Abstract
Relationships between renal function and medical costs for deceased donor kidney transplant recipients are not fully quantified post-transplant. We describe these relationships with renal function measured by estimated glomerular filtration rate (eGFR) and graft failure. The United States Renal Data System identified adults receiving single-organ deceased donor kidneys 2012–2015. Inpatient, outpatient, other facility costs and eGFRs at discharge, 6 and 12 months were included. A time-history of costs was constructed for graft failures and monthly costs in the first year post-transplant were compared to those without failure. The cohort of 24,021 deceased donor recipients had a 2.4% graft failure rate in the first year. Total medical costs exhibit strong trends with eGFR. Recipients with 6-month eGFRs of 30–59 ml/min/1.73m2 have total costs 48% lower than those <30 ml/min/1.73m2. For recipients with graft failure monthly costs begin to rise 3–4 months prior to failure, with incremental costs of over $38,000 during the month of failure. Mean annual total incremental costs of graft failure are over $150,000. Total costs post-transplant are strongly correlated with eGFR. Graft failure in the first year is an expensive, months-long process. Further reductions in early graft failures could yield significant human and economic benefits.
Introduction
Kidney transplantation is the preferred treatment for end-stage kidney disease (ESKD) with greater patient survival than dialysis as well as far lower annual costs (1–3) Early achievement and maintenance of good renal function in the first year post-transplant has been shown to be associated with both improved graft survival and lower costs in subsequent time periods. Kidney function within the first year of transplantation has been consistently identified as a key factor that affects longer term graft survival in observational and experimental settings (4–17) Further, clinical and administrative claims data from the USRDS have shown that 12-month estimated glomerular filtration rate (eGFR) is strongly associated with costs in the second and third years post-transplant among those surviving to at least 1 year post-transplant (18, 19) Additionally, eGFR at 6 months post-transplant has also demonstrated a strong association with hospitalizations in the following 12 month period (20).
This body of evidence has helped inform patient management practices aimed at maintaining renal function in the first months post-transplant to help realize the recent gains in long-term human and economic benefits (21, 22) Chief among these efforts has been improved immunosuppressant management to minimize acute rejection, which are costly events impairing short-term renal function and which increase long-term failure risks (23).
Graft failure is relatively rare in the first year post-transplant. However it is a very costly clinical event by itself in addition to the subsequent return to dialysis and, perhaps, later transplant. Overall, the economic costs of failure in terms comparing the costs of those with functioning grafts versus those on maintenance dialysis have been well-characterized over the years (24–27) For example in the US, reference data from the USRDS Annual Reports demonstrates that when a kidney transplant fails, the incremental cost to Medicare is approximately $95,000 in the first full-year of failure and annual costs for continuing maintenance dialysis patients are over 3 times greater than for those grafts for more than 1 year (28) Factors likely driving these costs include increased healthcare utilization peri-failure as well as the increased costs of the return to dialysis and, less frequently, rapid re-transplantation. While mean renal function in a cohort of transplant recipients stabilizes by 3 months post-transplant, there remains substantial variation in individual patient eGFR outcomes in the first year (14, 29–33).
Thus, the time-dependence of the relationships between graft function and medical costs in the first year post-transplant are not well studied: what are the short-term economic implications of patient management practices that improve renal function in the first year post-transplant? Our aims were to describe relationships between total medical costs in the first year following deceased donor kidney transplant with overall renal function as measured by: 1) eGFR at different time points for recipients with graft survival of at least 12 months, and; 2) graft failure for those with survival ≤12 months. We used the USRDS claims dataset to assess these real-world economic relationships within 1 year post kidney transplantation.
Materials and Methods
Data Sources
A retrospective cohort of single-organ, deceased donor kidney transplantation recipients was identified using the United States Renal Data System (USRDS) database from 1 January 2012 to 31 December 2015. One year follow-up was allowed to 31 December 2016. The USRDS database is a joint effort of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the Centers for Medicare and Medicaid Services (CMS) that tracks many descriptive elements for all patients in the US with end-stage kidney disease (ESKD). USRDS registries integrate information from the Organ Procurement and Transplantation Network (OPTN), CMS, and Medicare billing claims records (2) These elements are linked with a unique encrypted patient identifier, permitting investigators to combine patient-specific information from multiple tables without revealing patient identity. Data provided by USRDS are deidentified thus this analysis was exempt from IRB review.
Study Population
This study population included adult (18+) deceased donor kidney transplant patients with Medicare as their primary payer at time of first transplantation from 2012 to 2015. If patients had multiple kidney transplant procedures in that time period, the first was included. Patients with other organ transplants prior to the index transplantation were excluded from this study.
Patients without serum creatinine measures at discharge, at 180 and 365 days post-transplantation were excluded from this analysis (Table 1). Patients who died with a functioning graft during the first year were excluded from the analysis following their death.
TABLE 1
| Category | All patients | Graft failurea | No graft failurea | |||
|---|---|---|---|---|---|---|
| Overall, N (% of total) | 24,021 | 100% | 586 | 2.4% | 23,435 | 97.6% |
| Age Group, N (%) | ||||||
| <30 | 1,213 | 5.1% | 33 | 5.6% | 1,180 | 5.0% |
| 30–45 | 4,488 | 18.7% | 89 | 15.2% | 4,399 | 18.8% |
| 45–59 | 8,811 | 36.7% | 214 | 36.5% | 8,597 | 36.7% |
| 60–74 | 8,859 | 36.9% | 222 | 37.9% | 8,637 | 36.9% |
| ≥75 | 650 | 2.7% | 28 | 4.8% | 622 | 2.7% |
| Age | ||||||
| Mean (SD) | 54.0 | 13.09 | 55.2 | 13.54 | 54.0 | 13.08 |
| Gender | ||||||
| Male | 14,705 | 61.2% | 359 | 61.3% | 14,346 | 61.2% |
| Female | 9,316 | 38.8% | 227 | 38.7% | 9,089 | 38.8% |
| Race | ||||||
| Black | 8,735 | 36.4% | 239 | 40.8% | 8,496 | 36.3% |
| Non-Black | 15,286 | 63.6% | 347 | 59.2% | 14,939 | 63.8% |
| BMI [kg/m2] | ||||||
| Mean (SD) | 28.6 | 5.45 | 29.3 | 5.75 | 28.6 | 5.44 |
| Cause of ESRD | ||||||
| Polycystic kidney disease | 1,607 | 6.7% | 19 | 3.2% | 1,588 | 6.8% |
| Diabetes | 7,291 | 30.4% | 180 | 30.7% | 7,111 | 30.3% |
| Glomerulonephritis | 5,144 | 21.4% | 142 | 24.2% | 5,002 | 21.3% |
| Hypertension | 6,580 | 27.4% | 169 | 28.8% | 6,411 | 27.4% |
| Other | 3,399 | 14.2% | 76 | 13.0% | 3,323 | 14.2% |
| Year of Transplant | ||||||
| 2012 | 5,774 | 24.0% | 146 | 24.9% | 5,628 | 24.0% |
| 2013 | 5,949 | 24.8% | 149 | 25.4% | 5,800 | 24.8% |
| 2014 | 5,993 | 25.0% | 140 | 23.9% | 5,853 | 25.0% |
| 2015 | 6,305 | 26.3% | 151 | 25.8% | 6,154 | 26.3% |
Recipient demographic and clinical characteristics.
Within 12 months post-transplant.
Patient Baseline Characteristics
The following patient demographics and clinical variables were assessed in this study (Table 1): age at transplantation, gender, assigned race (Black vs. Non-Black), body mass index (BMI), and cause of ESKD. Baseline patient demographics were assessed for the entire sample population and stratified further for patients who experienced graft failure and those who did not during the first 12 months post-transplant.
eGFR and Cost Variable Definitions
Costs were derived from Parts A and B claims including inpatient, emergency, outpatient, and skilled nursing facility costs. Costs were included following discharge from the initial transplant hospitalization. Note that, for patients with graft failure, dialysis costs are not reported separately by USRDS and are largely incorporated into the Outpatient cost category. eGFRs were available at discharge, and at 6 and 12 months. Thus, eGFR:Cost relationships are described for several month-based time periods post-discharge: 0–3, 3–6, and 6–12 months. Serum creatinine measures were included in they were within 2 weeks of the discharge, 6, or 12 month time periods. All costs were adjusted to 2019 USD. In this study, eGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, using serum creatinine (34, 35).
Statistical Analysis
Descriptive statistics were used to describe the baseline demographic and clinical variables. Continuous variables were described with mean and standard deviation (SD). Categorical variables were described with counts and percentage. Data were missing for calculation of BMI for a few patients (146/24,021, 0.6%) and these missing values were imputed using the mean from patients with complete data. Both descriptive and multivariate generalized linear models with standard gamma distribution and log link function were used to define the relationship between eGFR measures and total medical costs.
For recipients with graft failure a time-history of medical costs was constructed with failure as the index date (Month 0 in Figure 3). Descriptive analyses were conducted in which monthly costs of those with graft failure in the first year post-transplant were compared to those without failure. Monthly costs for those without graft failure in the first year were a composite measure. This composite was created for each possible month pre- and post-index date by assigning the mean medical cost of non-failures to the month post-transplant in which failures occurred. This matching process appropriately reflects the higher costs experienced by all recipients in the first months post-transplant. This reporting followed the STROBE guidelines.
Results
Demographic and Clinical Characteristics
Patients who received a single-organ deceased donor kidney transplant from 2012 to 2015 experienced a 1-year graft failure rate of 2.4% (Table 1). Compared to those without early graft failure, these recipients tended to be slightly older (mean age: 55.2 vs. 54.0 years) and had marginally higher BMI (29.3 vs. 28.6 kg/m2). A greater proportion of recipients with early graft failure were Black (40.8% vs. 36.3%) and were less likely to have PKD (3.2% vs. 6.8%) and more likely to have glomerulonephritis (24.2% vs. 21.3%) as cause of ESRD. Among those without graft failure in the first year post-transplant over 96% had serum creatinine measurements at discharge, and at 6 and 12 months post-transplant.
Overall Medical Costs
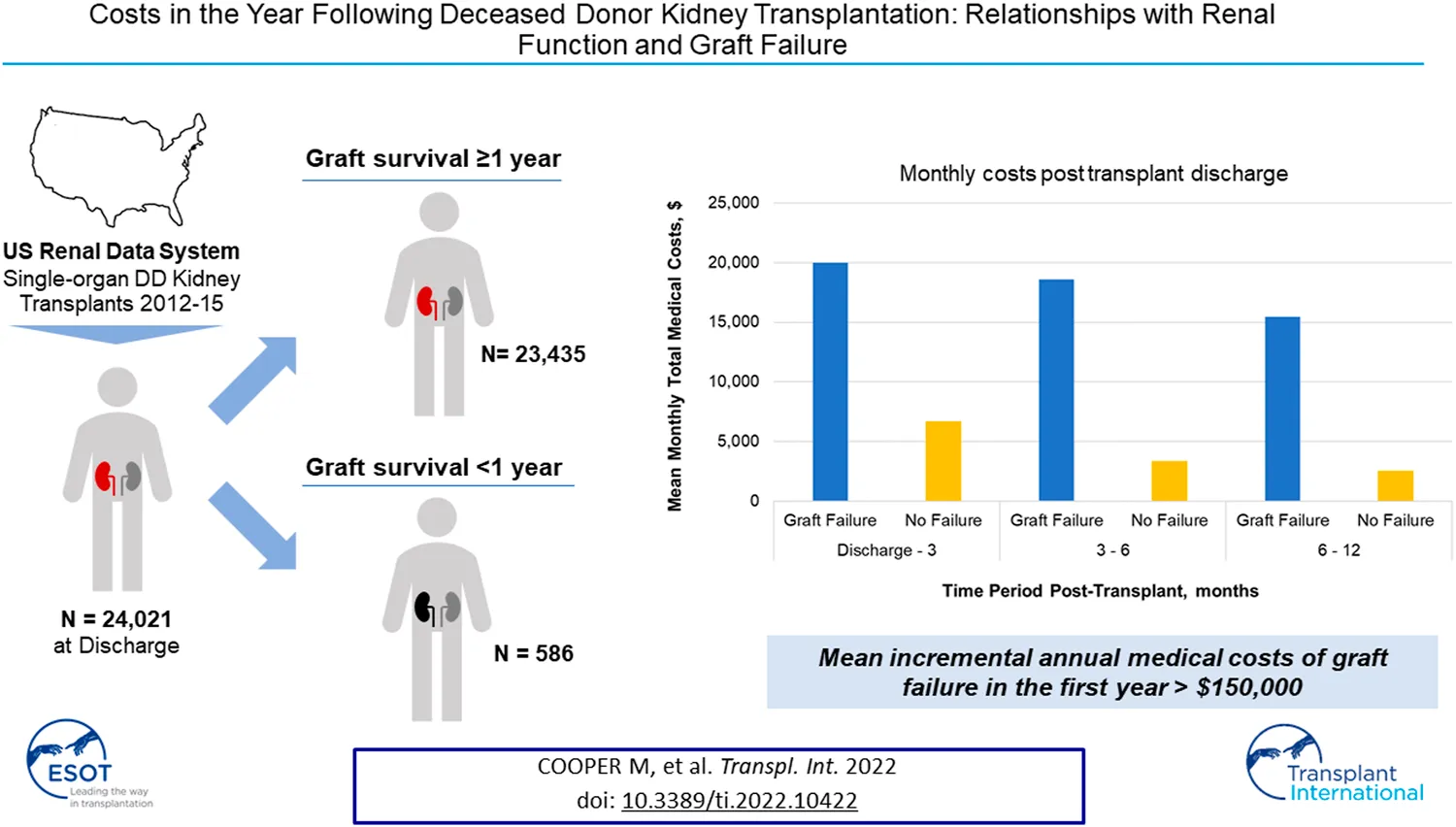
Mean monthly medical costs in the first year post-transplant (Figure 1) are substantially higher for recipients who had graft failure while monthly costs for both groups show similar downward trends over the first year post-transplant. Mean monthly costs in the early time period, discharge to 3 months, are 3.0 times higher for graft failures versus those without failure ($19,992 vs. $6,681). This ratio increases to a factor of 6.0 in the 6–12 month timeframe ($15,436 vs. $2,555).
FIGURE 1
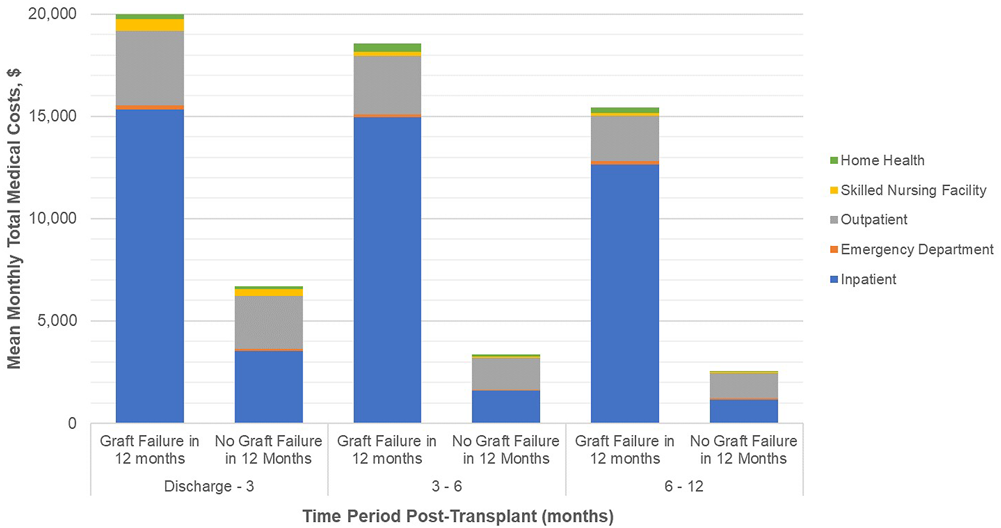
Healthcare costs (PPPM) post transplant discharge date, by time period post-surgical discharge. Footnotes: Mean hospice costs are ≤ $3 per month and are not reported here. Please refer to Supplementary Table S3. All differences in total monthly medical costs between those with and without graft failure in any given time period are significant at P<0.001.
By treatment setting, inpatient costs are the largest driver of cost differentials between patient groups. In the discharge to 3 month period, mean monthly inpatient costs are 4.4 times higher in the graft failure group ($15,341 vs. $3,520) and 10.9 times higher ($12,637 vs. $1,163) in the 6–12 month period. Outpatient costs are consistently higher for those with graft failure in the first year; during the early period mean monthly medical costs are 42% higher than non-failures ($3,638 vs. $2,562) and, while absolute OP costs decrease overall, the differential rises to 81% higher during the 6–12 month period ($2,218 vs. $1,226). Other medical costs (skilled nursing facility, emergency department, and home health) are small components of total costs but are consistently higher for those with graft failure.
eGFR and Cost Outcomes
The relationships between monthly medical costs and eGFR measurements at different timepoints demonstrate consistently strong trends (Figure 2). Both unadjusted and adjusted (Supplementary Table S4) relationships are similar in that eGFR is strongly correlated with medical costs in all time periods (p < 0.001 for each trend). The trend in the discharge to 3 month time period appears to be the least strong likely due to high variance among serum creatinine values at discharge (Supplementary Table S2) and continuing variability in renal function soon after transplant. For time periods after 3 months, the relationship between eGFR measurements and mean monthly medical costs are quite similar. Adjusted costs are highest ($7,157 to $7,826 per month) for those with a functioning graft and eGFR<15 ml/min/1.73 m2. In the 6–12 month time period, for example, adjusted monthly medical costs are over 40% lower for those with 6-month eGFRs 15–29 ml/min/1.73 m2 than for those with 6-month eGFRs <15 ml/min/1.73 m2 ($4,546 vs. $7,799) and costs are another 35% lower for 30–44 ml/min/1.73 m2 as compared to 15–29 ml/min/1.73m2 ($2,919 vs. $4,546). In addition to eGFR measures, age, gender, race, BMI, and cause of ESRD are important determinants of mean monthly costs in most time periods (Supplementary Table S5). The model fit parameters indicate that 12-month eGFR measurements are somewhat more strongly correlated with mean monthly costs in the 6–12 month time period than are 6-month eGFR measurements in the adjusted analyses.
FIGURE 2
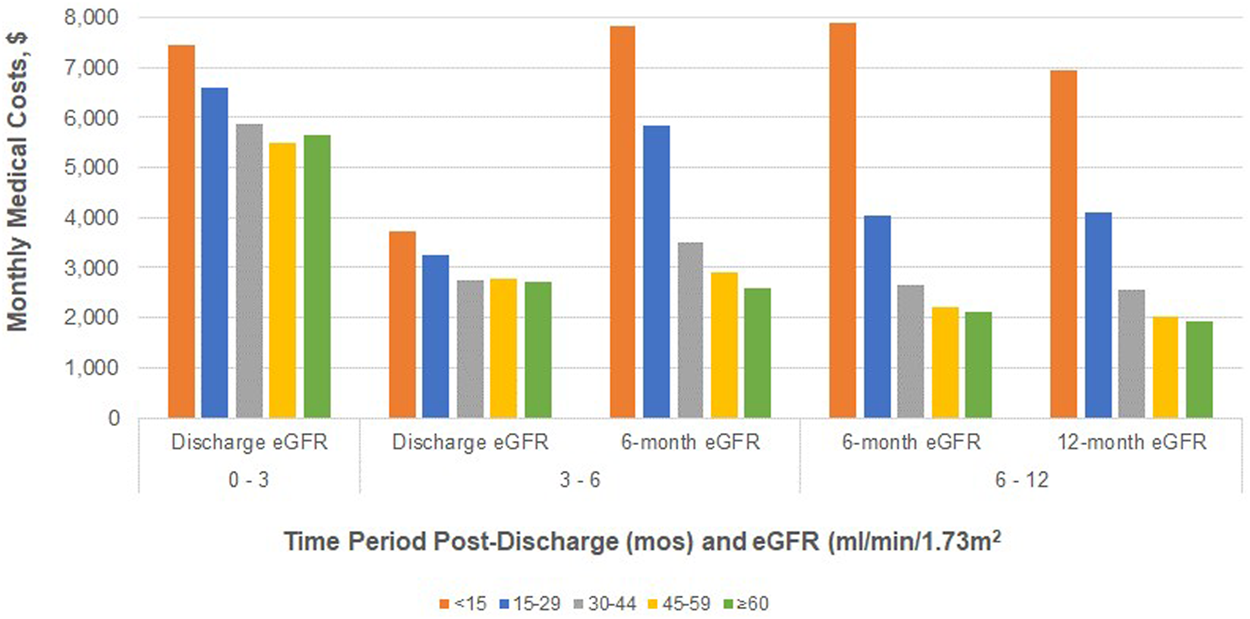
Adjusted total medical costs (PPPM) post-transplant discharge date for patients without graft failure, by eGFR measurement and time period post-surgical discharge.
Early Graft Failure and Cost Outcomes
For recipients with graft failure monthly costs begin to rise 3, 4 months prior to failure, with a spike of over $38,000 during the month of failure (Figure 3 and Supplementary Table S6). Costs appear to stabilize 3, 4 months post-failure suggesting a failure process that is several months long. Mean monthly costs ≥6 months post-failure are about 3 times those for patients without failure. Compared to the monthly costs of patients without graft failure weighted for the month post-transplant, costs for those experiencing graft failure are higher at each month observed. Centering on the median month of failure, 6 months post-transplant, the incremental costs of graft failure are $153,000 in the first year, as compared to those without graft failure.
FIGURE 3
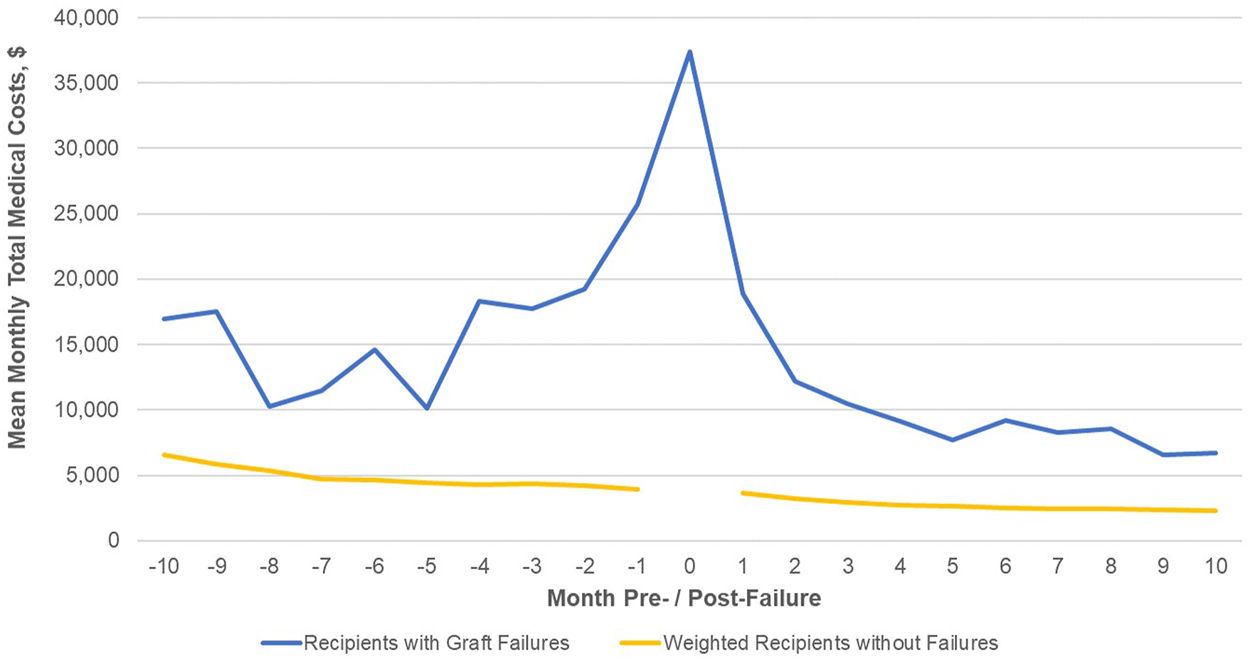
Total medical costs (PPPM) post-transplant discharge date for patients with graft failure. Footnote: No cost data exist for the index month (Month 0) for the comparison group of patients without graft failure.
Discussion
This analysis of deceased donor recipients with Medicare as primary payer demonstrates the strong relationship between medical costs and graft function in the first year post-transplant. For those recipients without early graft failure, there is a consistent trend toward lower total medical costs among patients with higher eGFR. Among non-failures, adjusted total medical costs are comparable for those with eGFRs above 45 ml/min/1.73 m2 and increase exponentially for those with eGFRs below 45 ml/min/1.73 m2. These patterns hold from 3 months post-transplant and regardless whether 6-month or 12-month eGFR is used as the reference point, reflecting the relative stability of renal function for the majority of patients without early graft failure. These results confirm that current patient management practices aimed at achieving and maintaining good renal function post-transplant also provide net economic benefits as well. As a contrasting point, current organ allocation/procurement policies have improved broader/more equitable distribution of organs with resulting longer CIT, higher rates of DGF, and higher sCr early post-transplant which, collectively, may tend to reduce eGFR among the cohort of transplant recipients. Our findings therefore suggest there may be an unrecognized economic cost of our constrained organ supply.
Time histories of medical costs for recipients who do experience early graft loss illustrate the high overall costs of failure. These trends also indicate that, from a healthcare utilization standpoint, graft failure is a process that may begin as early as several months prior to the failure event itself and that continues for 2–3 months as patients return to dialysis or retransplantation.
Regardless of treatment setting, mean medical costs are higher for those with early failure than those without. The dominant factor driving the higher total costs is inpatient utilization where costs are a factor of 4x to 10x in the early time period (0–3 months) and later time period (6–12 months), respectively. The peri-failure period is a time of particularly complex and intensive care management. Evidence that there is slightly higher patient mortality risk for each 1 ml/min/1.73 m2 higher eGFR at dialysis reinitiation (36, 37) underscores the criticality; declaring failure too early is detrimental to patient outcomes as is waiting too long. Therefore, constant monitoring is required to identify appropriate time to initiate dialysis, with admissions, more biopsies and more frequent OP visits to monitor changes in SCr. In addition, most patients will require care to prepare for dialysis, including evaluation and placement of vascular access. For a relative few, evaluation for pre-emptive re-transplantation requires additional care. Post-failure, nephrectomy may be indicated in many patients to eliminate risks of graft intolerance syndrome as well as graft rupture or hemorrhage. This complex peri-failure care management is also highly-dependent on an individual patient’s history, status, and preferences. That management places a significant burden on patients in direct out-of-pocket costs for care, but also indirect costs of time, travel, and family support. Disadvantaged patients may therefore not receive optimal care, thereby increasing the total cost of care and the time required to return to stable care post-failure. This is an under-studied area. An interesting area of future research would include an analysis of how the relationship between renal function and medical costs as patient management have improved over time.
Important limitations of this study are those typical for retrospective analyses of observational data, including the potential for residual confounding associated with factors not available in the study database. Additionally, given the study inclusion criteria, costs may not be generalizable to all DD recipient populations. For example, we excluded patients without Medicare as primary payer and our results may not apply to patients with employer group health plans. More generally, USRDS data lacks detailed laboratory and histology data that may be appropriate to include in our regression analyses of the eGFR:cost relationship regarding certain data elements and this lack of specificity may be important for prospective study. While additional control variables, if included, might be shown to be statistically significant determinants of first-year costs, our analysis suggests that they would not achieve the dominant role of renal function.
Our findings demonstrate that total medical costs in the first year post-transplant are substantially correlated with renal function, measured both as graft failure and as eGFR among those with surviving grafts at various times post-discharge. These results indicate that continued focus on improving renal function and reducing early graft failures could yield significant human and economic benefits.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Access to USRDS data requires a formal application and acceptance of a Data Use Agreement. Requests to access these datasets should be directed to USRDS, https://www.usrds.org/for-researchers/standard-analysis-files.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors were involved in study design. MC, ZW, WW, and RN were involved in data analysis. MC and RN were involved in initial medical writing. All authors were involved in data review and interpretation, manuscript review and editing, and approval of the final manuscript.
Conflict of interest
Authors ZW and WW are employed by company Genesis Research LLC; RJN is employed by Beta6 Consulting Group, LLC. MC received research funding and/or consultancy fees unrelated to this study from Angion Biomedica. RJN was an employee of Angion Biomedica at the time of writing.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Angion Biomedica provided funding for the analysis supporting this research. Genesis Research performed the data analysis under contract to Angion Biomedica. Funding for open access fees has not been provided.
Author disclaimer
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10422/full#supplementary-material
Abbreviations
BMI, body mass index; CIT, cold ischemia time; CKD-EPI, chronic kidney disease epidemiology collaboration; CMS, centers for medicare and medicaid services; DGF, delayed graft function; eGFR, estimated glomerular filtration ratio; ESKD, end-stage kidney disease; NIDDK, national institute of diabetes and digestive and kidney disease; OPTN, organ procurement and transplantation network; PKD, polycystic kidney disease; USRDS, United States renal data system.
References
1.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transpl. 2009;9 Suppl 3(Suppl. 3):S1–155. 10.1111/j.1600-6143.2009.02834.x
2.
U.S. Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2020).
3.
Abecassis M Bartlett ST Collins AJ Davis CL Delmonico FL Friedewald JJ et al Kidney Transplantation as Primary Therapy for End-Stage Renal Disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (Nkf/Kdoqitm) Conference. Clin J Am Soc Nephrol (2008) 3(2):471–80. 10.2215/CJN.05021107
4.
Hariharan S Mcbride MA Cherikh WS Tolleris CB Bresnahan BA Johnson CP . Post-Transplant Renal Function in the First Year Predicts Long-Term Kidney Transplant Survival. Kidney Int (2002) 62(1):311–8. 10.1046/j.1523-1755.2002.00424.x
5.
Ishikawa A Flechner SM Goldfarb DA Myles JL Modlin CS Boparai N et al Quantitative Assessment of the First Acute Rejection as A Predictor of Renal Transplant Outcome. Transplantation (1999) 68(9):1318–24. 10.1097/00007890-199911150-00017
6.
Nicol D MacDonald AS Lawen J Belitsky P . Early Prediction of Renal Allograft Loss beyond One Year. Transpl Int (1993) 6(3):153–7. 10.1111/j.1432-2277.1993.tb00636.x10.1007/bf00336359
7.
Pilch NA Rohan V Rao V Mauldin PD Su Z Dubay DA et al Renal Function Variability: An Independent Risk Factor for Graft Loss and Death Following Kidney Transplantation. Am J Nephrol (2018) 47(3):191–9. 10.1159/000487714
8.
DuBay DA Su Z Morinelli TA Baliga P Rohan V Bian J et al Development and Future Deployment of a 5 Years Allograft Survival Model for Kidney Transplantation. Nephrology (2019) 24(8):855–62. 10.1111/nep.13488
9.
Kasiske BL Israni AK Snyder JJ Skeans MA Peng Y Weinhandl ED . A Simple Tool to Predict Outcomes after Kidney Transplant. Am J Kidney Dis (2010) 56(5):947–60. 10.1053/j.ajkd.2010.06.020
10.
Kasiske BL Israni AK Snyder JJ Skeans MA . The Relationship between Kidney Function and Long-Term Graft Survival after Kidney Transplant. Am J Kidney Dis (2011) 57(3):466–75. 10.1053/j.ajkd.2010.10.054
11.
Loupy A Aubert O Orandi BJ Naesens M Bouatou Y Raynaud M et al Prediction System for Risk of Allograft Loss in Patients Receiving Kidney Transplants: International Derivation and Validation Study. BMJ (2019) 366(sep16 7):L4923. 10.1136/bmj.l4923
12.
Schnitzler MA Lentine KL Gheorghian A Axelrod D Trivedi D L’Italien G . Renal Function Following Living, Standard Criteria Deceased and Expanded Criteria Deceased Donor Kidney Transplantation: Impact on Graft Failure and Death. Transpl Int (2012) 25(2):179–91. 10.1111/j.1432-2277.2011.01395.x
13.
Schnitzler MA Johnston K Axelrod D Gheorghian A Lentine KL . Associations of Renal Function at 1-Year after Kidney Transplantation with Subsequent Return to Dialysis, Mortality, and Healthcare Costs. Transplantation (2011) 91(12):1347–56. 10.1097/TP.0b013e31821ab993
14.
Durrbach A Pestana JM Pearson T Vincenti F Garcia VD Campistol J et al A Phase III Study of Belatacept versus Cyclosporine in Kidney Transplants from Extended Criteria Donors (BENEFIT-EXT Study). Am J Transpl (2010) 10(3):547–57. 10.1111/j.1600-6143.2010.03016.x
15.
Vincenti F Rostaing L Grinyo J Rice K Steinberg S Gaite L et al Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med (2016) 374(4):333–43. 10.1056/NEJMoa1506027
16.
Ekberg H Tedesco-Silva H Demirbas A Vítko Š Nashan B Gürkan A et al Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation. N Engl J Med (2007) 357(25):2562–75. 10.1056/NEJMoa067411
17.
Weiner DE Carpenter MA Levey AS Ivanova A Cole EH Hunsicker L et al Kidney Function and Risk of Cardiovascular Disease and Mortality in Kidney Transplant Recipients: The FAVORIT Trial. Am J Transpl (2012) 12(9):2437–45. 10.1111/j.1600-6143.2012.04101.x
18.
Gheorghian A Schnitzler MA Axelrod DA Kalsekar A L’italien G Lentine KL . The Implications of Acute Rejection and Reduced Allograft Function on Health Care Expenditures in Contemporary US Kidney Transplantation. Transplantation (2012) 94(3):241–9. 10.1097/TP.0b013e318255f839
19.
Schnitzler MA Gheorghian A Axelrod D L’Italien GG Lentine KL . The Cost Implications of First Anniversary Renal Function after Living, Standard Criteria Deceased and Expanded Criteria Deceased Donor Kidney Transplantation. J Med Econ (2013) 16(1):75–84. 10.3111/13696998.2012.722571
20.
Keong FM Afshar YA Pastan SO Chowdhury R Binongo JN Patzer RE . Decreasing Estimated Glomerular Filtration Rate Is Associated with Increased Risk of Hospitalization after Kidney Transplantation. Kidney Int Rep (2016) 1(4):269–78. 10.1016/j.ekir.2016.08.008
21.
Poggio ED Augustine JJ Arrigain S Brennan DC Schold JD . Long‐term Kidney Transplant Graft Survival-Making Progress when Most Needed. Am J Transpl (2021) 21(8):2824–32. 10.1111/ajt.16463
22.
Schold JD Arrigain S Flechner SM Augustine JJ Sedor JR Wee A et al Dramatic Secular Changes in Prognosis for Kidney Transplant Candidates in the United States. Am J Transpl (2019) 19(2):414–24. 10.1111/ajt.15021
23.
Hart A Zaun D Itzler R Schladt D Israni A Kasiske B . Cost, Healthcare Utilization, and Outcomes of Antibody-Mediated Rejection in Kidney Transplant Recipients in the US. J Med Econ (2021) 24(1):1011–7. 10.1080/13696998.2021.1964267
24.
Fu R Sekercioglu N Berta W Coyte PC . Cost-effectiveness of Deceased-Donor Renal Transplant versus Dialysis to Treat End-Stage Renal Disease: A Systematic Review. Transpl Direct (2020) 6(2):E522. 10.1097/TXD.0000000000000974
25.
Klarman HE Francis JOS Rosenthal GD . Cost Effectiveness Analysis Applied to the Treatment of Chronic Renal Disease. Med Care (1968) 6(1):48–54. 10.1097/00005650-196801000-00005
26.
Karlberg I Nyberg G . Cost-Effectiveness Studies of Renal Transplantation. Int J Technol Assess Health Care (2009) 11(3):611–22. 10.1017/S026646230000876X
27.
Karlberg I Nyberg G . Cost-effectiveness Studies of Renal Transplantation. Int J Technol Assess Health Care (1995) 11(3):611–22. 10.1017/s026646230000876x
28.
U.S. Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Reference Tables K.10-K.12. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2019).
29.
Law JP Borrows R McNulty D Sharif A Ferro CJ . Early Renal Function Trajectories, Cytomegalovirus Serostatus and Long-Term Graft Outcomes in Kidney Transplant Recipients. BMC Nephrol (2021) 22(1):102. 10.1186/s12882-021-02285-2
30.
Vincenti F Tedesco Silva H Busque S O’Connell P Friedewald J Cibrik D et al Randomized Phase 2b Trial of Tofacitinib (CP-690,550) in De Novo Kidney Transplant Patients: Efficacy, Renal Function and Safety at 1 Year. Am J Transpl (2012) 12(9):2446–56. 10.1111/j.1600-6143.2012.04127.x
31.
Haynes R Haynes R Blackwell L Staplin N Herrington WG Emberson J et al Campath, Calcineurin Inhibitor Reduction, and Chronic Allograft Nephropathy (The 3C Study) - Results of a Randomized Controlled Clinical Trial. Am J Transpl (2018) 18(6):1424–34. 10.1111/ajt.14619
32.
de Fijter JW Holdaas H Øyen O Sanders J-S Sundar S Bemelman FJ et al Early Conversion from Calcineurin Inhibitor- to Everolimus-Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. Am J Transpl (2017) 17(7):1853–67. 10.1111/ajt.14186
33.
Berger SP Sommerer C Witzke O Tedesco H Chadban S Mulgaonkar S et al Two‐year Outcomes in De Novo Renal Transplant Recipients Receiving Everolimus‐facilitated Calcineurin Inhibitor Reduction Regimen from the TRANSFORM Study. Am J Transpl (2019) 19(11):3018–34. 10.1111/ajt.15480
34.
Levey AS Stevens LA Schmid CH Zhang Y Castro AF Feldman HI et al A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med (2009) 150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006
35.
CKD-EPI Adults (Conventional Units). National Institute of Diabetes and Digestive Kidney Diseases NIDDK (2009). Available from: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate-calculators/ckd-epi-adults-conventional-units (Accessed August 20, 2021).
36.
Gill JS Abichandani R Kausz AT Pereira BJG . Mortality after Kidney Transplant Failure: The Impact of Non-immunologic Factors. Kidney Int (2002) 62(5):1875–83. 10.1046/j.1523-1755.2002.00640.x
37.
Molnar MZ Streja E Kovesdy CP Hoshino J Hatamizadeh P Glassock RJ et al Estimated Glomerular Filtration Rate at Reinitiation of Dialysis and Mortality in Failed Kidney Transplant Recipients. Nephrol Dial Transpl (2012) 27(7):2913–21. 10.1093/ndt/gfs004
Summary
Keywords
kidney transplant, graft failure, estimated glomerular filtration rate, graft function, cost
Citation
Cooper M, Schnitzler M, Nilubol C, Wang W, Wu Z and Nordyke RJ (2022) Costs in the Year Following Deceased Donor Kidney Transplantation: Relationships With Renal Function and Graft Failure. Transpl Int 35:10422. doi: 10.3389/ti.2022.10422
Received
10 February 2022
Accepted
28 April 2022
Published
27 May 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Cooper, Schnitzler, Nilubol, Wang, Wu and Nordyke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert J. Nordyke, bob@beta6consulting.com, orcid.org/0000-0003-2424-7852
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.