2023.
Edited by:
Fakhreddin Jamali, University of Alberta, CanadaCopyright © 2023 Nakao, Koseki, Kato, Yamada, Tsuboi, Takahashi and Mizuno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Mizuno, dG9tb2hpcm8ubWl6dW5vQGZ1aml0YS1odS5hYy5qcA==
†These authors have contributed equally to this work
 Hiroka Nakao1†
Hiroka Nakao1† Takenao Koseki
Takenao Koseki Tomohiro Mizuno
Tomohiro Mizuno