Abstract
Corynebacterium spp. are associated with respiratory infections in immunocompromised hosts. A link with bronchial complications after lung transplantation (LTx) has been suggested. We aimed to assess the link between respiratory sampling of Corynebacterium spp. and significant bronchial complication (SBC) after LTx. We performed a single center retrospective study. Inclusion of LTx recipients with at least one respiratory Corynebacterium spp. sample (July 2014 to December 2018). Subjects were matched to unexposed LTx recipients. Primary outcome was SBC occurrence after Corynebacterium spp. isolation. Secondary outcomes were Corynebacterium spp. persistent sampling, chronic lung allograft dysfunction (CLAD) onset and all-cause mortality. Fifty-nine patients with Corynebacterium spp. sampling with 59 without isolation were included. Corynebacterium spp. identification was not associated with SBC occurrence (32.4% vs. 21.6%, p = 0.342). Previous SBC was associated with further isolation of Corynebacterium spp. (OR 3.94, 95% CI [1.72–9.05]). Previous SBC and corticosteroids pulses in the last 3 months were the only factors associated with increased risk of Corynebacterium spp. isolation in multivariate analysis. Corynebacterium spp. sampling was significantly associated with CLAD onset (27.1% vs. 6.9%, p = 0.021). Corynebacterium spp. isolation was not associated with SBC but with higher risk of CLAD. Whether CLAD evolution is affected by Corynebacterium spp. eradication remains to be investigated.
Introduction
Bronchial complications after lung transplantation (LTx) are a major burden, leading to severe morbidity and mortality (1), occurring in nearly 10% of LTx recipients (LTRs) (2,3). The multiple risk factors might include characteristics of the harvested organ (duration of mechanical ventilation, previous bronchial colonization, duration of cold ischemia) or surgical issues (duration of surgery, anastomosis techniques) (2). Early post-operative complications have also been reported as risk factors for bronchial issues (2). Bronchial complications usually require close bronchoscopic assessment, and in up to 25% of cases (3), interventional bronchoscopy for bronchial stent placement, or balloon dilatation. In a longer perspective, these bronchial complications can lead to functional loss (1). Moreover, repeated respiratory infections and bronchial complications may be linked, as a cause or a consequence (2). For instance, isolation of Pseudomonas aeruginosa or Staphylococcus aureus has been found associated with bronchial stenosis (4,5). Corynebacterium spp. can induce various clinically significant respiratory infections, notably in immunocompromised patients or patients with severe respiratory diseases (6-8). In LTRs, being both immunocompromised and with structural bronchial abnormalities, Corynebacterium spp. have been suspected to be associated with bronchial complications (9). In this report, the presence of a bronchial stent was a significant risk factor for persistence of Corynebacterium spp. infection.
We aimed to unravel the possible link between respiratory isolation of Corynebacterium species and significant bronchial complications (SBCs) in a cohort of LTRs in the Paris-Bichat Lung Transplant Program, France. Our objectives were to investigate the association of Corynebacterium spp. isolation and the occurrence of an SBC and to describe the Corynebacterium spp. epidemiology, the course of Corynebacterium spp. infection and its risk factors and long-term prognosis.
Patients and Methods
Patients and Settings
We retrospectively included all adult LTx recipients with at least one lower-respiratory-tract specimen in which a Corynebacterium spp. was isolated between July 2014 and December 2018 in the Paris-Bichat Lung Transplant Program. Cases were identified in the local microbiology department database, where all respiratory samples are recorded. Each case was matched to a non-exposed control, selected as the next LTx patient in chronological order. The matching was according to age at LTx ±5 years, mono- or bipulmonary status, underlying respiratory disease (defined in four categories: chronic obstructive pulmonary disease [COPD]/emphysema, interstitial lung disease, bronchial dilatation, miscellaneous). Data were collected anonymously, and the electronic files were used according to French law (Informatique et Libertés). The Evaluation Committee for observational research protocols of the French Respiratory Society (SPLF, CEPRO 2020-044) approved the study and waived informed consent.
Clinical and Microbiological Collected Data
All LTx candidates and recipients have been prospectively included in Paris-Bichat Lung Transplant database since 2006. This database includes demographical and anamnestic data, details on LTx surgery and post-operative course, bronchoscopic findings, and respiratory function.
All lower-respiratory-tract samples (sputum, tracheal aspirate, bronchoalveolar lavage [BAL], or protected distal aspiration) taken during usual care were immediately sent to the bacteriology laboratory. They were inoculated onto routine agar plates incubated for 48 h at 35°C under aerobic and anaerobic conditions. Bacteria were identified at the species level by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) Microflex LT Biotyper (Bruker Daltonics, Bremen, Germany). Bacterial susceptibility to antibiotics was determined with the disk-diffusion method according to EUCAST guidelines (www.eucast.org).
From the bacteriology laboratory database, we retrieved all cases of LTRs in whom Corynebacterium spp. had been isolated in at least one lower-respiratory-tract specimen. In patients with sputum and tracheal aspirates, we included only specimens showing ≥25 leukocytes/field and ≤10 upper respiratory epithelial cells/field, as assessed by the scoring system of Murray and Washington (10). The usual thresholds were applied for interpreting quantitative cultures (i.e., ≥104, ≥105 and ≥107 colony formation units/mL for BAL specimens, tracheal aspiration and sputum culture, respectively). Microbiological data are described in terms of the first Corynebacterium spp. isolation.
LTx Management
Usual management of LTx in our center is highly protocolized and reported elsewhere (11). In brief, intraoperative veno-arterial extra-corporeal membrane oxygenation (ECMO) was initiated according to hemodynamics and respiratory findings during surgery (12), with peripheral cannulation. All patients receive the same initial immunosuppressive regimen (mycophenolate mofetil, corticosteroids and tacrolimus). All patients receive life-long proton pump inhibitors. Antibiotic prophylaxis with cefazoline is administered for 48 h, then adapted to postoperative microbiological analysis. A first bronchoscopy is systematically performed within the first hours after LTx. During the post-operative course, surveillance bronchoscopy and BAL are performed in case of clinically suspected respiratory infection. In case of abnormalities in bronchial healing, these bronchoscopies are repeated, and microbiological samples are taken if an infection is suspected, thus allowing for longitudinal study of colonization.
Transbronchial biopsies are performed in case of clinically suspected acute cellular or antibody mediated rejection (AMR). Acute cellular rejection (ACR) was defined according to established criteria (13), as was AMR (14).
Study Definitions
We defined an SBC as the presence of a bronchial fistula diagnosed by chest CT-scan or bronchoscopy or the need for interventional bronchoscopy for dilatation or bronchial stenting (2). Persistent respiratory colonization was defined by isolation on a respiratory sample on at least three occasions at least 1 month apart in less than 1 year (15). Chronic lung allograft dysfunction (CLAD) was defined according to ISHLT recommendations (16) as a decline in forced expiratory volume per second (FEV1) ≥ 20% from baseline, persisting at 3-month intervals, excluding other causes. Baseline FEV1 was the mean of the 2 best post-transplant FEV1 measurements. The diagnosis of infection (pneumonia or bronchitis or colonization) was retrospectively defined according to Centers for Disease Control and Prevention definitions (17) by the review of all the medical files by a blinded adjudication committee.
Study Outcomes
The primary outcome was the occurrence of an SBC. The date of the event was the date of the first SBC. Secondary outcomes were 1) persistent respiratory colonization with Corynebacterium spp., 2) CLAD occurrence and its delay after transplantation, 3) all-cause mortality during follow-up, and 4) Corynebacterium species and their distribution.
Statistical Analysis
First, a descriptive analysis was performed in the entire cohort population and according to exposed or unexposed status. Categorical variables were summarized as counts (percentage) and frequency distributions were compared with the Mac Nemar test. Continuous variables were expressed as median (IQR) and differences were tested with the Wilcoxon test.
Second, we compared occurrence of SBC between exposed and unexposed patients in the subset of patients with respiratory Corynebacterium spp. isolation before the occurrence of SBC and their matched controls, using the Mac Nemar test. We also searched for factors associated with SBC among the following variables: Corynebacterium spp. isolation, underlying respiratory disease, invasive mechanical ventilation duration, ECMO necessity, by a univariate analysis. Third, factors associated with with Corynebacterium spp. isolation were investigated by univariate then multivariate logistic regression in the entire cohort population, using the same approach.
Time between LTx and occurrence of CLAD or death was compared between the exposed and unexposed groups by means of survival curves (Kaplan-Meier method) and tested by means of a log-rank test. If the patient was alive or without chronic rejection at the end of the study, the patient was censored at the study end date (December 31, 2018). Those analyses excluded patients with CLAD before isolation of Corynbacterium spp. Statistical tests were 2-sided with a significance level of 0.05. All analyses were performed using R software (version 4.0.3).
Results
Characteristics of the Cohort
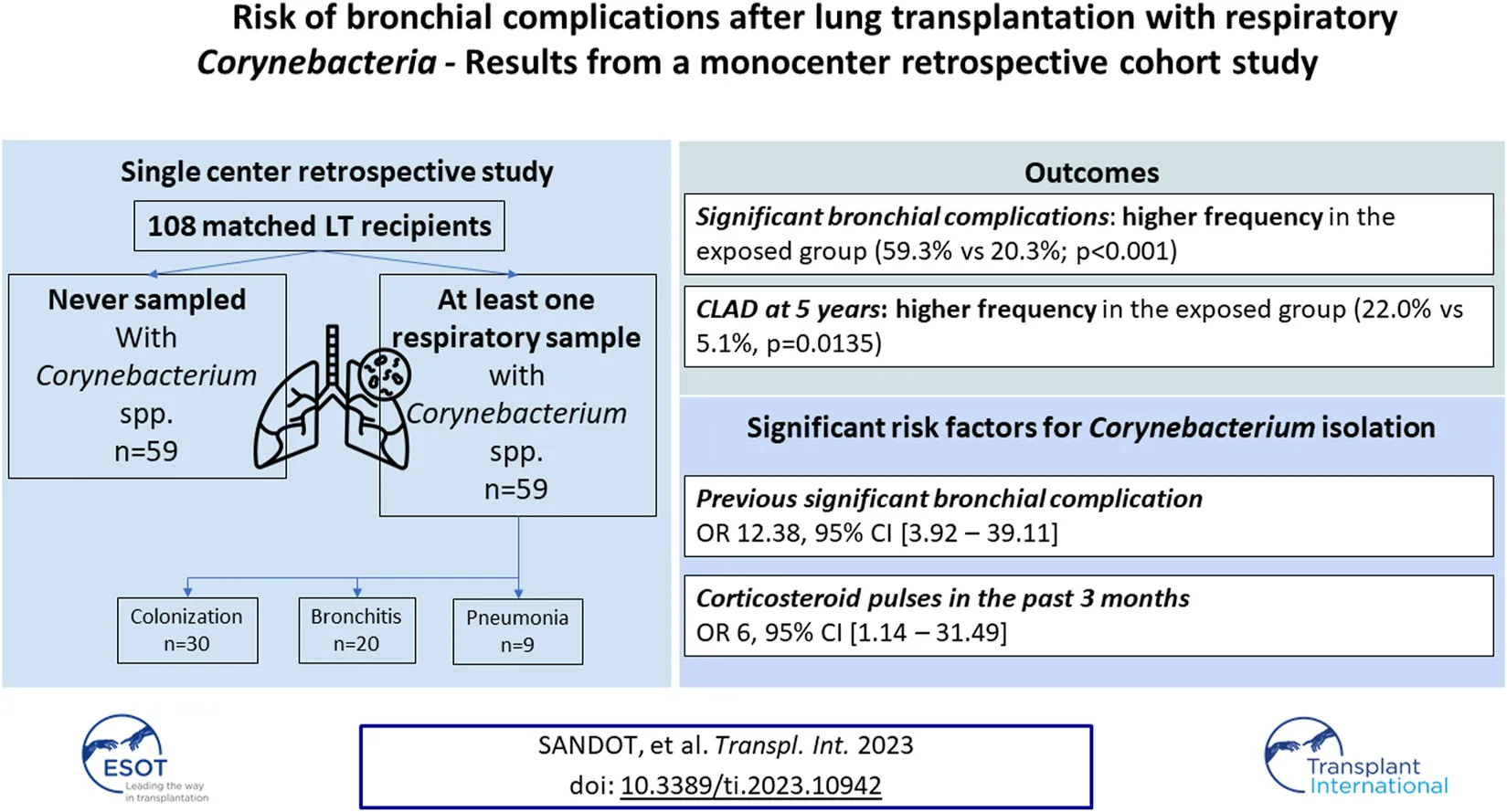
Over the study period, the cohort of LTx recipient represented 367 patients (Figure 1). Corynebacterium spp. were isolated in 60/367 LTRs (16.3% of the cohort) after a median of 128 days [interquartile range 38–503] after LTx. One patient was excluded from the analysis because Corynebacterium spp. had also been isolated before LTx on a systematic bronchoscopy, which left 59 LTR as the “exposed” cohort; these were matched to 59 non-exposed LTRs. Patient characteristics are in Table 1.
FIGURE 1
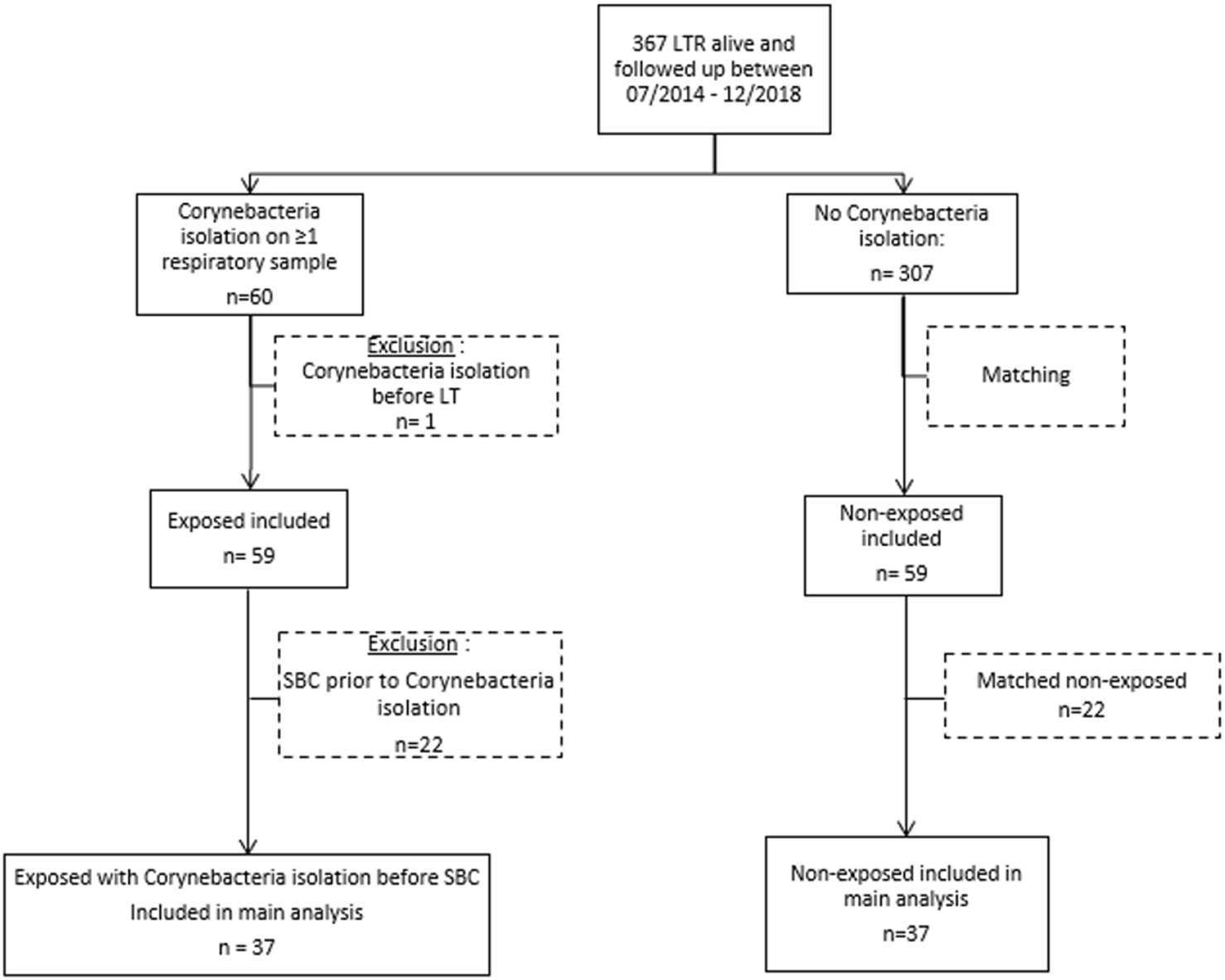
Flowchart of the study. LTR, lung transplantation recipient; SBC, significant bronchial complication.
TABLE 1
| Exposed, n = 59 | Non-exposed, n = 59 | p-valuea | |
|---|---|---|---|
| Recipient characteristics | |||
| Male sex, n (%) | 46 (78.0) | 43 (72.9) | 0.60 |
| Age (years) | 56.1 [50.4–61.5] | 56.5 [53.2–59.5] | 0.057 |
| Underlying respiratory disease, n (%) | |||
| Emphysema/COPD | 25 (42.4) | 28 (47.5) | 0.25 |
| Interstitial lung disease | 28 (47.5) | 28 (47.5) | |
| Bronchiectasis | 2 (3.4) | 2 (3.4) | |
| Other | 4 (6.9) | 1 (1.7) | |
| Type of lung transplantation n (%) | |||
| Right single-lung | 15 (25.4) | 17 (28.8) | 0.719 |
| Left single-lung | 9 (15.3) | 8 (13.6) | 0.870 |
| Double lung | 35 (59.3) | 34 (57.6) | 1 |
| Highly emergent LTx | 7 (11.9) | 9 (15.3) | 0.75 |
| Intraoperative veno-arterial extracorporeal membrane oxygenation, n (%) | 42 (71.2) | 31 (52.5) | 0.03 |
| Post-operative duration of mechanical ventilation (days) | 6.0 [1.0–13.0] | 1.0 [1.0–4.0] | 0.117 |
| Tracheostomy, n (%) | 20 (33.9) | 13 (22.0) | 0.21 |
| ICU length of stay (days) | 16.0 [11.0–34.0] | 14.0 [9.5–22.5] | 0.521 |
| Primary lung graft dysfunction, n (%) | 15 (25.4) | 12 (20.7) | 0.68 |
| CMV mismatch, n (%) | 9 (15.3) | 9 (16.1) | 1 |
| Immunosuppressive therapy | 5 | ||
| Corticosteroids | 59 (100) | 9 (100) | |
| Corticosteroids dosagea (mg) | 20.0 [7.5–30.0] | 25.0 [10.0–37.5] | 0.18 |
| Mycophenolate mofetil | 53 (89.8) | 50 (87.7) | 1.00 |
| Calcineurins inhibitor | 56 (94.9) | 56 (98.2) | 0.47 |
| Other immunosuppressive therapy in the last 6 months | 7 (11.9) | 1 (1.8) | 0.04 |
| Antimicrobial therapy in the last 3 months | 35 (59.3) | 31 (52.5) | 0.42 |
| Immunological complications | |||
| Acute cellular rejection | 13 (22.0) | 12 (20.3) | 1 |
| Corticosteroids pulses in the last 3 months | 24 (40.7) | 18 (30.5) | 0.26 |
| Allo-immunization or antibody mediated rejection | 16 (27.1) | 5 (8.5) | 0.015 |
Baseline characteristics of lung transplantation (LTx) recipients with a lower-respiratory-tract specimen in which a Corynebacterium spp. was isolated (exposed) and non-exposed recipients.
Prednisone equivalent.
Data are median (interquartile range) unless otherwise indicated.
Baseline data were collected on the date of Corynebacterium spp. isolation for exposed patients and on the date with equivalent time to transplantation for non-exposed patients.
Primary lung graft dysfunction was diagnosed according to Snell et al. (43); Acute cellular rejection was diagnosed according to Stewart et al. (13); antibody-mediated rejection was diagnosed according to Levine et al. (18).
Counts presented as n (%); medians presented with interquartile range for non-normally distributed data.
p-value for the Wilcoxon or Mc Nemar non-parametric tests as appropriate—logistic regression for categorical variable with more than 2 modalities except for underlying respiratory disease.
COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; CMV, cytomegalovirus.
Strict matching for the underlying respiratory disease leading to transplantation was not possible for 3 cases with rare lung diseases (histiocytosis, lymphangioleiomyomatosis and pulmonary graft-versus-host disease) who were matched with patients transplanted for COPD. Matching on mono- or bipulmonary status was favored whenever possible, to eliminate the risk of confounding colonization or infection of the native lung on isolation of Corynebacterium spp.
All patients were receiving systemic corticosteroid therapy, with no difference between exposed and non-exposed patients in median dose of corticosteroids or use of antimetabolites or anticalcineurins. Patients with Corynebacterium spp. isolated significantly more frequently received other types of immunosuppressive therapies than non-exposed patients (7/59, 11.9% vs. 1/59, 1.8%, p = 0.04; mammalian target of rapamycin inhibitors for 4 patients, belatacept for 2, and rituximab in the previous 6 months for 1 vs. belatacept for 1) (Table 1).
A history of AMR was more common in the exposed than non-exposed group (16/59, 27.1% vs. 5/59, 8.47%, p = 0.015) (Table 1). None of the included patients underwent fundoplication surgery.
Risk Factor for Significant Bronchial Complication
Presence of a SBC was significantly more frequent in the exposed than non-exposed group: 35/59 (59.3%) and 12/59 (20.3%) (p < 0.001). Likewise, an interventional bronchoscopy procedure and placement of a bronchial stent were more frequently required in exposed patients (31/59, 52.5% vs. 10/59, 16.9% and 19/59, 32.2% vs. 2/59, 3.4%; both p < 0.001). Among the patients with bronchial stents, 12 in the exposed group had a mechanical stent obstruction requiring bronchoscopy and no patient in the non-exposed group.
We analyzed data for 74 patients (Table 2) to evaluate whether Corynebacterium spp. was a risk factor for SBC: 21 patients in the exposed group had an SBC before Corynebacterium spp. isolation and were excluded from the analysis with their matched control. One non-exposed patient had an SBC before the matched exposed counterpart had Corynebacterium spp. isolated. Therefore, he was excluded. The respiratory isolation of a Corynebacterium spp. was not associated with increased frequency of further SBC (OR 2.33, IC95 0.60–9.02, p = 0.220). The time to onset of SBC did not significantly differ between the two groups. None of each SBC type was significantly more frequent in patients with an Corynebacterium spp. isolated.
TABLE 2
| n/Na | OR (95% CI) | p-value | |
|---|---|---|---|
| Previous Corynebacterium spp. isolation | 37/74 | 2.33 (0.60–9.02) | 0.220 |
| URD - Pulmonary fibrosis | 38/74 | 0.92 (0.17–5.13) | 0.928 |
| URD - COPD | 34/74 | 0.88 (0.16–4.99) | 0.888 |
| URD - Bronchiectasis | 2/74 | 4.64 (0.04–530.81) | 0.526 |
| Intubation length >24 h | 63/74 | 1.01 (0.91–1.12) | 0.814 |
| Highly emergent transplantation | 11/74 | 1.85 (0.18–18.91) | 0.602 |
| Intra-operative Extra corporeal membrane oxygenation | 49/74 | 1.10 (0.23–5.19) | 0.903 |
Factors associated with significant bronchial complication in univariate logistic regression.
n, frequency; N, number observed; URD: underlying respiratory disease.
Risk Factors for the Isolation of Corynebacterium spp.
We compared data for exposed and unexposed patients by univariate then multivariate logistic regression (Table 3).
TABLE 3
| Covariates | n/Na | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Crude OR | CI 95% | p-value | Adjusted OR | CI 95% | p-value | ||
| Previous significant bronchial complication | 30/118 | 10.83 | 3.47–33.80 | <0.001 | 12.38 | 3.92–39.11 | <0.001 |
| Interstitial lung disease | 56/118 | 1.00 | 0.49–2.06 | 1.000 | |||
| Emphysema/COPD | 53/118 | 0.81 | 0.39–1.68 | 0.579 | |||
| Bronchiectasis | 4/118 | 1.00 | 0.14–7.35 | 1.00 | |||
| CMV mismatch | 18/115 | 0.94 | 0.34–2.57 | 0.904 | |||
| Tracheotomy | 33/118 | 1.81 | 0.80–4.11 | 0.154 | |||
| Primitive lung graft dysfunction | 27/117 | 1.31 | 0.55–3.10 | 0.544 | |||
| Parenteral corticosteroids <3 months | 9/118 | 3.84 | 0.76–19.30 | 0.103 | 6.00 | 1.14–31.49 | 0.0034 |
| Antibiotic treatment <3 months | 69/118 | 1.63 | 0.78–3.42 | 0.192 | |||
| Previous CLAD | 6/118 | 5.37 | 0.61–47.45 | 0.131 | |||
| Previous AMR | 16/118 | 3.51 | 1.06–11.62 | 0.040 | |||
| Previous ACR | 25/118 | 1.11 | 0.46–2.68 | 0.822 | |||
Factors associated with Corynebacterium spp. sampling in univariate then multivariate analysis.
n, Frequency; N, number of observation; OR: odds ratio; 95% CI, 95% confidence interval.
COPD, chronic obstructive pulmonary disease; CMV, cytomegalovirus; CLAD, Chronic Lung Allograft Dysfunction (diagnosed accorded to Verleden et al. (16) ACR, acute cellular rejection (diagnosed according to Stewart et al. (13); AMR, antibody mediated rejection (diagnosed according to Levine et al. (18).
Primary lung graft dysfunction was diagnosed according to Christie et al. (19).
The presence of a previous SBC and history of corticosteroids pulses in the last 3 months were the only factors associated with an increased risk of Corynebacterium spp. isolation in multivariate analysis (OR 12.38, 95% CI [3.92–39.11]; p < 0.001, and 6 [1.14–31.49]; p = 0.0034). Prior antibiotic therapy exposure and history of chronic allograft dysfunction were not associated with isolation of Corynebacterium spp.
Clinical Features of Corynebacterium spp. Isolation
The presence of Corynebacterium spp. in a respiratory sample was associated with a lower respiratory-tract-infection pattern or functional decline in 34/59 (57.6%) LTRs, including 9 (15.3%) with a monomicrobial isolation (Supplementary Table S1). Nine (15.3%) had radiological pneumonia, 2 (3.4%) with monomicrobial isolation. Overall, 20 (33.9%) patients had signs of bronchitis: cough in 14 (23.7%), sputum or bronchoscopic evidence of purulent secretion in 13 (22%) and both in 7 (11.9%). Five (8.5%) had functional decline associated with dyspnea. Corynebacterium spp. isolation was associated with functional decline in 14 (24.6%) patients, 5 (8.5%) with monomicrobial isolation. In total, 25 (42.4%) patients had no clinical or biological sign of infection and were therefore considered colonized.
In 42/59 (71%) patients, the first Corynebacterium spp. respiratory isolation occurred during the hospital stay, including 14 (24.7%) in the intensive care and 7 during the immediate post-LTx stay. Among the 7 patients admitted to the intensive care unit, 3 (21.4%) had acute respiratory failure and 4 (28.6%) respiratory-related sepsis. Oxygen therapy was needed for 19 (32.2%) patients, invasive mechanical ventilation for 11 (18.6%) patients, and non-invasive ventilation for one patient.
Description of First Corynebacterium spp. Isolation
C. striatum was the most frequently retrieved species, accounting for 71.2% of patients (n = 42), followed by C. amycolatum (14 patients, 23.7%) (Supplementary Table S2); C. pseudodiphteriticum, C. accolens, and C. propinquum were isolated from one patient each. In 41/59 (69.5%) patients, at least one other bacterium was isolated from the respiratory sample, with 25 (43.1%) above the significance threshold. P. aeruginosa was the main bacterium isolated from the plurimicrobial samples, in 18 (30.5%) patients.
Microbiological Outcomes
In total, 18 (30.5%) patients received effective antimicrobial therapy against Corynebacterium spp. infection based on antibiotics susceptibility testing.
Only 31 patients (52.5%) received antibiotic therapy on first isolation; only 18 (58.1%) of these received effective antibiotic therapy for Corynebacterium spp. based on antimicrobial susceptibility testing.
Forty-two patients (71.2%) had persistent Corynebacterium spp. colonization; among them, 16/18 (88.9%) patients who received an antibiotic course that was deemed effective on in vitro data. The antimicrobial therapies are detailed in the Supplementary Table S3. The median duration of Corynebacterium spp. carriage was 58 days [interquartile range 7–412]. We found no association between the administration of an effective antibiotic therapy and the presence of persistent colonization.
Long-Term Outcomes
The frequency of ACR episodes did not significantly differ between the exposed and non-exposed groups. An AMR episode occurred in 16/59 exposed patients, significantly more often than in the non-exposed patients (27.1% vs. 8.5%, p = 0.015). The frequency of CMV reactivations did not differ according to Corynebacterium spp. exposure (exposed vs. non-exposed 18.6% vs. 8.5%, p = 0.178). The length of follow-up did not significantly differ between the groups, but the occurrence of CLAD at 5 years of follow up was significantly higher in exposed than non-exposed patients (27.1% vs. 6.9%; p = 0.02122.0% vs. 5.1%, p = 0.0135). In the exposed group, CLAD was diagnosed after a median time of 497 days [346–888] of Corynebacterium spp. first isolation. Conversely, survival without CLAD differed significantly between the two groups (Figure 2; p = 0.012), with earlier onset in exposed versus non-exposed patients, but all-cause mortality did not significantly differ.
FIGURE 2
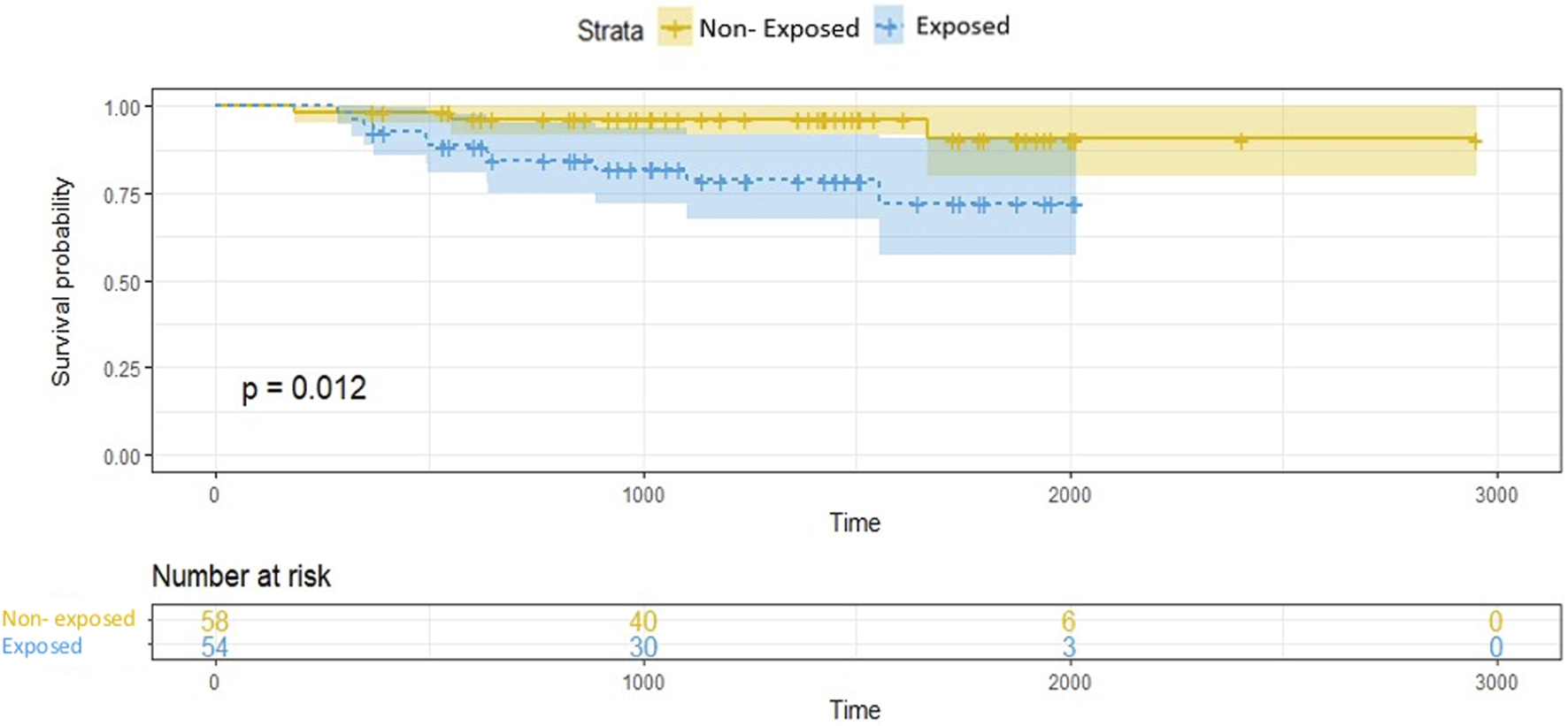
Survival without chronic lung allograft dysfunction. Kaplan-Meier analysis. Survival was measured from the date of transplantation to the end of the collection period. Non-exposed = 0: not exposed to Corynebacterium spp.; exposed = 1: exposed to Corynebacterium spp.
Discussion
In this retrospective case–control study, we aimed to investigate the suspected association between Corynebacterium spp. isolation in the respiratory tract and the occurrence of an SBC in 118 LTRs. Our findings can be summarized as follows: 1) although a pre-existing SBC was found an independent risk factor for detection of Corynebacterium spp., colonization by a Corynebacterium spp. was not associated with probability of a subsequent SBC; 2) the presence of a Corynebacterium spp. in the lower respiratory tract was associated with clinical manifestations of lower-respiratory-tract infection in 57.6% of cases, 68.6% being associated with another pathogen bacterial species; 3) although 18 (30.5%) patients received an antimicrobial course deemed effective by antimicrobial susceptibility testing, 16 (88.9%) of these had persistent colonization; and 4) survival was higher without CLAD in patients in whom a Corynebacterium spp. was never isolated.
The sole series of Corynebacterium spp. infection in LTRs reported the course and outcomes of 27 patients with Corynebacterium spp. isolation during a 2-year period (9). The low number of patients limits the relevance of the findings. In this series (9), more than half of the patients (53%) had a bronchial complication. The authors defined bronchial complication as the presence of mucosal plaques or purulent secretions at the bronchial suture. Despite guidelines for staging bronchial anomalies (20,21), the description of ischemic bronchitis and its extent is subjective and depends on the evaluator. In our center, fiberoptic bronchoscopies are performed by various physicians (namely LT pulmonologists, general pulmonologists, critical care physicians), not all of them skilled for reporting bronchial complications. A standardized assessment of bronchial complications (20,21) was therefore not available for all the patients. In our work, we deliberately chose an objective, and relevant endpoint, to limit a possible classification bias. Therefore, an SBC was defined as the occurrence of a bronchial fistula or the need for stenting or dilatation. This definition is undisputable as, during the study period, the bronchoscopists with skills on the evaluation of LT bronchial abnormalities were identical, indications for dilatation or bronchial stenting remained unchanged, and all the suspected dehiscence recorded by any physician were confirmed by a single skilled bronchoscopist. We do acknowledge the lack of formal guidelines on the timing or indication of interventional bronchoscopy or the procedure (dilatation or stenting), and the management of these complications may vary between centers. In our center, practices remained unchanged during the study period, with all indications performed by a single expert operator, which highly limits this classification bias. Using this definition, we report that Corynebacterium spp. isolation was associated with a pre-existing SBC in 21 (35.6%) patients.
Nevertheless, an interventional bronchoscopy procedure and the placement of a bronchial stent were more frequently needed in patients with Corynebacterium spp. isolation. Moreover, the presence of an SBC was an independent risk factor for Corynebacterium spp. isolation (OR 12.38 (3.92–39.11, p < 0.001).
In the recent study by Los-Arcos et al. (9), the clinical symptoms of lower-respiratory-tract infection were few. Only 12 (50%) patients had signs of respiratory infection (bronchitis, no pneumonia) and 9 when restricted to LTRs with exclusive isolation of Corynebacterium spp. and no other pathogen. Data concerning microbiological success or longer-term evolution, especially bronchial evolution, are not reported.
In our series, systemic infection was rare, with only 9 pneumonia (15.3%) cases and 20 (33.9%) with bronchitis.
The isolation of Corynebacterium spp. has been reported in immunocompromised hosts [solid-organ transplantation (8), connective tissue diseases under immunosuppressive therapy (22)], and patients with severe underlying respiratory disease (7,23,24). In these settings, infectious episodes related to Corynebacterium spp. have quite a silent course, more often appearing as bronchial or tracheobronchial than parenchymal infection (24). In a series of 18 patients with cystic fibrosis (23), 10 (76.9%) had worsening respiratory symptoms and none had pneumonia. In 10 hospitalized patients with COPD (7) 6 had pneumonia and 4 had exacerbations. Of note, in 6 of the 10 samples, the Corynebacterium spp. was the sole pathogen isolated and therefore responsible for the clinical symptoms.
In our series, the poor clinical picture may be explained by several combined factors. LTRs receive a high immunosuppressive regimen, thus impairing the T-cell and B-cell response (25-27). Moreover, owing to post-operative anatomical factors, the local host response to infection is decreased (28): modification of bronchial innervation secondary to the surgery decreasing cough reflex (29), impaired lymphatic drainage (30,31) etc.
Corynebacterium spp. are usually reported as skin and nasal mucosa commensal bacteria. Their isolation in a respiratory specimen is frequently considered a simple colonization, even though several recent studies suggest a varied pathogenicity at different sites (respiratory, endocarditis (32), brain abscess and meningal (33) infections) as well as the possibility of cross transmission of resistant strains between patients (34,35). These factors, associated with a limited number of symptomatic patients, might explain why Corynebacterium spp. isolation, even when reaching the microbiological threshold of significance, was not systematically considered to dictate antimicrobial therapy.
The finding of even moderate ischemic bronchitis frequently leads to the prescription of antibiotic therapy, potentially leading to the selection of antibiotic-resistant strains. Among the patients with available antimicrobial susceptibility testing, only 58% had received effective in vitro antibiotic therapy (cf. data in supplementary appendix).
In this series, we evidenced an association of Corynebacterium spp. isolation following corticosteroids pulses. These results, based on limited effectives (only 9 patients), should be taken carefully. However, these findings are consistent with evidence of increased occurrence of bacterial and fungal infection after immunosuppression intensification (36), and with higher frequency of Corynebacterium spp. isolation in LTRs receiving other types of immunosuppressive therapies than the conventional immunosuppressive regimen.
We found an association with a previous history of AMR in univariate analysis (OR 3.41 – IC 1.06–11.62), disappearing in multivariate analysis. To our knowledge, the association between respiratory infection or colonization and the occurrence of AMR has not been reported. AMR treatment relies on a heavy immunosuppression regimen (18), which is known to increase the risk of further infection.
Of note, increased risk of ACR has been described after a viral (37, 38) or bacterial (39) infection. In our series, Corynebacterium spp. isolation was not associated with more frequent occurrence of ACR, although ACR is suspected to promote bronchial complications (20).
In our series, the occurrence of CLAD was significantly higher in patients who had at least one positive Corynebacterium spp. respiratory sample (27.1% vs. 6.9% in non-exposed patients, p = 0.021). Some viral (37) or bacterial (39) lower-respiratory-tract infections or colonization have been reported as risk factors for CLAD (37-39). A single-center retrospective study (40) of 64 patients with post-LTx isolation of P. aeruginosa reported a higher frequency of CLAD occurrence within 2 years post-transplantation (23.4% vs. 7.7%, p = 0.006) in patients with P. aeruginosa colonization. Likewise, another study (41) included 95 LTRs with at least one P. aeruginosa isolation. CLAD-free survival was significantly higher in patients with successful eradication than in prolonged colonized patients (p = 0.018). These findings support the hypothesis of an inflammatory role of the bacteria, promoting airway damage, and leading to the generation of CLAD (42). Some evidence suggests that a similar mechanism may be involved in Corynebacterium spp. infection (42). Obviously, experimental evidence to support these hypotheses are necessary.
Although including a large number of LTRs with a positive Corynebacterium spp. lower-respiratory-tract sample, this work has several limitations. This was a single-center study, therefore limiting the significance of its conclusions in other centers. Indeed, in our center, the patients referred for LTx mostly have interstitial lung disease and emphysema. The findings might have been different in a center in which the main underlying respiratory condition would be cystic fibrosis, for example. Nevertheless, the single-center design allows for limiting the confounding factors: the perioperative management and post-LTx follow-up remained identical throughout the study period; the bronchoscopy findings and the indications for endoscopic management of bronchial complications remained unchanged; and the rigorous endoscopic and microbiological follow-up of patients with ischemic bronchitis allowed for reducing the classification bias. All the included LTRs were identified from our center’s microbiology laboratory database. The possibility to have missed the identification of a LTR with a documented Corynebacterium spp. in a respiratory sample outside our hospital is unlikely because the management of LTR is highly centralized in our center, for infectious events or for bronchial issues. We decided to match cases and controls according to the underlying respiratory disease, and single or double LTx in order to limit the role of possible pre-existing colonization at LTx. In addition, we referred to published definitions (43) for the various other variables of interest, thus allowing for a homogeneous collection. Because of its retrospective design, neither the susceptibility profile of all Corynebacteria strains nor their phylogenetic relation could be extensively studied.
In conclusion, in this single-center series of 118 LTRs, the isolation of a Corynebacterium spp. was not associated with a subsequent SBC but occurred more frequently in patients who already had a complication. We found increased frequency and earlier occurrence of CLAD in patients with Corynebacterium spp. respiratory isolation. Although we suggest the responsibility of chronic airway inflammation and an association with increased occurrence of AMR, the exact pathophysiology remains to be clarified. The impact of Corynebacterium spp. eradication on the occurrence of CLAD should be evaluated in future studies.
Investigators of the Paris-Bichat Lung Transplant Program
Agnès Abadie, Enora Atchade, Sandrine Boudinet, Pierre Cerceau, Adrian Crutu, Diego Ferreira, Gwenn Frere, Lucie Genet, Tiphaine Goletto, Aurélie Gouel, Sylvain Jean-Baptiste, Gilles Jebrak, Brice Lortat-Jacob, Armelle Marceau, Chahine Medraoui, Lise Morer, Domitille Mouren, Quentin Pellenc, Arnaud Roussel, Mathilde Salpin, Alice Savary, Aurélie Snauwaert, Sebastien Tanaka, Parvine Tashk, Charlotte Thibaut de Menonville, Sandrine Tissot, Sabrina Trigueiros, Nathalie Zappella.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by CEPRO 2020-044.
Author contributions
AS, NG, VB, PE, and JM participated in research design; AS, TR, and JM participated in the writing of the paper; all the authors participated in the performance of the research, data analysis and critically reviewed the manuscript.
Acknowledgments
We thank the nurses and nurse assistants involved in the Paris Bichat Lung Transplant Program for their commitment to the program, and the microbiology laboratory technicians for their work.
Conflict of interest
VB received advisory board fees from Novartis and Takeda; GW received advisory board fees from CSLBehring; PhM received advisory board and speaking fees from Pfizer, MSD, and Menarini; JM received congress reimbursement fees from Biotest and CSLBehring.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.10942/full#supplementary-material
References
1.
Moreno P Alvarez A Algar FJ Cano JR Espinosa D Cerezo F et al Incidence, Management and Clinical Outcomes of Patients with Airway Complications Following Lung Transplantation. Eur J Cardiothorac Surg (2008) 34(6):1198–205. 10.1016/j.ejcts.2008.08.006
2.
Varela A Hoyos L Romero A Campo-Cañaveral JL Crowley S . Management of Bronchial Complications after Lung Transplantation and Sequelae. Thorac Surg Clin (2018) 28(3):365–75. 10.1016/j.thorsurg.2018.04.006
3.
Yserbyt J Dooms C Vos R Dupont LJ Van Raemdonck DE Verleden GM . Anastomotic Airway Complications after Lung Transplantation: Risk Factors, Treatment Modalities and Outcome-A Single-centre Experience. Eur J Cardiothorac Surg (2016) 49(1):e1–8. 10.1093/ejcts/ezv363
4.
Santacruz JF Mehta AC . Airway Complications and Management after Lung Transplantation: Ischemia, Dehiscence, and Stenosis. Proc Am Thorac Soc (2009) 6(1):79–93. 10.1513/pats.200808-094GO
5.
Thistlethwaite PA Yung G Kemp A Osbourne S Jamieson SW Channick C et al Airway Stenoses after Lung Transplantation: Incidence, Management, and Outcome. J Thorac Cardiovasc Surg (2008) 136(6):1569–75. 10.1016/j.jtcvs.2008.08.021
6.
Otsuka Y Ohkusu K Kawamura Y Baba S Ezaki T Kimura S . Emergence of Multidrug-Resistant Corynebacterium Striatum as a Nosocomial Pathogen in Long-Term Hospitalized Patients with Underlying Diseases. Diagn Microbiol Infect Dis (2006) 54(2):109–14. 10.1016/j.diagmicrobio.2005.08.005
7.
Díez-Aguilar M Ruiz-Garbajosa P Fernández-Olmos A Guisado P Del Campo R Quereda C et al Non-diphtheriae Corynebacterium Species: an Emerging Respiratory Pathogen. Eur J Clin Microbiol Infect Dis (2013) 32(6):769–72. 10.1007/s10096-012-1805-5
8.
Burke GJ Malouf MA Glanville AR . Opportunistic Lung Infection with Corynebacterium Pseudodiphtheriticum after Lung and Heart Transplantation. Med J Aust (1997) 166(7):362–4. 10.5694/j.1326-5377.1997.tb123164.x
9.
Los-Arcos I Len O Martín-Gómez MT Baroja A Berastegui C Deu M et al Clinical Characteristics and Outcome of Lung Transplant Recipients with Respiratory Isolation of Corynebacterium Spp. J Clin Microbiol (2018) 56(8):e00142–18. 10.1128/JCM.00142-18
10.
Murray PR Washington JA . Microscopic and Baceriologic Analysis of Expectorated Sputum. Mayo Clin Proc (1975) 50(6):339–44.
11.
Renard R Girault A Avramenko-Bouvier A Roussel A Cerceau P Pellenc Q et al Outcome of Lung Transplantation Using Grafts from Donors over 65 Years of Age. Ann Thorac Surg (2021) 112:1142–9. 10.1016/j.athoracsur.2020.10.018
12.
Ius F Sommer W Tudorache I Avsar M Siemeni T Salman J et al Five-year Experience with Intraoperative Extracorporeal Membrane Oxygenation in Lung Transplantation: Indications and Midterm Results. J Heart Lung Transpl (2016) 35(1):49–58. 10.1016/j.healun.2015.08.016
13.
Stewart S Fishbein MC Snell GI Berry GJ Boehler A Burke MM et al Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Heart Lung Transpl (2007) 26(12):1229–42. 10.1016/j.healun.2007.10.017
14.
Roux A Levine DJ Zeevi A Hachem R Halloran K Halloran PF et al Banff Lung Report: Current Knowledge and Future Research Perspectives for Diagnosis and Treatment of Pulmonary Antibody-Mediated Rejection (AMR). Am J Transplant (2019) 19(1):21–31. 10.1111/ajt.14990
15.
Chalmers JD Aliberti S Blasi F . Management of Bronchiectasis in Adults. Eur Respir J (2015) 45(5):1446–62. 10.1183/09031936.00119114
16.
Verleden GM Glanville AR Lease ED Fisher AJ Calabrese F Corris PA et al Chronic Lung Allograft Dysfunction: Definition, Diagnostic Criteria, and Approaches to treatment―A Consensus Report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant (2019) 38(5):493–503. 10.1016/j.healun.2019.03.009
17.
ATS/IDSA. ATS/IDSA Publishes Clinical Guideline on Community Acquired Pneumonia. (2018). Available at: https://www.thoracic.org/about/newsroom/press-releases/journal/2019/ats-idsa-publishes-clinical-guideline-on-community-acquired-pneumonia.php. (Accessed April 8, 2022).
18.
Levine DJ Glanville AR Aboyoun C Belperio J Benden C Berry GJ et al Antibody-mediated Rejection of the Lung: A Consensus Report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2016) 35(4):397–406. 10.1016/j.healun.2016.01.1223
19.
Christie JD Carby M Bag R Corris P Hertz M Weill D et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2005) 24(10):1454–9. 10.1016/j.healun.2004.11.049
20.
Crespo MM McCarthy DP Hopkins PM Clark SC Budev M Bermudez CA et al ISHLT Consensus Statement on Adult and Pediatric Airway Complications after Lung Transplantation: Definitions, Grading System, and Therapeutics. J Heart Lung Transplant (2018) 37(5):548–63. 10.1016/j.healun.2018.01.1309
21.
Dutau H Vandemoortele T Laroumagne S Gomez C Boussaud V Cavailles A et al A New Endoscopic Standardized Grading System for Macroscopic central Airway Complications Following Lung Transplantation: the MDS Classification. Eur J Cardiothorac Surg (2014) 45(2):e33–38. 10.1093/ejcts/ezt499
22.
Donaghy M Cohen J . Pulmonary Infection with Corynebacterium Hofmannii Complicating Systemic Lupus Erythematosus. J Infect Dis (1983) 147(5):962. 10.1093/infdis/147.5.962
23.
Bittar F Cassagne C Bosdure E Stremler N Dubus JC Sarles J et al Outbreak of Corynebacterium Pseudodiphtheriticum Infection in Cystic Fibrosis Patients, France. France Emerg Infect Dis (2010) 16(8):1231–6. 10.3201/eid1608.100193
24.
Shariff M Aditi A Beri K . Corynebacterium Striatum: an Emerging Respiratory Pathogen. J Infect Dev Ctries (2018) 12(7):581–6. 10.3855/jidc.10406
25.
Barnes PJ . Corticosteroid Effects on Cell Signalling. Eur Respir J (2006) 27(2):413–26. 10.1183/09031936.06.00125404
26.
Staatz CE Tett SE . Clinical Pharmacokinetics and Pharmacodynamics of Mycophenolate in Solid Organ Transplant Recipients. Clin Pharmacokinet (2007) 46(1):13–58. 10.2165/00003088-200746010-00002
27.
Parekh K Trulock E Patterson GA . Use of Cyclosporine in Lung Transplantation. Transpl Proc (2004) 36(2):318S–322S. 10.1016/j.transproceed.2004.01.056
28.
Burguete SR Maselli DJ Fernandez JF Levine SM . Lung Transplant Infection. Respirology (2013) 18(1):22–38. 10.1111/j.1440-1843.2012.02196.x
29.
Veale D Glasper PN Gascoigne A Dark JH Gibson GJ Corris PA . Ciliary Beat Frequency in Transplanted Lungs. Thorax (1993) 48(6):629–31. 10.1136/thx.48.6.629
30.
Speich R van der Bij W . Epidemiology and Management of Infections after Lung Transplantation. Clin Infect Dis (2001) 33(1):S58–65. 10.1086/320906
31.
Verleden GM Vos R van Raemdonck D Vanaudenaerde B . Pulmonary Infection Defense after Lung Transplantation: Does Airway Ischemia Play a Role?Curr Opin Organ Transpl (2010) 15(5):568–71. 10.1097/MOT.0b013e32833debd0
32.
Keijman JMG Luirink MR Ramsay G Jacobs JA . Native Valve Endocarditis Due to Corynebacterium Striatum. Clin Microbiol Newsl (2000) 22(16):125–7. 10.1016/S0196-4399(01)80002-7
33.
Kammoun MM Regaieg K Bahloul M Ammar R Bouaziz M . Corynebacterium Striatum Meningitis. Med Mal Infect (2016) 46(8):454–6. 10.1016/j.medmal.2016.06.007
34.
Silva-Santana G Silva CMF Olivella JGB Silva IF Fernandes LMO Sued-Karam BR et al Worldwide Survey of Corynebacterium Striatum Increasingly Associated with Human Invasive Infections, Nosocomial Outbreak, and Antimicrobial Multidrug-Resistance, 1976-2020. Arch Microbiol (2021) 203(5):1863–80. 10.1007/s00203-021-02246-1
35.
Zasada AA Mosiej E . Contemporary Microbiology and Identification of Corynebacteria Spp. Causing Infections in Human. Lett Appl Microbiol (2018) 66(6):472–83. 10.1111/lam.12883
36.
Remund KF Best M Egan JJ . Infections Relevant to Lung Transplantation. Proc Am Thorac Soc (2009) 6(1):94–100. 10.1513/pats.200809-113GO
37.
Vilchez RA McCurry K Dauber J Iacono A Keenan R Zeevi A et al The Epidemiology of Parainfluenza Virus Infection in Lung Transplant Recipients. Clin Infect Dis (2001) 33(12):2004–8. 10.1086/324348
38.
Shah PD McDyer JF . Viral Infections in Lung Transplant Recipients. Semin Respir Crit Care Med (2010) 31(2):243–54. 10.1055/s-0030-1249120
39.
Glanville AR Gencay M Tamm M Chhajed P Plit M Hopkins P et al Chlamydia Pneumoniae Infection after Lung Transplantation. J Heart Lung Transpl (2005) 24(2):131–6. 10.1016/j.healun.2003.09.042
40.
Botha P Archer L Anderson RL Lordan J Dark JH Corris PA et al Pseudomonas aeruginosa Colonization of the Allograft after Lung Transplantation and the Risk of Bronchiolitis Obliterans Syndrome. Transplantation (2008) 85(5):771–4. 10.1097/TP.0b013e31816651de
41.
De Muynck B Van Herck A Sacreas A Heigl T Kaes J Vanstapel A et al Successful Pseudomonas aeruginosa Eradication Improves Outcomes after Lung Transplantation: a Retrospective Cohort Analysis. Eur Respir J (2020) 56(4):2001720. 10.1183/13993003.01720-2020
42.
Gregson AL Wang X Weigt SS Palchevskiy V Lynch JP 3rd Ross DJ et al Interaction between Pseudomonas and CXC Chemokines Increases Risk of Bronchiolitis Obliterans Syndrome and Death in Lung Transplantation. Am J Respir Crit Care Med (2013) 187(5):518–26. 10.1164/rccm.201207-1228OC
43.
Snell GI Yusen RD Weill D Strueber M Garrity E Reed A et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, Part I: Definition and Grading—A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2017) 36(10):1097–103. 10.1016/j.healun.2017.07.021
Summary
Keywords
lung transplant, infection, chronic lung allograft dysfunction (CLAD), bronchial complications, corynebacteria
Citation
Sandot A, Grall N, Rodier T, Bunel V, Godet C, Weisenburger G, Tran-Dinh A, Montravers P, Mordant P, Castier Y, Eloy P, Armand-Lefevre L, Mal H and Messika J (2023) Risk of Bronchial Complications After Lung Transplantation With Respiratory Corynebacteria. Results From a Monocenter Retrospective Cohort Study. Transpl Int 36:10942. doi: 10.3389/ti.2023.10942
Received
01 October 2022
Accepted
14 February 2023
Published
01 March 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Sandot, Grall, Rodier, Bunel, Godet, Weisenburger, Tran-Dinh, Montravers, Mordant, Castier, Eloy, Armand-Lefevre, Mal and Messika.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Messika, j.messika@hopital-foch.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.