Abstract
We aimed to identify plasma biomarkers that predict changes in bone mineral density (BMD) and increase the understanding of impaired BMD after heart transplantation (HT). Twenty-eight adult patients were included. Data, including densitometry and 29 plasma proteins, before and 1 year after HT were analyzed. Pre-HT plasma levels of fibroblast growth factor 23 (FGF23) correlated with post-HT T score in lumbar spine, adjusted for age, gender, and BMI (1.72 [95% CI 1.33; 2.22], p = 0.011). Change (∆; post-HT—pre-HT) in plasma levels of melusin correlated to ∆T score from the lumbar spine (p = 0.028). ∆plasma levels of TR-AP, ITGB2, and Stromelysin-1 correlated to ∆T score from the femoral neck (p < 0.05). However, no correlations remained after adjustments for age, gender, and BMI. In conclusion, elevated plasma FGF23 pre-HT predicted an increase in lumbar BMD after HT. However, the results are surprising since FGF23 is known to be inversely correlated with BMD. This may partly be explained by the complex pathophysiology in this particular cohort. Due to the explorative nature of the study and the small sample size, further investigations of biochemical markers on bone metabolism in this patient population are encouraged.
Introduction
Osteoporosis is a common condition in patients who have undergone heart transplantation (HT) [1]. It may arise as a side effect of the immunosuppressive therapy given after HT, or as a consequence of various factors related to the heart failure prior to HT, including immobilization, impaired renal function, and heart failure medications [2-7]. Osteoporosis increases the risk of bone fractures which increase morbidity and mortality rates, of which the excess mortality rate within the first year after a hip fracture has been found to range from 8.4% to 36% [8-10]. Also, about 50% of patients who suffer a hip fracture are not able to walk independently afterwards long-term [10]. It has previously been reported that the mortality rate increases 1.5-fold for each standard deviation (SD) decrease in bone mineral density (BMD) in patients with osteoporosis [11]. Hence, impaired bone health constitutes a major limitation for survival and quality of life after HT. Early identification and treatment of osteoporosis are therefore of great clinical interest.
Emerging indicators of bone disease are biochemical markers which reflect the dynamics of bone metabolism, i.e., the process of bone formation and bone resorption [12]. Markers for bone formation reflect the function and recruitment of osteoblasts, including alkaline phosphatase (total and bone-specific), osteocalcin, and procollagen type I N-terminal propeptide, which all can be measured in plasma [13]. Markers for bone resorption, on the other hand, reflect the byproducts of osteoclast activity and include hydroxyproline, pyridinoline, and deoxypyridinoline, which are found in urine, whereas N-terminal and C-terminal crosslinked peptides can be found in both plasma and urine [13].
The current gold standard for assessment of bone strength is BMD which is measured using Dual-energy X-ray absorptiometry (DXA) [8]. Although DXA is widely available and provides a non-invasive method of bone strength assessment, it is also considered a static measurement of bone strength and a relatively expensive investigation, with a reported median cost of $98 per investigation in the Unites States in 2010 [13, 14]. It has been hypothesized that biochemical markers of bone metabolism may prove to be more useful than DXA as they are non-invasive, relatively inexpensive, and due to increasing availability of clinical chemistry analyzers in laboratories [15].
Whether biochemical biomarkers on bone metabolism before HT are useful in assessing bone health after HT is, however, unclear. Therefore, we aimed to identify plasma biomarkers that may predict changes in BMD and increase the understanding of impaired BMD after HT.
Patients and Methods
Study Design and Patient Selection
In the present observational cohort study, 29 patients with advanced heart failure were enrolled between October 2011 and July 2015. Patients were evaluated before and 1-year after HT, during the routine clinical evaluations at Skåne University Hospital, Lund, Sweden. Inclusion criteria were adult patients (≥18 years old) available in Lund Cardio Pulmonary Registry (LCPR), a prospective cohort of blood samples and clinical data, and a part of Region Skåne Biobank. Blood samples were collected at the time of inclusion and at the 1-year follow-up. Diagnostic and transplantation procedures were conducted at Skåne University hospital in Lund, Sweden, in accordance with the prevailing guidelines of The International Society for Heart and Lung Transplantation at the time of inclusion [16, 17].
Written informed consents were acquired from all patients upon enrollment. The study was approved by the local ethical board in Lund, Sweden (diary numbers: 2010/114; 2010/442; 2011/368; 2011/777; 2014/92 and 2015/270) and was conducted in agreement with the declarations of Helsinki and Istanbul.
Blood Sampling and Protein Analysis
Between October 2011 and February 2017, venous, non-fasting, blood samples were collected in ethylenediaminetetraacetic acid (EDTA) vacutainer tubes from patients during the routine clinical evaluations before- and at the 1-year follow-up after HT. The blood samples were thereafter centrifuged at 2,000 rpm × 10 min at 20°C and plasma aliquots subsequently stored in LCPR at −80°C.
Twenty-nine proteins related to bone metabolism were analysed in May 2017 using the following multiplex immunoassay panels (Proseek Multiplex cardiovascular II, cardiovascular III and Oncology II panels, Olink Proteomics, Uppsala, Sweden). Proximity extension assay is based on protein specific oligonucleotide-linked antibodies and quantitative microfluidic PCR for protein detection. When a pair of antibodies are in proximity due to binding to the target protein, their respective oligonucleotide strands hybridize, forming a protein-unique DNA reporter sequence, which is subsequently used to quantify the proteins using real-time PCR [18].
The twenty-nine proteins analysed were cadherin-5, CCN family member 1 (CCN1), collagen alpha-1(I) chain (COL1A1), decorin, fibroblast growth factor 23 (FGF23), glypican-1, integrin alpha-V (ITGAV), integrin beta-2 (ITGB2), integrin beta-5 (ITGB5), matrilysin, matrix extracellular phosphoglycoprotein (MEPE), matrix metalloproteinase (MMP)2, MMP9, MMP12, melusin, metalloproteinase inhibitor 4 (TIMP4), osteoclast-associated immunoglobulin-like receptor (hOSCAR), osteonectin, osteopontin, osteoprotegerin, perlecan, prolargin, receptor activator of nuclear factor κ-B (RANK), stromelysin-1, syndecan-1, tartrate-resistant acid phosphatase type 5 (TR-AP), thrombospondin-2, transmembrane glycoprotein NMB (GPNMB), and WNT1-inducible-signaling pathway protein 1 (WISP1).
The proteins’ levels were expressed in a log2 normalized protein expression scale (NPX) as arbitrary units, corresponding to the inverted Ct-values, unless otherwise stated, i.e., linear NPX [18]. All panels are validated regarding sensitivity, dynamic range, specificity, precision, and scalability. Information about panel specific validation can be found at www.olink.com/downloads.
Bone Mineral Density and Other Data Collection
Measurements of BMD was collected from clinical records during the transplantation assessment before HT and from the routine check-up 1 year after HT. BMD was expressed in T score (SD) and was obtained using DXA from the lumbar spine and femoral neck.
Other data collected included age (recipient), gender, body mass index (BMI [kg/m2]), primary indications for HT, and administration of systemic corticosteroids (CS). Glomerular filtration rate (GFR [ml/min/1.73 m2]) was based on measurement of iohexol clearance or serum levels of creatinine (i.e., estimated [e]GFR). The eGFR was calculated using the CKD-EPI formula, in accordance with the current guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) working formulation [19].
Study Setup
To explore the predictive value of protein levels and BMD, correlations between pre-HT protein levels and post-HT T score in lumbar spine and femoral neck were performed. Next, to reflect the dynamics of protein levels in relation to the dynamics of BMD, correlations of Δ (delta; post-HT—pre-HT values) protein levels vs. ΔT score in lumbar spine and femoral neck were performed.
Statistical Analysis
Linear regression models were employed to describe the relation between each of the plasma protein levels pre-HT and T score from the lumbar spine and femoral neck post-HT, respectively. Similarly, the relation between Δplasma protein levels and ΔT score from the lumbar spine and femoral neck was investigated in linear regression models. We adjusted for multiple testing using the Benjamini and Hochberg (false discovery rate) correction (Q = 5%). We used Pearson correlation coefficients to evaluate the relationship between pre-HT plasma protein levels and post-HT T scores as well as the relationship between Δplasma protein levels and ΔT scores. Simple linear regressions were calculated in order to predict pre-HT plasma levels of FGF23 by GFR based on iohexol clearance and serum levels of creatinine. All analyses were performed in R v.4.1 (R Core Development Team 2021), and a p-value of <0.05 was considered statistically significant. The median and interquartile range (IQR) were calculated for continuous variables.
Results
Study Population
Of the 29 patients, one was retransplanted and was therefore excluded. Of the remaining included 28, pre-HT data was collected at a median of 115 (70; 237) days before HT and post-HT data was collected at a median of 395 (369; 429) days after HT. The most frequent primary indication for HT was dilated cardiomyopathy (68%). Baseline characteristics, as well as follow-up data 1 year after HT, are displayed in Table 1.
TABLE 1
| Recipient characteristics | Pre-HT | Post-HT | ||||
|---|---|---|---|---|---|---|
| N = 28 | Missing | N = 28 | Missing | |||
| Age (years) | 50 | (45; 60) | 0 | 52 | (47; 62) | 0 |
| Female, N (%) | 6 | (21) | 0 | 6 | (21) | 0 |
| BMI (kg/m2) | 27 | (24; 28) | 1 | 26 | (23; 30) | 0 |
| Serum creatinine (µmol/L) | 106 | (88; 121) | 0 | 114 | (97; 142) | 0 |
| Creatinine based eGFR | 65 | (57; 82) | 0 | 54 | (45; 75) | 0 |
| Iohexol-GFR (ml/min/1.73 m2) | 56 | (45; 69) | 13 | 53 | (46; 78) | 2 |
| Daily administration of systemic CS, N (%) | 1 | (4) | 0 | 27 | (96) | 0 |
| Primary indication for HT | 0 | |||||
| Dilated cardiomyopathy | 19 | (68) | ||||
| Hypertrophic cardiomyopathy | 2 | (7) | ||||
| Ischemic cardiomyopathy | 2 | (7) | ||||
| Other | 5 | (18) | ||||
| BMD (g/m2) | ||||||
| Lumbar spine | 1.135 | (1.028; 1.272) | 1.113 | (0.944; 1.188) | 3 | |
| Femoral neck | 1.001 | (0.946; 1.063) | 0.904 | (0.818; 0.966) | 3 | |
| T score (SD) | ||||||
| Lumbar spine | −0.7 | (−1.6; 0.4) | −1.0 | (−2.3; −0.2) | 2 | |
| Femoral neck | −0.7 | (−1.0; −0.1) | −1.4 | (−1.9; −0.9) | 2 | |
Patient characteristics.
Values for continuous variables are expressed as median (IQR), whereas categorical values are expressed as number (%). BMD, bone mineral density; BMI, body mass index; CS, corticosteroids; HT, heart transplantation; (e)GFR, (estimated) glomerular filtration rate; IQR, interquartile range; SD, standard deviation.
Maintenance Immunosuppressive Therapy
Immunosuppressive agents were tapered after HT in accordance with local guidelines, previously described elsewhere [20]. A total of 64% received a combination of prednisolone, tacrolimus, and mycophenolate mofetil; 14% received prednisolone, cyclosporine, and mycophenolate mofetil; 14% received prednisolone, tacrolimus, and azathioprine; whereas 7% received other combinations. Only one patient was completely free of systemic corticosteroids at the 1-year post-HT check-up.
Pre-HT FGF23 Correlated Independently With Post-HT T Score in the Lumbar Spine
In linear regression analyses, pre-HT plasma levels of FGF23 correlated with post-HT T score in lumbar spine adjusted for age, gender, and BMI (1.72 [95% CI 1.33; 2.22], p = 0.011). All correlations between pre-HT levels of proteins and post-HT T score from the lumbar spine and femoral neck are presented in Table 2. Protein levels from both pre- and post-HT are displayed in boxplots in Figure 1 (FGF23) and in Supplementary Figure S1 (remainder). Correlations between pre-HT plasma protein levels and post-HT T score from the lumbar spine and femoral neck are shown in Supplementary Figure S2.
TABLE 2
| (A) Plasma protein | β | (95% CI) | p | Adjusted pa |
|---|---|---|---|---|
| FGF23 | 1.72 | (1.33; 2.22) | <0.001* | 0.011* |
| Osteopontin | 2.44 | (1.40; 4.27) | 0.005* | 0.066 |
| Osteoprotegerin | 3.21 | (1.30; 7.91) | 0.018* | 0.111 |
| Perlecan | 2.24 | (1.22; 4.11) | 0.016* | 0.111 |
| RANK | 2.02 | (1.17; 3.49) | 0.019* | 0.111 |
| COL1A1 | 2.20 | (1.14; 4.27) | 0.028* | 0.135 |
| ITGB2 | 2.18 | (1.03; 4.63) | 0.053 | 0.219 |
| Melusin | 1.22 | (1.00; 1.50) | 0.066 | 0.240 |
| WISP1 | 1.78 | (0.94; 3.36) | 0.087 | 0.258 |
| ITGB5 | 2.32 | (0.92; 5.88) | 0.089 | 0.258 |
| Stromelysin-1 | 1.51 | (0.93; 2.43) | 0.107 | 0.259 |
| MEPE | 1.79 | (0.91; 3.54) | 0.106 | 0.259 |
| MMP2 | 2.59 | (0.80; 8.42) | 0.126 | 0.261 |
| MMP9 | 1.52 | (0.92; 2.51) | 0.117 | 0.261 |
| Prolargin | 3.43 | (0.64; 18.40) | 0.164 | 0.317 |
| Syndecan-1 | 1.71 | (0.79; 3.70) | 0.184 | 0.334 |
| Matrilysin | 1.39 | (0.78; 2.47) | 0.270 | 0.459 |
| TIMP4 | 1.48 | (0.73; 2.99) | 0.285 | 0.459 |
| Osteonectin | 2.10 | (0.46; 9.58) | 0.349 | 0.533 |
| Glypican-1 | 1.60 | (0.54; 4.72) | 0.403 | 0.584 |
| ITGAV | 1.85 | (0.35; 9.80) | 0.478 | 0.660 |
| Cadherin-5 | 1.38 | (0.47; 4.07) | 0.566 | 0.746 |
| Decorin | 1.25 | (0.50; 3.13) | 0.631 | 0.766 |
| HOSCAR | 1.52 | (0.28; 8.32) | 0.634 | 0.766 |
| TR-AP | 0.83 | (0.32; 2.16) | 0.703 | 0.778 |
| Thrombospondin-2 | 0.79 | (0.22; 2.90) | 0.724 | 0.778 |
| MMP12 | 1.08 | (0.76; 1.54) | 0.681 | 0.778 |
| GPNMB | 1.11 | (0.16; 7.57) | 0.917 | 0.950 |
| CCN1 | 1.00 | (0.41; 2.44) | 0.994 | 0.994 |
| (B) Plasma protein | ||||
| Melusin | 1.15 | (1.02; 1.28) | 0.029* | 0.758 |
| GPNMB | 0.40 | (0.14; 1.12) | 0.095 | 0.758 |
| HOSCAR | 0.44 | (0.18; 1.12) | 0.100 | 0.758 |
| ITGB5 | 1.54 | (0.90; 2.66) | 0.130 | 0.758 |
| Osteopontin | 1.33 | (0.92; 1.92) | 0.138 | 0.758 |
| COL1A1 | 1.35 | (0.90; 2.03) | 0.159 | 0.758 |
| Thrombospondin-2 | 0.60 | (0.29; 1.24) | 0.183 | 0.758 |
| FGF23 | 1.10 | (0.91; 1.33) | 0.313 | 0.947 |
| Syndecan-1 | 0.82 | (0.52; 1.29) | 0.405 | 0.947 |
| MMP12 | 0.92 | (0.75; 1.13) | 0.436 | 0.947 |
| ITGB2 | 1.19 | (0.75; 1.90) | 0.465 | 0.947 |
| TR-AP | 0.82 | (0.47; 1.41) | 0.478 | 0.947 |
| Prolargin | 0.72 | (0.26; 1.96) | 0.525 | 0.947 |
| Osteonectin | 1.33 | (0.55; 3.23) | 0.531 | 0.947 |
| Stromelysin-1 | 1.08 | (0.81; 1.44) | 0.618 | 0.947 |
| Cadherin-5 | 0.86 | (0.46; 1.61) | 0.642 | 0.947 |
| MMP9 | 1.07 | (0.79; 1.45) | 0.682 | 0.947 |
| MEPE | 0.92 | (0.61; 1.39) | 0.696 | 0.947 |
| CCN1 | 1.11 | (0.66; 1.85) | 0.704 | 0.947 |
| Perlecan | 0.93 | (0.63; 1.38) | 0.732 | 0.947 |
| Osteoprotegerin | 1.10 | (0.61; 1.97) | 0.760 | 0.947 |
| ITGAV | 1.16 | (0.44; 3.06) | 0.767 | 0.947 |
| Matrilysin | 0.96 | (0.68; 1.34) | 0.799 | 0.947 |
| Glypican-1 | 0.93 | (0.49; 1.75) | 0.816 | 0.947 |
| RANK | 1.04 | (0.73; 1.49) | 0.819 | 0.947 |
| Decorin | 1.04 | (0.62; 1.77) | 0.874 | 0.947 |
| WISP1 | 1.02 | (0.69; 1.51) | 0.913 | 0.947 |
| MMP2 | 0.97 | (0.48; 1.99) | 0.941 | 0.947 |
| TIMP4 | 0.99 | (0.65; 1.49) | 0.947 | 0.947 |
Regression analyses between pre-HT levels of plasma proteins measured in normalized protein expression scale, expressed in AU, and post-HT T score from the lumbar spine (A) and femoral neck (B). aAdjusted with Benjamini & Hochberg (false discovery rate) correction.
Adjusted with Benjamini & Hochberg (false discovery rate) correction.
AU, arbitrary units; CCN1, CCN family member 1; CI, confidence interval; COL1A1, collagen alpha-1(I) chain; FGF23, fibroblast growth factor 23; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; MEPE, matrix extracellular phosphoglycoprotein; MMP, matrix metalloproteinase; TIMP4, metalloproteinase inhibitor 4; hOSCAR, osteoclast-associated immunoglobulin-like receptor; RANK, receptor activator of nuclear factor κ-B; TR-AP, tartrate-resistant acid phosphatase type 5; GPNMB, transmembrane glycoprotein NMB; WISP1, WNT1-inducible-signaling pathway protein 1. *Indicates statistical significance.
FIGURE 1
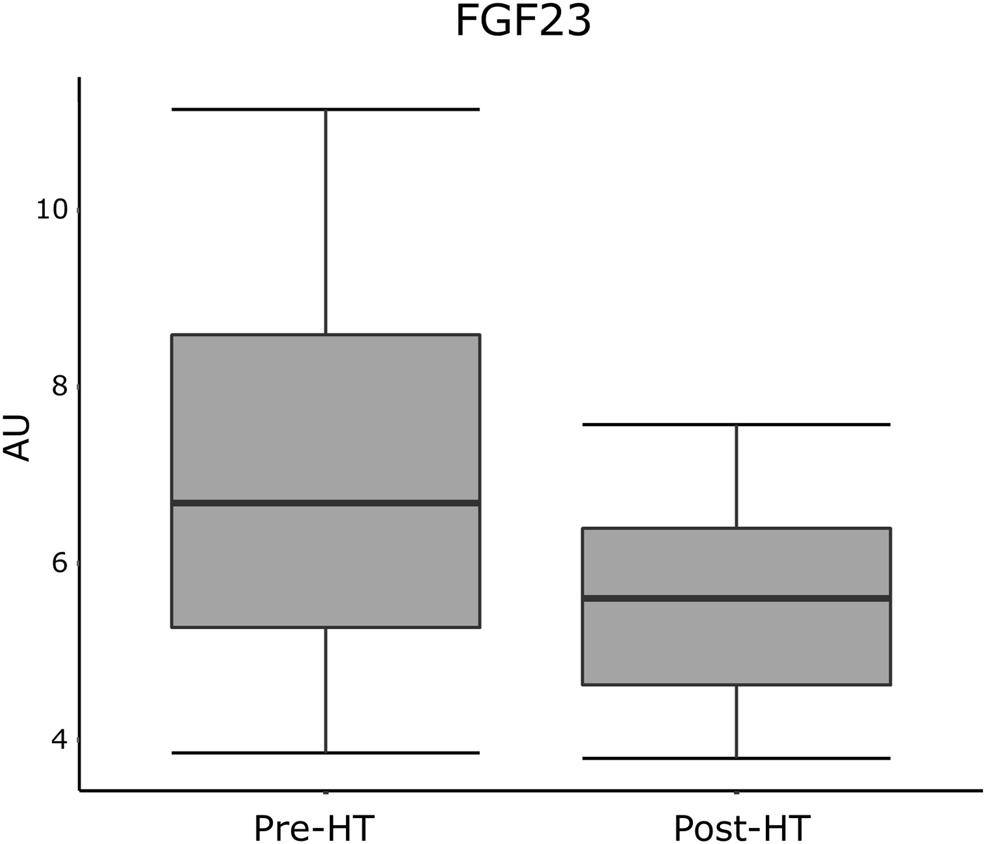
Plasma levels of FGF23 from pre- and post-HT measured in normalized protein expression scale, expressed in AU. AU, arbitrary units; FGF23, fibroblast growth factor 23; HT, heart transplantation.
In a sub -analysis, pre-HT plasma levels of FGF23 were inversely correlated with pre-HT iohexol based GFR, suggesting a FGF23 factor decrease of 0.99 (95% CI 0.97; 1.00) arbitrary units (AU) (p = 0.029). Likewise, pre-HT FGF23 levels decreased with a factor of 0.99 (95% CI 0.97; 1.00) AU by every unit increase in pre-HT creatinine-based GFR, however, this relationship was statistically not significant (p = 0.072).
Dynamics of Plasma Protein Levels in Relation to Bone Mineral Density Evolution
Regression analyses between Δplasma protein levels and ∆T score from the lumbar spine and femoral neck are shown in Table 3. In the unadjusted analysis, ∆plasma levels of melusin correlated to ∆T score from the lumbar spine (1.20 [95% CI 1.03; 1.40], p = 0.028). ∆plasma levels of TR-AP, ITGB2, and Stromelysin-1 correlated to ∆T score from the femoral neck (1.23 [95% CI 1.07; 1.42], 1.25 [95% CI 1.03; 1.52], and 0.90 [95% CI 0.81; 0.99], respectively, all with p < 0.05). However, after adjustments for age, gender, and BMI, no significant correlations remained. Correlations between Δplasma protein levels and ∆T score from the lumbar spine and femoral neck are shown in Supplementary Figure S3.
TABLE 3
| (A) Plasma protein | β | (95% CI) | p | Adjusted pa |
|---|---|---|---|---|
| ΔMelusin | 1.20 | (1.03; 1.40) | 0.028* | 0.809 |
| ΔOsteoprotegerin | 1.01 | (0.48; 2.14) | 0.974 | 0.993 |
| ΔCCN1 | 0.96 | (0.48; 1.94) | 0.912 | 0.993 |
| ΔWISP1 | 1.02 | (0.62; 1.68) | 0.935 | 0.993 |
| ΔCOL1A1 | 1.34 | (0.75; 2.39) | 0.337 | 0.993 |
| ΔITGB2 | 0.84 | (0.42; 1.69) | 0.624 | 0.993 |
| ΔITGAV | 0.53 | (0.15; 1.88) | 0.334 | 0.993 |
| ΔDecorin | 0.58 | (0.13; 2.61) | 0.485 | 0.993 |
| ΔMMP2 | 1.04 | (0.54; 2.02) | 0.908 | 0.993 |
| ΔStromelysin-1 | 0.97 | (0.67; 1.40) | 0.868 | 0.993 |
| ΔMatrilysin | 0.81 | (0.38; 1.72) | 0.589 | 0.993 |
| ΔOsteonectin | 3.25 | (0.55; 19.20) | 0.206 | 0.993 |
| ΔOsteopontin | 1.25 | (0.86; 1.81) | 0.259 | 0.993 |
| ΔTR-AP | 1.14 | (0.67; 1.94) | 0.641 | 0.993 |
| ΔMMP9 | 1.31 | (0.97; 1.77) | 0.089 | 0.993 |
| ΔITGB5 | 1.55 | (0.55; 4.35) | 0.417 | 0.993 |
| ΔSyndecan-1 | 1.23 | (0.84; 1.81) | 0.298 | 0.993 |
| ΔCadherin-5 | 0.34 | (0.09; 1.25) | 0.117 | 0.993 |
| ΔGlypican-1 | 0.84 | (0.31; 2.25) | 0.725 | 0.993 |
| ΔThrombospondin-2 | 0.77 | (0.29; 2.07) | 0.615 | 0.993 |
| ΔMMP12 | 1.02 | (0.65; 1.62) | 0.926 | 0.993 |
| ΔProlargin | 0.78 | (0.22; 2.79) | 0.706 | 0.993 |
| ΔPerlecan | 1.27 | (0.49; 3.25) | 0.628 | 0.993 |
| ΔGPNMB | 0.49 | (0.06; 3.97) | 0.512 | 0.993 |
| ΔhOSCAR | 0.88 | (0.19; 4.17) | 0.877 | 0.993 |
| ΔTIMP4 | 1.02 | (0.59; 1.77) | 0.934 | 0.993 |
| ΔFGF23 | 1.04 | (0.88; 1.22) | 0.669 | 0.993 |
| ΔMEPE | 1.00 | (0.42; 2.39) | 0.993 | 0.993 |
| ΔRANK | 1.47 | (0.86; 2.49) | 0.169 | 0.993 |
| (B) Plasma protein | ||||
| ΔTR-AP | 1.23 | (1.07; 1.42) | 0.007* | 0.189 |
| ΔITGB2 | 1.25 | (1.03; 1.52) | 0.032* | 0.435 |
| ΔStromelysin-1 | 0.90 | (0.81; 0.99) | 0.045* | 0.435 |
| ΔMMP2 | 0.83 | (0.69; 1.00) | 0.063 | 0.457 |
| ΔGPNMB | 0.60 | (0.33; 1.06) | 0.094 | 0.481 |
| ΔTIMP4 | 0.88 | (0.75; 1.03) | 0.116 | 0.481 |
| ΔFGF23 | 0.96 | (0.92; 1.01) | 0.106 | 0.481 |
| ΔOsteonectin | 1.47 | (0.88; 2.46) | 0.158 | 0.528 |
| ΔMelusin | 1.04 | (0.99; 1.09) | 0.164 | 0.528 |
| ΔProlargin | 0.77 | (0.53; 1.12) | 0.184 | 0.534 |
| ΔCCN1 | 1.14 | (0.93; 1.39) | 0.213 | 0.547 |
| ΔCOL1A1 | 0.90 | (0.76; 1.07) | 0.238 | 0.547 |
| ΔITGB5 | 1.20 | (0.89; 1.62) | 0.245 | 0.547 |
| ΔWISP1 | 0.92 | (0.80; 1.06) | 0.280 | 0.557 |
| ΔDecorin | 1.28 | (0.82; 2.01) | 0.288 | 0.557 |
| ΔITGAV | 0.83 | (0.57; 1.21) | 0.341 | 0.618 |
| ΔThrombospondin-2 | 0.88 | (0.65; 1.18) | 0.387 | 0.624 |
| ΔPerlecan | 0.88 | (0.66; 1.17) | 0.381 | 0.624 |
| ΔOsteopontin | 1.04 | (0.93; 1.17) | 0.471 | 0.719 |
| ΔMatrilysin | 0.94 | (0.75; 1.17) | 0.569 | 0.745 |
| ΔGlypican-1 | 0.92 | (0.69; 1.23) | 0.575 | 0.745 |
| ΔMMP12 | 1.04 | (0.91; 1.19) | 0.591 | 0.745 |
| ΔRANK | 1.05 | (0.89; 1.24) | 0.564 | 0.745 |
| ΔOsteoprotegerin | 0.95 | (0.75; 1.18) | 0.627 | 0.758 |
| ΔMEPE | 0.94 | (0.73; 1.22) | 0.658 | 0.763 |
| ΔSyndecan-1 | 0.98 | (0.87; 1.10) | 0.714 | 0.796 |
| ΔCadherin-5 | 0.95 | (0.63; 1.43) | 0.801 | 0.830 |
| ΔhOSCAR | 1.07 | (0.67; 1.71) | 0.791 | 0.830 |
| ΔMMP9 | 1.01 | (0.91; 1.11) | 0.910 | 0.910 |
Regression analyses between Δplasma protein levels measured in normalized protein expression scale, expressed in AU, and ∆T score from the lumbar spine (A) and femoral neck (B).
Adjusted with Benjamini & Hochberg (false discovery rate) correction.
Δ, delta (post-HT—pre-HT values); AU, arbitrary units; CCN1, CCN family member 1; CI, confidence interval; COL1A1, collagen alpha-1(I) chain; FGF23, fibroblast growth factor 23; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; MEPE, matrix extracellular phosphoglycoprotein; MMP, matrix metalloproteinase; TIMP4, metalloproteinase inhibitor 4; hOSCAR, osteoclast-associated immunoglobulin-like receptor; RANK, receptor activator of nuclear factor κ-B; TR-AP, tartrate-resistant acid phosphatase type 5; GPNMB, transmembrane glycoprotein NMB; WISP1, WNT1-inducible-signaling pathway protein 1. *Indicates statistical significance.
Discussion
Impaired BMD is commonly found in patients who have undergone HT, leading to significant impact on morbidity and mortality [9, 10]. Early risk stratification and prevention of the development of osteoporosis is therefore of great interest. Emerging indicators of bone disease are plasma bone turnover markers which reflect the dynamics of bone metabolism. Such biochemical markers are considered beneficial with regard to availability and cost-effectiveness when compared to DXA which constitutes the current gold standard method for assessment of BMD [15]. Hence, identification of biochemical markers for the prediction of osteoporosis after HT is of particular interest.
The present single-center observational cohort study aimed to identify plasma biomarkers that may predict changes in BMD and increase the understanding of impaired BMD after HT. This may enable better prediction of impaired skeletal health and improve outcome in this patient population. The present study showed that plasma levels of FGF23 before HT correlated with T score in the lumbar spine after HT, independent of age, gender, and BMI. However, no correlations between changes in plasma levels of biochemical markers and T scores were found. The findings suggest that post-HT BMD loss may be predicted by pre-HT measurements of serum FGF23.
A positive correlation was found between pre-HT levels of FGF23 and post-HT T score in lumbar spine. FGF23, mainly secreted by osteocytes and osteoblasts in bone, plays a significant role in bone mineralization by stimulating phosphaturia, as well as suppressing the production of 1,25-dihydroxyvitamin D, resulting in inhibited bone mineralization [21]. Thus, the findings of the present study are contradictory. In a study by Valentin et al. (2013) on mutant mice, it was concluded that FGF23 plasma levels strongly correlates with circulating calcium levels, suggesting that suppressed FGF23 levels protects from hypocalcemia by reduced inhibitory effect on 1,25-dihydroxyvitamin D production [22]. To our knowledge, no previous study on the correlation of FGF23 levels and BMD after HT have been conducted. However, Jovanovich et al. (2013) found in a prospective, longitudinal study of community-dwelling adults aged 65 or older, including >3,000 participants with a median follow-up of 9.6 (IQR = 5.1; 11.0) years, that FGF23 was weakly associated with increased BMD in both lumbar spine and hip, but no associations were detected between FGF23 levels and fracture risk [23]. Similarly, FGF23 correlated positively with BMD in lumbar spine and hip in a study by Marsell et al. (2008), including >3,000 male participants aged 69–80 years [24]. However, the correlations were discovered to be dependent on BMI. Thus, these results partly support those of the present study. In addition, FGF23 levels are also known to increase in relation to progression of kidney dysfunction, which is common in HT candidates [25]. In a previous study at our center, it was concluded that the occurrence of kidney dysfunction, measured by iohexol clearance, increased over time after HT, reaching 25% with CKD stage ≥4 by the fifth post-operative year [26]. It is furthermore known that DXA from the lumbar spine might be overestimated in cases of vascular calcification, which is a common feature in patients with chronic kidney disease [27, 28]. All in all, FGF23 predicted a higher lumbar T score after HT, which may partly be explained by the complex pathophysiological mechanisms in this particular patient cohort.
Plasma levels of FGF23 correlated positively with T score in the lumbar spine, but not with T score in the femoral neck. In a cross-sectional study, Rupp et al. assessed levels of FGF23 and bone microarchitecture in 82 patients with osteoporosis [29]. They concluded that increased levels of FGF23 were associated with impaired trabecular but not cortical bone microarchitecture, after adjusting for age and BMI. This is contradicting to our results, but may be partly explained by overestimations of T score in the lumbar spine, as outlined above, as well as the potential impact of renal dysfunction as pre-HT levels of FGF23 correlated with both measured and estimated GFR pre-HT.
A correlation between the change from pre-HT to post-HT in plasma levels of melusin, a muscle-specific integrin beta1-interacting protein, and the change in lumbar T score was found in the unadjusted analysis. However, no correlation remained after adjustments for age, gender, and BMI. It is well known that beta1 integrins are required for proper bone formation and homeostasis by playing a main role in the recruitment, differentiation, and mineralization of osteoblasts [30-32]. Brunner et al. (2018) reported that, for proper bone formation, beta1 integrins are required at the early stages of osteoblast differentiation in vivo [33]. Thus, the findings of the present study may reflect the pathophysiology behind beta1 integrins and their impact on bone formation.
Further, in the unadjusted analysis, the change from pre-HT to post-HT in plasma levels of ITGB2, stromelysin-1, and TR-AP correlated with the change in femoral T score. After adjustments for age, gender, and BMI, however, these correlations were lost. Although TR-AP has been considered a marker for osteoclastic activity, Halling Linder et al. (2017) demonstrated that TR-AP exhibits an inhibitory effect on osteopontin mediated mineralization delay, which is supported by the findings of this study [34, 35]. Also, ITGB2, which is involved in cell adhesion and in promoting intracellular signaling events, has been found to play a key role in the osteogenic processes [36, 37]. Miura et al. (2005) showed that mice lacking CD18, one of the members in beta-2 integrin family, exhibited features of osteoporosis, including decreased BMD, and impaired trabecular microarchitecture. This is consistent with the positive correlation between ∆plasma levels of ITGB2 and ∆T score in the femoral neck that was found in the present study [37]. Stromelysin-1 is an activator of procollagenases which promotes cartilage degeneration [38]. In a study by Blom et al. (2007), stromelysin-1-knockout mice demonstrated a significant reduction in cartilage degeneration after induction of osteoarthritis [39]. Whether stromelysin-1 has an impact on the development of osteoporosis in HT patients remains to be established.
The present study provides explorative data on novel biochemical plasma markers for bone metabolism in 28 patients after HT. The major strength of this study was the application of multiplex proximity extension assay, which is known for its high sensitivity and specificity in plasma [18]. Data was independent of fasting and was adjusted for age, gender, and BMI. Moreover, the study was performed at a single-center which facilitated data collection. Due to the explorative nature of the study, the small size of the patient cohort, as well as absence of a validation cohort, generalizability of the results is limited. Furthermore, the small size of the study restricted statistical adjustments with additional variables, such as comorbidities, medications, CS dose, time on waiting list, vitamin D intake and serum level, as well as calcium and phosphate levels in serum and urine, potentially influencing the BMD and levels of biochemical markers.
In conclusion, the present study showed that elevated plasma levels of FGF23 pre-HT predicted an increase in lumbar BMD after HT, which may be partly explained by the complex pathophysiological mechanisms in relation to the comorbid burden and immunosuppressive therapy in this patient cohort. Further investigations of biochemical markers on bone metabolism, especially FGF23, in larger HT populations are highly encouraged.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Local Ethical Board in Lund, Sweden (diary numbers: 2010/114; 2010/442; 2011/368; 2011/777; 2014/92 and 2015/270) and was conducted in agreement with the declarations of Helsinki and Istanbul. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EL: Study design, data collection, data analysis, and writing of the article. SA: Study design, data collection, and writing and reviewing of the article. AA: Study design, data collection, and writing and reviewing of the article. GR: Study design, data acquisition, and writing and reviewing of the article.
Funding
This work was funded by unrestricted research grants from the Anna-Lisa & Sven-Eric Lundgren Foundation, as well as from ALF's Foundation, Lund, Sweden. The contributors had no role in the collection, analysis or interpretation of the data, and had no right to restrict the dissemination or publication of the results.
Acknowledgments
We acknowledge the support of the staff at the Hemodynamic Lab, the Section for Heart Failure and Valvular Disease, VO. Heart and Lung Medicine, Skåne University Hospital, Lund, Sweden, and the Department of Clinical Sciences Lund, Cardiology, at Lund University, Sweden. Exceptional gratitude to Anna Åkesson for her statistical expertise which was invaluable during the analysis and interpretation of the data. We also acknowledge the biobank services and retrieval of blood samples from LCPR at Lab medicine Skåne, University and Regional Laboratories, Region Skåne, Sweden. A special thanks to Anneli Ahlqvist for her efforts in LCPR administration and blood sample management.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10161/full#supplementary-material
Supplementary Figure 1Plasma levels of proteins from pre- and post-HT measured in normalized protein expression scale, expressed in AU. AU, arbitrary units; CCN1, CCN family member 1; COL1A1, collagen alpha-1(I) chain; HT, heart transplantation; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; MEPE, matrix extracellular phosphoglycoprotein; MMP, matrix metalloproteinase; TIMP4, metalloproteinase inhibitor 4; hOSCAR, osteoclast-associated immunoglobulin-like receptor; RANK, receptor activator of nuclear factor κ-B; TR-AP, tartrate-resistant acid phosphatase type 5; GPNMB, transmembrane glycoprotein NMB; WISP1, WNT1-inducible-signaling pathway protein 1.
Supplementary Figure 2(A-AC) Correlations between pre-HT plasma protein levels measured in normalized protein expression scale, expressed in AU, and post-HT lumbar and femoral T scores. AU, arbitrary units; CCN1, CCN family member 1; COL1A1, collagen alpha-1(I) chain; FGF23, fibroblast growth factor-23; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; MEPE, matrix extracellular phosphoglycoprotein; MMP, matrix metalloproteinase; TIMP4, metalloproteinase inhibitor 4; hOSCAR, osteoclast-associated immunoglobulin-like receptor; RANK, receptor activator of nuclear factor κ-B; TR-AP, tartrate-resistant acid phosphatase type 5; GPNMB, transmembrane glycoprotein NMB; WISP1, WNT1-inducible-signaling pathway protein 1.
Supplementary Figure 3Correlations between Δplasma protein levels measured in normalized protein expression scale, expressed in AU, and ∆T score from the lumbar spine and femoral neck. AU, arbitrary units; CCN1, CCN family member 1; COL1A1, collagen alpha-1(I) chain; FGF23, fibroblast growth factor-23; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; MEPE, matrix extracellular phosphoglycoprotein; MMP, matrix metalloproteinase; TIMP4, metalloproteinase inhibitor 4; hOSCAR, osteoclast-associated immunoglobulin-like receptor; RANK, receptor activator of nuclear factor κ-B; TR-AP, tartrate-resistant acid phosphatase type 5; GPNMB, transmembrane glycoprotein NMB; WISP1, WNT1-inducible-signaling pathway protein 1.
Abbreviations
AU, arbitrary units; BMD, bone mineral density; BMI, body mass index; CCN1, CCN family member 1; CI, confidence interval; COL1A1, collagen alpha-1(I) chain; CS, corticosteroids; DXA, Dual-energy X-ray absorptiometry; EDTA, ethylenediaminetetraacetic acid; FGF23, fibroblast growth factor 23; GFR, glomerular filtration rate; GPNMB, transmembrane glycoprotein NMB; hOSCAR, osteoclast-associated immunoglobulin-like receptor; HT, heart transplantation; ITGAV, integrin alpha-V; ITGB2, integrin beta-2; ITGB5, integrin beta-5; IQR, interquartile range; LCPR, Lund Cardio Pulmonary Registry; MEPE, matrix extracellular phosphoglycoprotein; MMP2, matrix metalloproteinase-2; MMP9, matrix metalloproteinase-9; MMP12, macrophage metalloelastase; RANK, receptor activator of nuclear factor κ-B; SD, standard deviation; TIMP4, metalloproteinase inhibitor 4; TR-AP, tartrate-resistant acid phosphatase type 5; WISP-1, WNT1-inducible-signaling pathway protein 1.
References
1.
Rodino MA Shane E . Osteoporosis after Organ Transplantation. Am J Med (1998). 104:459–69. 10.1016/s0002-9343(98)00081-3
2.
Aluoch AO Jessee R Habal H Garcia-Rosell M Shah R Reed G et al Heart Failure as a Risk Factor for Osteoporosis and Fractures. Curr Osteoporos Rep (2012). 10:258–69. 10.1007/s11914-012-0115-2
3.
Lim LS Fink HA Blackwell T Taylor BC Ensrud KE . Loop Diuretic Use and Rates of Hip Bone Loss and Risk of Falls and Fractures in Older Women. J Am Geriatr Soc (2009). 57:855–62. 10.1111/j.1532-5415.2009.02195.x
4.
Gajic-Veljanoski O Phua CW Shah PS Cheung AM . Effects of Long-Term Low-Molecular-Weight Heparin on Fractures and Bone Density in Non-pregnant Adults: A Systematic Review with Meta-Analysis. J Gen Intern Med (2016). 31:947–57. 10.1007/s11606-016-3603-8
5.
Kwok T Leung J Leung J Zhang YF Bauer D Ensrud KE et al Does the Use of ACE Inhibitors or Angiotensin Receptor Blockers Affect Bone Loss in Older Men? Osteoporos Int (2012). 23:2159–67. 10.1007/s00198-011-1831-7
6.
WHO. Prevention and Management of Osteoporosis : Report of a WHO Scientific Group. (2003). (WHO: Geneva, Switzerland).
7.
Löfdahl E Rådegran G . Osteoporosis Following Heart Transplantation and Immunosuppressive Therapy. Transplant Rev (2017). 31:232–9. 10.1016/j.trre.2017.08.002
8.
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA (2001). 285(6):785–95. 10.1001/jama.285.6.785
9.
Abrahamsen B van Staa T Ariely R Olson M Cooper C . Excess Mortality Following Hip Fracture: a Systematic Epidemiological Review. Osteoporos Int (2009). 20:1633–50. 10.1007/s00198-009-0920-3
10.
Cummings SR Melton LJ . Epidemiology and Outcomes of Osteoporotic Fractures. The Lancet (2002). 359:1761–7. 10.1016/s0140-6736(02)08657-9
11.
Teng GG Curtis JR Saag KG . Mortality and Osteoporotic Fractures: Is the Link Causal, and Is it Modifiable?Clin Exp Rheumatol (2008). 26:S125–37.
12.
Florencio-Silva R Sasso GR Sasso-Cerri E Simões MJ Cerri PS . Biology of Bone Tissue: Structure, Function, and Factors that Influence Bone Cells. Biomed Res Int (2015). 2015:421746. 10.1155/2015/421746
13.
Shetty S Kapoor N Bondu JD Thomas N Paul TV . Bone Turnover Markers: Emerging Tool in the Management of Osteoporosis. Indian J Endocrinol Metab (2016). 20:846–52. 10.4103/2230-8210.192914
14.
Nayak S Roberts MS Greenspan SL . Cost-effectiveness of Different Screening Strategies for Osteoporosis in Postmenopausal Women. Ann Intern Med (2011). 155:751–61. 10.7326/0003-4819-155-11-201112060-00007
15.
Burch J Rice S Yang H Neilson A Stirk L Francis R et al Systematic Review of the Use of Bone Turnover Markers for Monitoring the Response to Osteoporosis Treatment: the Secondary Prevention of Fractures, and Primary Prevention of Fractures in High-Risk Groups. Health Technol Assess (2014). 18:1–180. 10.3310/hta18110
16.
Costanzo MR Dipchand A Starling R Anderson A Chan M Desai S et al The International Society of Heart and Lung Transplantation Guidelines for the Care of Heart Transplant Recipients. J Heart Lung Transpl (2010). 29:914–56. 10.1016/j.healun.2010.05.034
17.
Mehra M Kobashigawa J Starling R Russell S Uber P Parameshwar J et al Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates-2006. J Heart Lung Transplant (2006). 25:1024–42. 10.1016/j.healun.2006.06.008
18.
Assarsson E Lundberg M Holmquist G Björkesten J Bucht Thorsen S Ekman D et al Homogenous 96-plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PloS one (2014). 9:e95192. 10.1371/journal.pone.0095192
19.
Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl (2013). 3:1.
20.
Löfdahl E Söderlund C Rådegran G . Bone mineral Density and Osteoporosis in Heart Transplanted Patients: A Single‐center Retrospective Study at Skåne University Hospital in Lund 1988‐2016. Clin Transpl (2019). 33:e13477. 10.1111/ctr.13477
21.
Guo Y-C Yuan Q . Fibroblast Growth Factor 23 and Bone Mineralisation. Int J Oral Sci (2015). 7:8–13. 10.1038/ijos.2015.1
22.
David V Dai B Martin A Huang J Han X Quarles LD . Calcium Regulates FGF-23 Expression in Bone. Endocrinology (2013). 154:4469–82. 10.1210/en.2013-1627
23.
Jovanovich A Bùžková P Chonchol M Robbins J Fink HA de Boer IH et al Fibroblast Growth Factor 23, Bone Mineral Density, and Risk of Hip Fracture Among Older Adults: The Cardiovascular Health Study. J Clin Endocrinol Metab (2013). 98:3323–31. 10.1210/jc.2013-1152
24.
Marsell R Mirza MAI Mallmin H Karlsson M Mellström D Orwoll E et al Relation between Fibroblast Growth Factor-23, Body Weight and Bone mineral Density in Elderly Men. Osteoporos Int (2009). 20:1167–73. 10.1007/s00198-008-0780-2
25.
Larsson T Nisbeth ULF Ljunggren Ö Jüppner H Jonsson KB . Circulating Concentration of FGF-23 Increases as Renal Function Declines in Patients with Chronic Kidney Disease, but Does Not Change in Response to Variation in Phosphate Intake in Healthy Volunteers. Kidney Int (2003). 64:2272–9. 10.1046/j.1523-1755.2003.00328.x
26.
Söderlund C Löfdahl E Nilsson J Reitan Ö Higgins T Rådegran G . Chronic Kidney Disease after Heart Transplantation: a Single-centre Retrospective Study at Skåne University Hospital in Lund 1988-2010. Transpl Int (2016). 29:529–39. 10.1111/tri.12710
27.
Palit S Kendrick J . Vascular Calcification in Chronic Kidney Disease: Role of Disordered mineral Metabolism. Cpd (2014). 20:5829–33. 10.2174/1381612820666140212194926
28.
Toussaint ND Lau KK Strauss BJ Polkinghorne KR Kerr PG . Associations between Vascular Calcification, Arterial Stiffness and Bone mineral Density in Chronic Kidney Disease. Nephrol Dial Transplant (2007). 23:586–93. 10.1093/ndt/gfm660
29.
Rupp T Butscheidt S Vettorazzi E Oheim R Barvencik F Amling M et al High FGF23 Levels Are Associated with Impaired Trabecular Bone Microarchitecture in Patients with Osteoporosis. Osteoporos Int (2019). 30:1655–62. 10.1007/s00198-019-04996-7
30.
Moursi AM Globus RK Damsky CH . Interactions between Integrin Receptors and Fibronectin Are Required for Calvarial Osteoblast Differentiation In Vitro. J Cel Sci. (1997). 110(Pt 18):2187–96. 10.1242/jcs.110.18.2187
31.
Wang L Zhao G Olivaresnavarrete R Bell B Wieland M Cochran D et al Integrin β1 Silencing in Osteoblasts Alters Substrate-dependent Responses to 1,25-dihydroxy Vitamin D3. Biomaterials (2006). 27:3716–25. 10.1016/j.biomaterials.2006.02.022
32.
Brunner M Millon-Frémillon A Chevalier G Nakchbandi IA Mosher D Block MR et al Osteoblast Mineralization Requires β1 integrin/ICAP-1-dependent Fibronectin Deposition. J Cel Biol (2011). 194:307–22. 10.1083/jcb.201007108
33.
Brunner M Mandier N Gautier T Chevalier G Ribba AS Guardiola P et al β1 Integrins Mediate the BMP2 Dependent Transcriptional Control of Osteoblast Differentiation and Osteogenesis. PloS one (2018). 13:e0196021. 10.1371/journal.pone.0196021
34.
Halling Linder C Ek-Rylander B Krumpel M Norgård M Narisawa S Millán JL et al Bone Alkaline Phosphatase and Tartrate-Resistant Acid Phosphatase: Potential Co-regulators of Bone Mineralization. Calcif Tissue Int (2017). 101:92–101. 10.1007/s00223-017-0259-2
35.
Andersson G Ek-Rylander B . The Tartrate-Resistant Purple Acid Phosphatase of Bone Osteoclasts-A Protein Phosphatase with Multivalent Substrate Specificity and Regulation. Acta Orthopaedica Scand (1995). 66:189–94. 10.3109/17453679509157689
36.
Thome S Begandt D Pick R Salvermoser M Walzog B . Intracellular β2integrin (CD11/CD18) Interacting Partners in Neutrophil Trafficking. Eur J Clin Invest (2018). 48:e12966. 10.1111/eci.12966
37.
Miura Y Miura M Gronthos S Allen MR Cao C Uveges TE et al Defective Osteogenesis of the Stromal Stem Cells Predisposes CD18-Null Mice to Osteoporosis. Proc Natl Acad Sci (2005). 102:14022–7. 10.1073/pnas.0409397102
38.
Lee M Shimizu E Krane SM Partridge NC . In: JPBilezikianLGRaiszTJMartin, editors. Principles of Bone Biology. 3rd ed.San Diego: Academic Press (2008). p. 367–84. Bone Proteinases. 10.1016/b978-0-12-373884-4.00038-0Chapter 19-Bone Proteinases)
39.
Blom AB van Lent PL Libregts S Holthuysen AE van der Kraan PM van Rooijen N et al Crucial Role of Macrophages in Matrix Metalloproteinase-Mediated Cartilage Destruction during Experimental Osteoarthritis : Involvement of Matrix Metalloproteinase 3. Arthritis Rheum (2007). 56:147–57. 10.1002/art.22337
Summary
Keywords
heart transplantation, plasma biomarkers, bone mineral density, osteoporosis, bone metabolism
Citation
Löfdahl E, Ahmed S, Ahmed A and Rådegran G (2022) Plasma Biomarkers for Clinical Assessment of Bone Mineral Density in Heart Transplanted Patients—A Single-Center Study at Skåne University Hospital in Lund. Transpl Int 35:10161. doi: 10.3389/ti.2022.10161
Received
29 October 2021
Accepted
10 February 2022
Published
28 March 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Löfdahl, Ahmed, Ahmed and Rådegran.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eveline Löfdahl, eveline.lofdahl@med.lu.se
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.