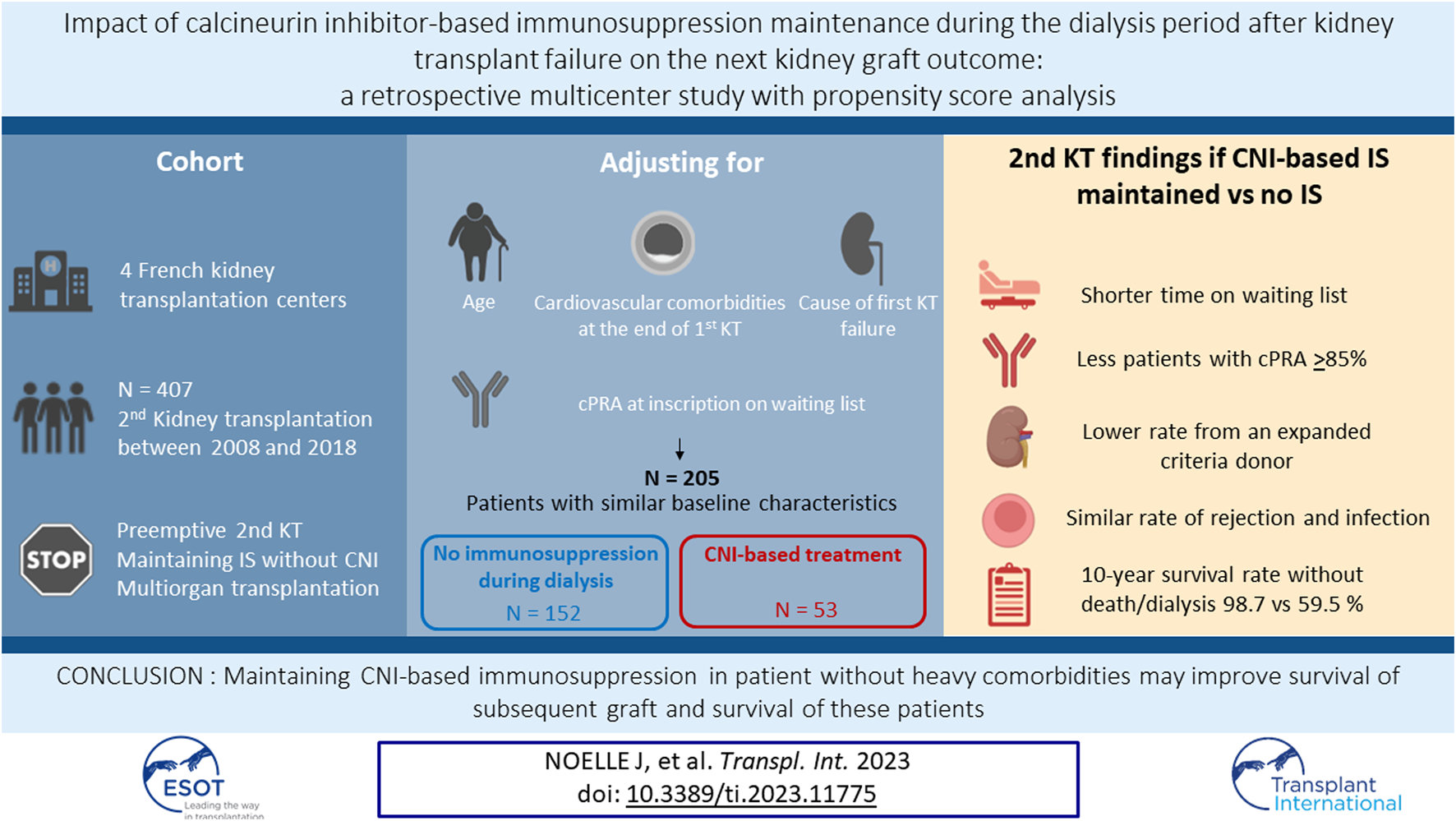
Abstract
The impact of immunosuppressive therapy (IS) strategies after kidney transplant failure (KTF) on potential future new grafts is poorly established. We assessed the potential benefit of calcineurin inhibitor (CNI)-based IS maintenance throughout the dialysis period on the outcome of the second kidney transplant (KT). We identified 407 patients who underwent a second KT between January 2008 and December 2018 at four French KT centers. Inverse probability of treatment weighting was used to control for potential confounding. We included 205 patients with similar baseline characteristics at KTF: a total of 53 received at least CNIs on the retransplant day (G-CNI), and 152 did not receive any IS (G-STOP). On the retransplant date, G-STOP patients experienced a longer pretransplant dialysis time, were more often hyperimmunized, and underwent more expanded-criteria donor KTs than G-CNI patients. During the second KT follow-up period, rejection episodes were similar in both groups. The 10-year survival rates without death and dialysis were 98.7% and 59.5% in G-CNI and G-STOP patients, respectively. In the multivariable analysis, CNI-based IS maintenance was associated with better survival (hazard ratio: 0.08; 95% confidence interval: 0.01–0.58, p = 0.01). CNI-based IS maintenance throughout the dialysis period after KTF may improve retransplantation outcomes.
Graphical Abstract
Introduction
Since the 2000s, the number of patients waiting for a second transplant after kidney transplant failure (KTF) has increased year after year. Currently, they represent 13%–23% of patients on the waiting list ([1–4]) and approximately 14% of the transplantations performed in France [5]. The majority of these patients develop anti-human leucocyte antigen (HLA) antibodies after KTF, and immunosuppressive therapy (IS) is gradually withdrawn, thus limiting their access to a new transplant [6, 7]. They represent more than half of the hyperimmunized patients on the waiting list, defined by a calculated panel reactive antibody (cPRA) level ≥85% [1, 8, 9]. A prolonged wait time [1, 10] is associated with poorer survival of the second transplant [11–14] and increased mortality [11, 15, 16].
The optimal management of IS after KTF in potential candidates for a second kidney transplant (KT) remains uncertain [17]. Until recently, expert recommendations suggested a sequential decrease in IS with cessation of antimetabolites in the event of KTF, gradual withdrawal of calcineurin inhibitors (CNIs) with cessation between 1 month and 3 months, and a delayed cessation of steroids depending on residual diuresis and the occurrence of symptoms related to graft intolerance [18–20]. Recently, an American expert transplant group suggested stopping immunosuppressive drugs in the absence of transplantation 1 year after KTF [21]. IS withdrawal aims to minimize infectious, cardiovascular [22, 23], and neoplastic [24] risks in patients with KTF. On the other hand, the British Transplantation Society suggests maintenance of IS when a living donor transplant is planned in the year following KTF [25]. Indeed, recent studies have suggested a decrease in immunization that may allow better access to a subsequent KT if CNIs are maintained after KTF [26, 27], without an increased risk of cardiovascular or infectious events [28]. These divergences undoubtedly explain the very scarce literature on retransplant outcomes in patients with IS maintained throughout the dialysis period [29].
The objective of the present retrospective, multicenter, observational study was thus to evaluate the impact of CNI-based IS maintenance during the dialysis period until the new transplantation on the outcome of the second graft.
Material and Methods
Study Population
This retrospective, multicenter study was performed at four French adult KT centers (Clermont-Ferrand, Bordeaux, Rouen and Poitiers). Patients were selected using the Cristal prospective database. The inclusion criteria were patients over 18 years old who had undergone a second KT between 1 January 2008 and 31 December 2020 at the Clermont-Ferrand, Rouen, or Poitiers transplant centers or between 1 January 2016 and 31 December 2020 at the Bordeaux transplant center (because of a change of the computerized patient record systems). The exclusion criteria consisted of second preemptive transplantations, continuation of IS treatment without CNIs, and multiorgan transplantations.
Data Collection
The following demographic, clinical, and biological data were collected: i) at the time of KTF—age, sex, body mass index, initial kidney disease, first transplant outcome and cause of allograft failure, PRA level, and the eventual presence of donor-specific anti-HLA antibodies (DSAs); ii) at the inscription on the waiting list for a second KT—PRA level and comorbidities (diabetes, stroke, ischemic heart disease, lower limb revascularization, neoplasia, and persistent post-KTF infection); iii) during the dialysis period—potential allograft nephrectomy, severe infection defined as an opportunistic infection [30] or requiring hospitalization [31], major cardiovascular events (hospitalization for acute coronary syndrome, cardiac arrhythmia, heart failure, lower limb revascularization, and stroke), and whether IS with CNIs was maintained; iv) at the retransplant initial hospitalization—induction therapy modalities, PRA level, eventual presence of DSAs (against the new KT), the type of donor (expanded criteria donor [32] or living donor), residual diuresis, and delayed graft function defined as the requirement of at least one dialysis session during the first week after transplantation [33]; and v) during the follow-up after the second KT—graft rejection episodes (Banff 2019 [34]), the appearance of DSAs, severe infection, major cardiovascular events, neoplasia, graft, and patient survival. Detection of anti-HLA antibodies was performed using the Luminex Single Antigen method (One Lambda, Canoga Park, CA) at the Clermont-Ferrand, Bordeaux, and Poitiers centers or Immucor Lifecodes (Immucor, Stamford, CT) at the Rouen center [35].
Oversight and study approval were provided by the Committee for Protection of Human Subjects (CPP SUD-EST VI) on 3 September 2019 (institutional review board 00008526) and by the National Consultative Committee on the Use of Health Research Information (14.510). No written consent was required for this study, but a non-opposition letter was sent to all patients in accordance with national legislation (MR-004 reference methodology) [36].
Definition of Groups
Two groups of patients were defined according to the modality of management of IS in the period between the two KTs: i) the CNI group (G-CNI), defined by the continuation of IS including CNIs either as monotherapy or in combination with other IS (i.e., steroids, mycophenolate mofetil, azathioprine, and mTOR pathway inhibitors) during the entire period between the two KTs, and ii) the stop group (G-STOP), defined by the cessation of all IS during the intertransplant period.
Statistics
Statistical analyses were performed with Stata software (version 15; StataCorp, College Station, Texas, USA). All tests were two-sided with an alpha level set at 5%. Categorical variables were expressed as number of patients and associated percentages, and continuous variables as mean ± standard deviation or median [25th; 75th percentiles], according to their statistical distribution.
Demographic and first transplant characteristics were compared between G-STOP and G-CNI using usual statistical tests: chi-squared test or Fisher’s exact test for categorical variables and Student’s t-test or Mann-Whitney test for continuous variables.
To assess the relationship between the group (G-STOP and G-CNI) and the primary and secondary endpoints, a propensity score (PS) analysis was implemented using the inverse probability of treatment weighting (IPTW) method [37, 38]. The PS was derived from the probability that treatment with a CNI would be continued for a given patient (G-CNI) conditional on confounders. The IPTW method consists of creating a “pseudo sample” of treated (G-CNI) and untreated (G-STOP) patients, weighting each patient by the inverse probability of receiving the treatment he or she actually received as follows: 1/PS in the G-CNI and 1/(1-PS) in the G-STOP. In practice, the probability of continuing CNI therapy was modeled using multiple logistic regression, and the estimated probability was used as the PS. Variables were selected for the PS based on clinical relevance: age at the end of the first transplant, cardiovascular comorbidities at the end of the first transplant, cause of first transplant failure, and cPRA level at the inscription on the waiting list for retransplant. Patients with missing cPRA levels at the inscription date were excluded from the analysis, as were patients with diabetes or infections because they were all G-STOP patients. Balance between groups was measured by standardized mean differences, calculated before and after weighting, and expressed as absolute values. A value greater than 0.2 was considered a sign of imbalance.
The primary outcome was a composite of dialysis and death after the second KT, presented as survival free of dialysis and death. This outcome was expressed as censored data and was estimated with the Kaplan-Meier method, and the groups were compared by the log-rank statistic. Multivariable analyses were performed with a Cox model (with the center as a random effect) considering covariables in terms of their significant results in univariate analysis (p < 0.10) as well as their clinical relevance14,32. The results are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs).
Secondary outcomes were compared in both groups by mixed models, considering the center as a random effect: linear mixed models were used for continuous outcomes and generalized linear mixed models with the logit link function were used for binary outcomes.
Finally, exposure-adjusted rates were calculated as the total number of event episodes (including recurrent events) over the total duration of follow-up and are expressed per 100 patient-years (p-y).
Results
Patient Characteristics at the First-Graft Failure
Among the 3246 KTs performed at the four centers during the study period, 407 patients (12.5%) received a second KT (Figure 1). Five patients with multiorgan transplantation were excluded, as well as 44 patients with preemptive KT, 31 patients with IS without CNIs, and 52 due to lack of data. A total of 275 patients were included, 216 in the G-STOP and 59 in the G-CNI. The median follow-up time after the second KT was 3.6 years [2.0; 7.0].
FIGURE 1
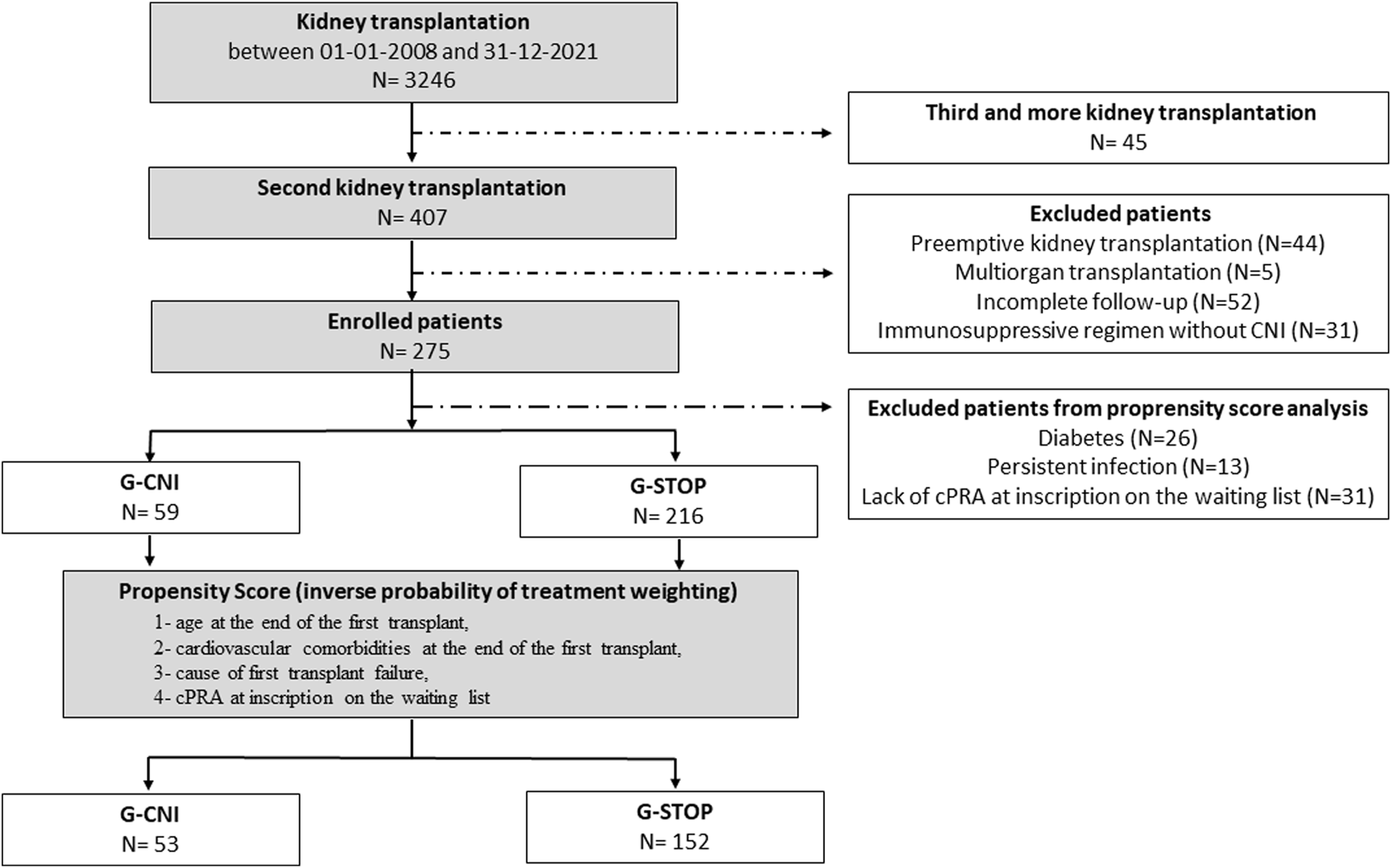
Flow chart of the study. CNI, calcineurin inhibitor; cPRA, calculated panel reactive antibody; G-CNI, group with immunosuppressive therapy maintenance; G-STOP, group with discontinued immunosuppressive therapy.
The characteristics of the patients before IPTW are depicted in Table 1. The 275 patients included were mainly men (64.7%), aged 49.7 ± 13.6 years at KTF. The primary cause of the first graft loss was rejection (61.8%). G-STOP patients compared to G-CNI patients had more diabetes at the end of the first KT (11.6% vs. 1.7%, p = 0.02), a shorter transplantation survival (92 months [34; 163] vs. 133 [87; 220], p = 0.003), and a higher cPRA at inscription on the waiting list for retransplant (51% [0; 86] vs. 5% [0; 70], p = 0.02). The rate of hyperimmunized patients (cPRA ≥85%) in the G-STOP and G-CNI was 26.7% and 13.2%, respectively (p = 0.04). Among G-CNI patients, 36 (61.0%) were treated with tacrolimus and 23 (39.0%) with cyclosporine. IS maintenance until the second KT consisted of CNI monotherapy in 19 patients (32.2%) and CNI combined with an antimetabolite or corticosteroid therapy in 30 patients (50.8%). Ten patients (17.0%) received triple IS.
TABLE 1
| Total (n = 275) | G-STOP (n = 216) | G-CNI (n = 59) | p | |
|---|---|---|---|---|
| Age at the end of G1 (years) | 49.7 ± 13.6 | 49.1 ± 13.6 | 52.0 ± 13.3 | 0.15 |
| Male sex | 178 (64.7) | 140 (64.8) | 38 (64.4) | 0.95 |
| Body mass index (kg/m2) | 24.4 ± 4.6 | 24.6 ± 4.7 | 23.7 ± 3.9 | 0.13 |
| Causal nephropathy | ||||
| Vascular nephropathy | 14 (5.1) | 13 (6.0) | 1 (1.7) | 0.14 |
| Genetic nephropathy | 57 (20.7) | 40 (18.5) | 17 (28.8) | |
| Glomerulonephritis | 126 (45.8) | 96 (44.4) | 30 (50.8) | |
| Diabetic nephropathy | 6 (2.2) | 6 (2.8) | 0 (0.0) | |
| Urological | 44 (16.0) | 39 (18.1) | 5 (8.5) | |
| Other | 28 (10.2) | 22 (10.2) | 6 (10.2) | |
| Comorbidities at the end of G1 | ||||
| Diabetes | 26 (9.5) | 25 (11.6) | 1 (1.7) | 0.02 |
| Cardiovascular diseasea | 37 (13.5) | 28 (13.0) | 9 (15.3) | 0.65 |
| Infectionsb | 13 (4.7) | 13 (6.0) | 0 (0.0) | 0.08 |
| Solid cancer | 25 (9.1) | 18 (8.3) | 7 (11.9) | 0.40 |
| Recurrent skin cancer | 6 (2.2) | 3 (1.4) | 3 (5.1) | 0.12 |
| Hemopathy | 5 (1.8) | 4 (1.9) | 1 (1.7) | 1.00 |
| G1 duration (months) | 106 [43; 176] | 92 [34; 163] | 133 [87; 220] | 0.003 |
| Cause of G1 failure | ||||
| Rejection | 170 (61.8) | 132 (61.1) | 38 (64.4) | 0.11 |
| Infection | 10 (3.6) | 10 (4.6) | 0 (0.0) | |
| IFTA | 28 (10.2) | 20 (9.3) | 8 (13.6) | |
| Vascular | 37 (13.5) | 33 (15.3) | 4 (6.8) | |
| Causal nephropathy recurrence | 30 (10.9) | 21 (9.7) | 9 (15.2) | |
| Presence of DSAs at the end of G1 | 62/228 (27.2) | 46/173 (26.6) | 16/55 (29.1) | 0.72 |
| cPRA at graft failure (%) (n = 244) | 48 [0; 83] | 51 [0; 86] | 5 [0; 70] | 0.02 |
| cPRA at graft failure ≥85% | 58/244 (23.8) | 51/191 (26.7) | 7/53 (13.2) | 0.04 |
Characteristics at the first kidney transplant failure date of patients with (G-CNI) or without (G-STOP) calcineurin inhibitor maintenance throughout the intergraft period.
Data are expressed as the number of patients (associated percentage), mean ± standard deviation or median [25th; 75th percentiles]. cPRA, calculated panel reactive antibody; DSA, donor-specific antibody; G1, first graft; G-CNI, group with immunosuppressive therapy maintenance; G-STOP, group with discontinued immunosuppressive therapy; IFTA, interstitial fibrosis and tubular atrophy.
Cardiovascular comorbidities: cerebrovascular accident, ischemic heart disease and/or obliterating arteriopathy of the lower limbs (surgical treatment).
Infections: numerous or persistent at the time of kidney transplant failure.
Patient Characteristics at the First-Graft Failure After IPTW
The characteristics at the time of KTF of the 205 patients included in the PS analysis are summarized in Table 2. After applying the IPTW method, the G-STOP and G-CNI were well balanced (standardized mean differences <20%) for the variables included in the IPTW model: age, cardiovascular comorbidities, the cause of first-transplant failure, and the cPRA level at inscription on the waiting list for retransplant.
TABLE 2
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| G-STOP (n = 152) | G-CNI (n = 53) | SMD | G-STOP | G-CNI | SMD | |
| Age at the end of G1 (years) | 47.9 ± 13.8 | 51.9 ± 13.2 | 0.29 | 48.9 ± 14.0 | 48.4 ± 12.8 | 0.04 |
| Male sex | 97 (63.8) | 35 (66.0) | 0.05 | (64.7) | (59.2) | 0.11 |
| Body mass index (kg/m2) | 24.4 ± 4.7 | 23.7 ± 3.7 | 0.18 | 24.4 ± 4.7 | 23.5 ± 3.6 | 0.22 |
| Causal nephropathy | ||||||
| Vascular nephropathy | 11 (7.2) | 1 (1.9) | 0.26 | (8.1) | (1.3) | 0.32 |
| Genetic nephropathy | 25 (16.5) | 15 (28.3) | 0.29 | (16.1) | (36.3) | 0.47 |
| Glomerulonephritis | 73 (48.0) | 26 (49.1) | 0.02 | (48.2) | (44.3) | 0.08 |
| Diabetic nephropathy | 0 (0.0) | 0 (0.0) | NA | (0.0) | (0.0) | NA |
| Urological | 26 (17.1) | 5 (9.4) | 0.23 | (16.4) | (8.0) | 0.26 |
| Other | 17 (11.2) | 6 (11.3) | 0.00 | (11.2) | (10.1) | 0.04 |
| Comorbidities at the end of G1 | ||||||
| Diabetes | 0 (0.0) | 0 (0.0) | NA | (0.0) | (0.0) | NA |
| Cardiovascular diseasea | 18 (11.8) | 8 (15.1) | 0.10 | (12.7) | (11.6) | 0.03 |
| Infectionsb | 0 (0.0) | 0 (0.0) | NA | (0.0) | (0.0) | NA |
| Solid cancer | 13 (8.6) | 7 (13.2) | 0.15 | (9.8) | (10.4) | 0.02 |
| Recurrent skin cancer | 1 (0.7) | 3 (5.7) | 0.29 | (0.7) | (4.1) | 0.22 |
| Hemopathy | 3 (2.0) | 1 (1.9) | 0.01 | (1.8) | (1.4) | 0.03 |
| G1 duration (months) | 92 [36; 167] | 133 [90; 217] | 0.44 | 104 [43; 172] | 120 [44; 205] | 0.16 |
| Cause of G1 failure | ||||||
| Rejection | 96 (63.2) | 34 (64.1) | 0.02 | (63.5) | (59.5) | 0.08 |
| Infection | 0 (0.0) | 0 (0.0) | NA | (0.0) | (0.0) | NA |
| IFTA | 14 (9.2) | 6 (11.3) | 0.07 | (9.6) | (7.7) | 0.07 |
| Vascular | 23 (15.1) | 4 (7.6) | 0.24 | (13.4) | (20.1) | 0.18 |
| Causal nephropathy recurrence | 19 (12.5) | 9 (17.0) | 0.13 | (13.5) | (12.7) | 0.02 |
| Presence of DSAs at the end of G1 | 30/120 (25.0) | 13/49 (26.5) | 0.04 | (22.9) | (29.7) | 0.15 |
| cPRA at graft failure (%) | 50 [0; 84] | 5 [0; 70] | 0.32 | 44 [0; 83] | 56 [0; 83] | 0.06 |
| cPRA at graft failure ≥85% | 37 (24.3) | 7 (13.2) | 0.29 | (22.2) | (23.8) | 0.04 |
Characteristics at the first kidney transplant failure date of patients with (G-CNI) or without (G-STOP) calcineurin inhibitor maintenance throughout the intergraft period before and after applying inverse probability weighting.
Data are expressed as the number of patients (associated percentage), mean ± standard deviation, or median [25th; 75th percentiles]. cPRA, calculated panel reactive antibody; DSA, donor-specific antibody; G1, first graft; G-CNI, group with immunosuppressive therapy maintenance; G-STOP, group with discontinued immunosuppressive therapy; IFTA, interstitial fibrosis and tubular atrophy; IPTW, inverse probability of treatment weighting; NA, not applicable; SMD, standardized mean difference.
Cardiovascular comorbidities: cerebrovascular accident, ischemic heart disease, and/or obliterating arteriopathy of the lower limbs (surgical treatment).
Infections: numerous or persistent at the time of kidney transplant failure.
Waiting Time and Characteristics of the Patients After the Second KT After IPTW
The median time on dialysis until the second KT was 21 months [11; 43]. This value was significantly lower in the G-CNI than in the G-STOP (16 [5; 26] vs. 37 [22; 64], respectively, p < 0.001). The waiting times from relisting to the second KT were 16 months [8; 23] in the G-CNI and 27 months [13; 48] in the G-STOP (p = 0.06) (Table 3).
TABLE 3
| Total | G-STOP | G-CNI | p | |
|---|---|---|---|---|
| Intergraft period | ||||
| Pretransplant dialysis time (months) | 21 [11; 43] | 37 [22; 64] | 16 [5; 26] | <0.001 |
| Time on the waiting list (months) | 19 [9; 37] | 27 [13; 48] | 16 [8; 23] | 0.06 |
| Transplantectomy and causes | (24.5) | (34.1) | (15.4) | 0.06 |
| Thrombosis | (39.5) | (34.6) | (48.3) | 0.94 |
| Graft intolerance syndrome | (52.2) | (52.5) | (51.7) | |
| Infection | (1.4) | (2.2) | (0.0) | |
| Surgical reason | (1.2) | (1.8) | (0.0) | |
| Other | (5.7) | (8.9) | (0.0) | |
| Second transplantation | ||||
| cPRA at D0 (%) | 76 [25; 93] | 87 [55; 96] | 67 [0; 84] | 0.001 |
| cPRA at D0 ≥ 85% | (39.4) | (55.2) | (23.9) | <0.001 |
| Anti-HLA antibodies at D0 | (82.0) | (91.5) | (72.9) | 0.047 |
| Presence of DSAs at D0 | (16.5) | (16.7) | (16.4) | 0.99 |
| HLA-A/B/DR antigen mismatches (0–6) | 4 [2; 4] | 3 [2; 4] | 4 [2; 4] | 0.92 |
| HLA- DR antigen mismatches, N = 2 | (14.5) | (16.9) | (12.3) | 0.39 |
| Cold ischemia time (minutes) | 940 [688; 1,110] | 960 [742; 1,208] | 935 [620; 1,051] | 0.03 |
| Living donor | (10.6) | (7.8) | (13.3) | 0.26 |
| Expanded criteria donor | (36.4) | (43.2) | (29.9) | 0.01 |
| Residual urine output ≥500 mL | (29.1) | (13.8) | (44.1) | 0.06 |
| Induction treatment | ||||
| No induction treatment | (0.5) | (1.0) | (0.0) | 0.27 |
| Thymoglobulin | (78.8) | (84.0) | (73.8) | |
| Basiliximab | (20.7) | (15.0) | (26.2) | |
| Delayed graft function | (17.7) | (25.8) | (9.9) | 0.001 |
| Evolution after second transplantation | ||||
| Rejection | (18.7) | (22.1) | (15.5) | 0.47 |
| Humoral | (14.0) | (14.6) | (13.4) | 0.95 |
| Cellular | (5.4) | (8.8) | (2.1) | <0.001 |
| Development of DSA | (9.7) | (10.4) | (8.9) | 0.52 |
| Return to dialysis and causes | (9.6) | (18.3) | (1.3) | 0.004 |
| Rejection | (45.1) | (48.4) | (0.0) | NA |
| Infection | (2.9) | (3.1) | (0.0) | |
| IFTA | (27.9) | (29.9) | (0.0) | |
| Vascular | (24.1) | (18.6) | (100.0) | |
| Causal nephropathy recurrence | (0.0) | (0.0) | (0.0) | |
| Death and causes | (4.7) | (9.7) | (0.0) | <0.001 |
| Infection | (19.4) | (19.4) | — | NA |
| Cancer | (7.8) | (7.8) | — | |
| Cardiovascular | (29.9) | (29.9) | — | |
| Other | (42.9) | (42.9) | — | |
Intergraft period and second transplantation outcomes after inverse probability weighting.
Data are expressed as the number of patients (percentage) or median [25th; 75th percentiles]. cPRA, calculated panel reactive antibody; D0, day of transplantation; DSA, donor-specific antibody; G-CNI, group with immunosuppressive therapy maintenance; G-STOP, group with discontinued immunosuppressive therapy; HLA, human leucocyte antigen; IFTA, interstitial fibrosis and tubular atrophy; NA, not applicable.
G-CNI patients, compared to G-STOP patients, had a lower median cPRA level at the time of the second KT (67% [0; 84] vs. 87% [55; 96], p = 0.001). The rate of hyperimmunized patients was also lower in the G-CNI: 23.9% versus 55.2% in the G-STOP (p < 0.001). The numbers of patients transplanted with preformed DSAs and induction treatment were comparable between the groups (Table 3).
Patients in the G-STOP were more frequently transplanted with an expanded criteria donor graft (43.2% vs. 29.9% in the G-CNI, p = 0.01). Hyperimmunized patients, compared with patients with cPRA levels <85%, were more likely to receive a kidney transplant from an expanded criteria donor [44.1% and 32%, respectively (p = 0.005)]. On the day of the second KT, 44.1% of G-CNI patients had a residual diuresis ≥500 mL compared to 13.8% in G-STOP (p = 0.06). The delayed graft function rate was 9.9% in the G-CNI and 25.8% in the G-STOP (p = 0.001). The numbers of patients transplanted with preformed DSA and an induction treatment were comparable between the groups (Table 3). Data before IPTW are presented in Supplementary Table SI.
Outcome After the Second KT After IPTW
After the second KT, 10 years survival free of dialysis and death was significantly better in the G-CNI than in the G-STOP (HR: 0.06, 95% CI: 0.01–0.30, p = 0.001) (Figure 2), with 10 years survival rates of 98.7% and 59.5%, respectively. In multivariable analysis after adjustment for expanded criteria donor, rejection, delayed graft function, age at second KT, graft survival time from the primary transplant, and rejection as etiology of first graft failure, continuation of CNIs between the two KTs was associated with a better 10 years survival free of dialysis and death (HR: 0.08, 95% CI: 0.01–0.58, p = 0.01) (Table 4). The difference in survival also remained significant after sensitivity analysis excluding second living donor transplants, with a 10 years survival rate of 98.5% in the G-CNI versus 56.4% in the G-STOP (HR: 0.06, 95% CI: 0.01–0.30, p = 0.001). Data on survival before IPTW are presented in Supplementary Figure S1.
FIGURE 2
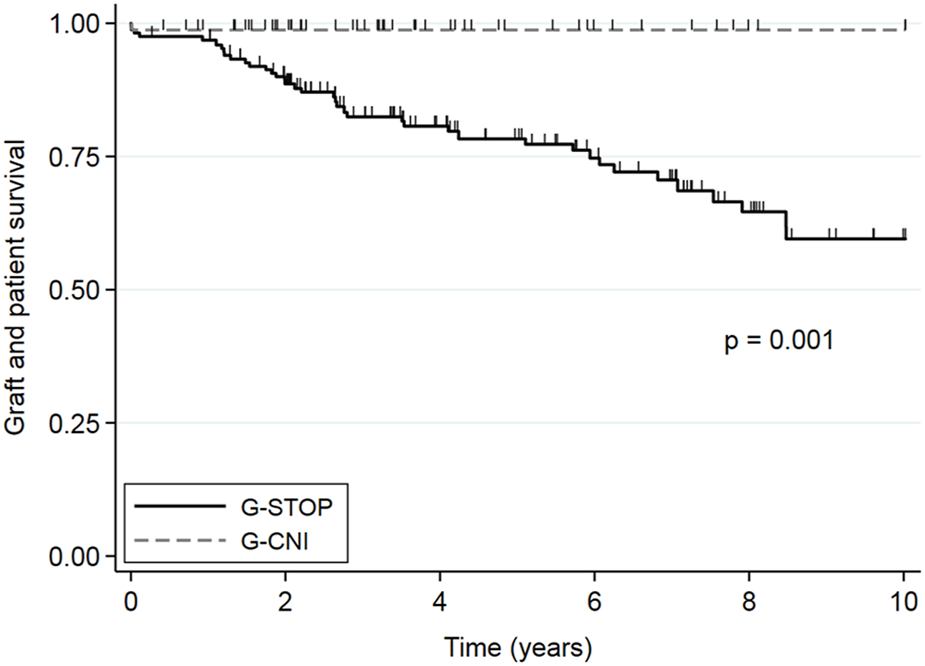
Overall second kidney transplant survival (without death or kidney transplant failure) in patients with immunosuppressive therapy maintenance until the second graft (G-CNI) or discontinued therapy (G-STOP). Data are presented after the inverse probability of treatment weighting.
TABLE 4
| HR | 95% CI | p | |
|---|---|---|---|
| Second transplant | |||
| CNI maintenance (vs. stop) | 12.50 | 1.72; 100.0 | 0.01 |
| Expanded criteria donor | 0.40 | 0.09; 1.79 | 0.23 |
| Recipient age (years) | 0.97 | 0.94; 1.01 | 0.06 |
| Rejection | 0.32 | 0.20; 0.52 | <0.001 |
| Cold ischemia time (minutes) | 1.00 | 1.00; 1.01 | 0.77 |
| First transplant | |||
| Graft survival (years) | 1.01 | 1.01; 1.01 | 0.002 |
| Rejection | 0.42 | 0.27; 0.67 | <0.001 |
Multivariable analysis of the factors associated with 10 years survival free of dialysis and death in patients after second renal transplantation after inverse probability weighting.
CI, confidence interval; CNI, calcineurin inhibitor; HR, hazard ratio.
A return to dialysis was observed in 18.3% of G-STOP patients compared to 1.3% of G-CNI patients (p = 0.004). The main cause of graft loss was rejection (45.1%). The number of humoral rejections and the occurrence of DSA were comparable in the two groups, but there was less cellular rejection in the G-CNI than in the G-STOP (2.1% vs. 8.8%, respectively, p < 0.001). All deaths were observed in the G-STOP (Table 3; Supplementary Table SI).
Major Cardiovascular, Infectious, and Neoplastic Events
In the period between the two KTs, the serious infectious event rates and their exposure-adjusted rates (patient-years) in the G-CNI and G-STOP were similar (Figure 3A; Supplementary Figure S2). The rates of cardiovascular events and neoplasia and their exposure-adjusted rates were significantly lower in the G-CNI than in the G-STOP (Figure 3A; Supplementary Figure S2).
FIGURE 3
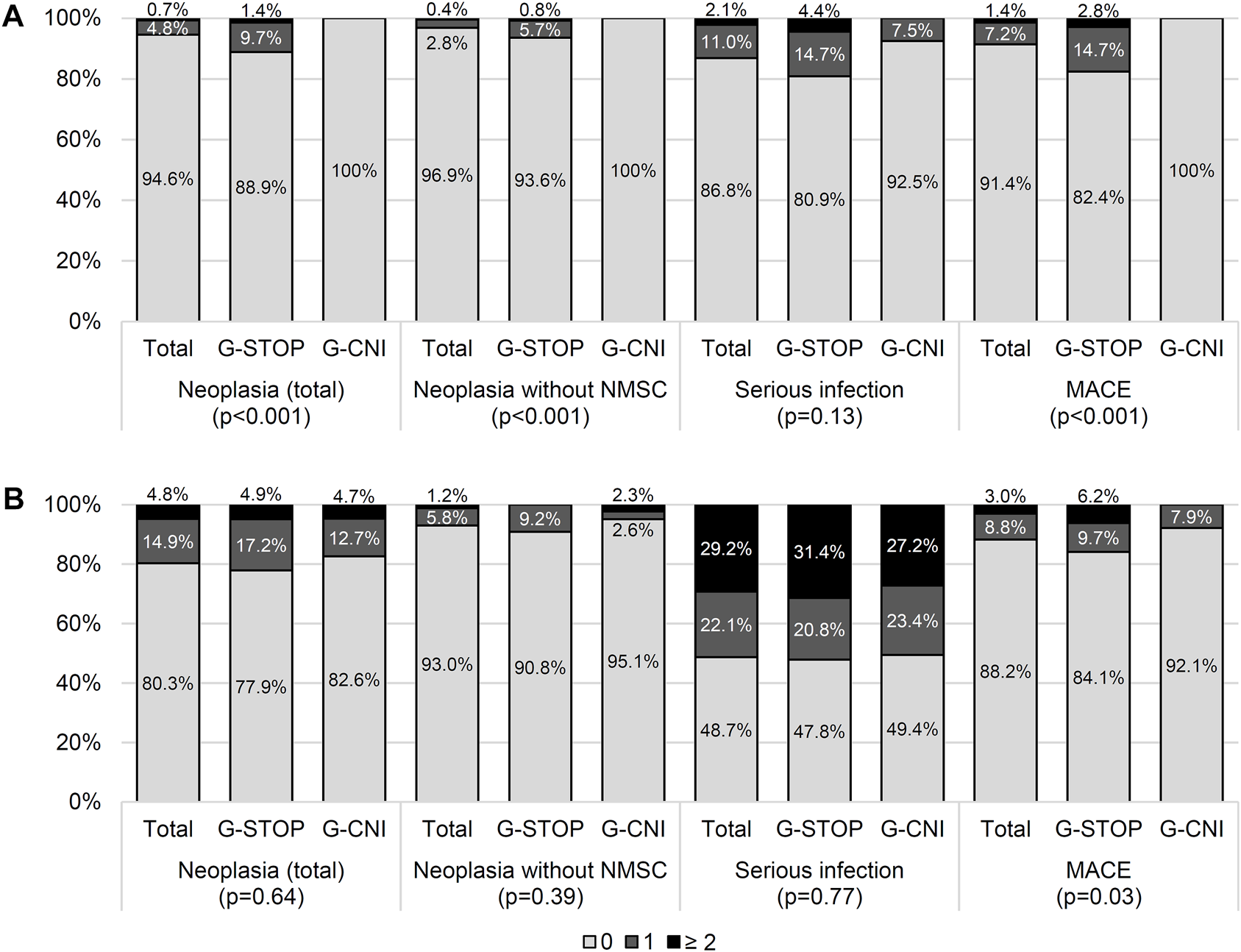
Adverse events between the two kidney transplants (A) and after the second transplant (B) in patients with immunosuppressive therapy maintenance (G-CNI) or discontinued therapy (G-STOP). Data are presented after the inverse probability of treatment weighting. MACE, major cardiovascular event; NMSC, non-melanoma skin cancer.
At the last follow-up after the second KT, the rates of patients with neoplastic events were similar in the G-CNI and G-STOP (Figure 3B). The rate of cardiovascular events was lower in the G-CNI than in the G-STOP (7.9% and 15.9%, respectively, p = 0.04) (Figure 3B). The serious infectious event rates were similar in the G-CNI and G-STOP (Figure 3B), but the exposure-adjusted rate was higher in the G-CNI than in the G-STOP (28.2/100 p-y and 22.8/100 p-y, respectively; p = 0.02) (Supplementary Figure S2).
Overall, after the first KTF, patients in the G-CNI and the G-STOP had a higher exposure-adjusted rate of serious infection (22.0/100 p-y and 15.3/100 p-y, respectively; p < 0.001) but a lower rate of major cardiovascular events (1.5/100 p-y and 4.8/100 p-y, respectively; p < 0.001). The exposure-adjusted rate of neoplasia was similar in both groups (Supplementary Figure S2).
Discussion
To our knowledge, this retrospective multicenter study is the first report relative to the impact of maintaining IS with CNIs in patients with KTF throughout the dialysis period on the second KT. Our results show that the maintenance of CNI-based IS therapy during the dialysis period is associated with a lower HLA immunization rate, lower waiting time before retransplantation, and less use of expanded criteria donors. Remarkably, G-CNI patients had a better survival free of dialysis and death at 10 years than G-STOP patients.
In the literature, the negative impact of the dialysis waiting time after KTF on the subsequent KT outcome and increased mortality is well documented [11, 14, 21]. In a recent study of 911 patients from the ANZDATA registry, each year spent on dialysis after KTF was associated with a 5% increase in the risk of death (mainly from cardiovascular or infectious events) as well as a greater risk of acute rejection and graft failure after the second KT [11]. The impact of the second KT on survival seems to be particularly beneficial when it takes place in the first 3 years after the return to dialysis [15]. One way to explain the two-fold shorter dialysis wait time in the G-CNI compared to the G-STOP in our study is the lower immunization after KTF in the G-CNI before and after IPTW. Indeed, despite a similar cPRA level at re-registration after PS analysis, G-CNI patients had a significantly lower median cPRA level at the time of the second KT. Moreover, the rate of hyperimmunized subjects was also lower in the G-CNI than in the G-STOP. These results are consistent with those previously reported in the literature. Thus, in 77 Spanish patients who experienced KTF, the cessation of CNIs in the first 6 months was significantly associated with the development of DSA with respect to the first graft (odds ratio: 23.2, 95% CI: 5.3–100.6, p < 0.001) [27]. In another study performed in the USA in 119 patients with KTF, 68% of patients with discontinued IS were hyperimmunized after 24 months, compared to 8% of patients with IS continuation that included a CNI (p < 0.001) [26]. The latter had better access to retransplantation (46% vs. 29%) and a shorter median waiting time between relisting and the second KT (17 [7; 55] vs. 36 [3; 72] months) 23. The significantly higher rate of hyperimmunized patients in the G-STOP group may explain in part why these patients received more expanded criteria deceased donor allografts [32]. Indeed, French biomedical agency gives priority access to KT for patients with PRA levels >85%. In comparison with other donor types, the use of expanded criteria deceased donor kidneys for transplantation has a significant negative impact on graft survival [39–41] and death [40], whether it is a first transplant or a retransplant [14].
While the rate of humoral rejection was similar in both groups, we observed a lower rate of cellular rejection in the G-CNI than in the G-STOP after the second KT. The rates of second transplants with preformed DSAs and de novo DSAs were similar in the two groups, which may explain the similar humoral rejection rates in the two groups [13]. Healthy et al. previously reported risk factors for acute rejection after retransplant as a shorter primary graft survival, rejection in the first KTF, and a long time spent on dialysis [14]. We can hypothesize the role of alloreactive memory T-cells [42, 43] acquired during the first allograft period but also during the dialysis period [29]. Indeed, a recent German retrospective study [29] reported a significantly lower rate of T-cell-mediated rejection of the second KT and better graft survival (p = 0.02) in patients with in situ previous transplants who also usually had CNI maintenance compared to patients with first allograft nephrectomy who also usually discontinued therapy. The authors observed less T-cell alloreactivity measured by ELISPOT assay against the pretransplant donor in the group with CNI maintenance for a prolonged period compared to patients with discontinued treatment due to transplantectomy [29].
The benefit-risk balance of IS maintenance until a new KT is widely debated in the literature. Some retrospective cohort studies have observed higher rates of major cardiovascular, infectious, or tumor events in patients with IS maintenance [22, 23]. However, the IS regimens continued after KTF are highly variable and could include only low-dose corticosteroids. In our previous work, we reported an increased risk of infection associated with the continuation of corticosteroids but not with CNI maintenance therapy [44]. In the present work, we did not observe an increase in these adverse events before the second KT in the G-CNI. Our results are similar to the most recent data available [26]. In a study of 102 patients with KTF, mortality was similar in patients in whom IS was discontinued early within 3 months after KTF (n = 52) and in patients (n = 50) in whom IS was continued with antimetabolites and/or CNIs [45]. A Canadian prospective registry did not observe any difference in the infectious rate between patients in whom IS was continued after KTF and those in whom IS was discontinued [28]. However, we observed higher exposure-adjusted rates (p-y) of serious infectious events in G-CNI after the second KT. Future studies will have to be vigilant regarding this point.
The current work includes several limitations. First, due to the retrospective nature of the study, major differences between the two groups were observed, such as the rates of diabetes at the end of the first KT, persistent infections at the time of KTF, and PRAs level at relisting in the G-CNI. We thus proposed a PS analysis using the IPTW method to reduce the effect of these confounding factors that may have influenced survival. However, we cannot exclude the existence of factors not accounted for [46]. Indeed, there seem to be patient profiles in which IS is more likely to be maintained, such as the persistence of significant diuresis [47] or a living donor transplant [48]. Recently, a prospective Canadian study showed a similar profile of patients on IS therapy after KTF. Other underlying confounding factors are probably unknown, such as social level [49] and ease of access to care [50, 51]. One of the main decision-making factors remains the prescribing habits of transplant nephrologists, as highlighted by recent surveys in the USA [48, 52] and France [44]. Only a prospective randomized study will be able to overcome the confounding factors. Second, as this study focused on patients who had access to a second KT, we cannot exclude the possibility that patients who had continued CNI-based IS after KTF experienced serious adverse events with abandonment of the retransplant plan or even death without being counted. Additionally, we were not able to access the date of cessation of IS treatment and thus establish its temporality in relation to the possible occurrence of an adverse event. However, we previously carried out a preliminary retrospective study of 119 KT patients relisted after KTF at four French adult KT centers. We did not report an increased risk of infectious, neoplastic, or cardiovascular events or death in patients in whom a CNI was continued for more than 3 months after KTF [44]. Furthermore, in the present cohort, according to the records, only one patient who was not immunized had IS interruption due to infection 2 months before retransplant. He subsequently developed acute antibody-mediated rejection with preformed DSAs against the new transplant. Finally, we chose to consider the maintenance of IS treatments only if CNIs were maintained. Indeed, only CNIs were associated with lower immunization during the inter-transplant period [27, 44, 53]. For the cohorts reported in the literature [48], G-CNI patients received heterogeneous treatments, with only one-third of patients on CNIs alone and almost one-fifth of patients on triple IS. Furthermore, residual CNI levels are rarely measured in patients after KTF and therefore were not collected. Only a recent English study of 48 adult KTF transplant recipients reported a residual tacrolimus level ≥3 ng/ml as protective against the development of alloimmunization [54]. Further studies are necessary to determine the optimal CNI-based IS protocols after KTF.
Our study shows that after KTF, maintaining CNI-based IS in a cohort of patients without heavy comorbidities may reduce the risk of immunization, shorten the waiting time, and provide better access to standard criteria donor grafts. These strategies may improve the survival of the subsequent graft and these patients.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Oversight and study approval were provided by the Committee for Protection of Human Subjects (CPP SUD-EST VI) on September 03, 2019 (institutional review board 00008526) and by the National consultative committee on the use of health research information (14.510)—No written consent was required for this study but a notice of non opposition letter was send to all patients in accordance with the national legislation (MR-004 reference methodology) [36]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JN collected data, analysed data, and wrote the paper. VM designed the research/study, analysed data, and wrote the paper. CéL, analysed data and contributed important reagents. LC collected data. BC collected data. AT collected data. LE collected data. DB collected data. ChL collected data. RL collected data. ClG collected data. MF collected data. A-EH collected data. P-OR designed the research/study and analysed data and contributed important reagents. CyG designed the research/study, analysed data, and wrote the paper.
Acknowledgments
Julien Aniort, Alba Atenza, Carole Philipponnet, Mohamed Hadj, Charlotte Uro-Coste, Aurélien Tiple, Ioana Enache, Julien Baudenon, Pessa Coulibaly, Arnaud Klisnick, Mohamed Mahieddine, Marc Bouiller, Céline Nadalin, Audrey Eyral, Clémentine Nicolo, Nathalie Charge, Fabien Duthé, Mickael Martin, Aurélie Plantie.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11775/full#supplementary-material
Supplementary Figure S1Overall second kidney transplant survival (without death or kidney transplant failure) in patients with immunosuppressive therapy maintenance until the second graft (G-CNI) or discontinued therapy (G-STOP). Data are presented before the inverse probability of treatment weighting.
Supplementary Figure S2Exposure-adjusted rates of serious complications in patients with immunosuppressive therapy maintenance until the second graft (G-CNI) or discontinued therapy (G-STOP). Data are presented after the inverse probability of treatment weighting. KT, kidney transplant; MACE, major cardiovascular event; NMSC, non-melanoma skin cancer. *p < 0.05.
Abbreviations
CI, confidence interval; CNI, calcineurin inhibitor; cPRA, calculated panel reactive antibody; DSA, donor-specific antibody; G-CNI, group with immunosuppressive therapy maintenance; G-STOP, group with discontinued immunosuppressive therapy; HLA, human leucocyte antigen; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IS, immunosuppressive therapy; KT, kidney transplant; KTF, kidney transplant failure; PS, propensity score; p-y, patient-years.
References
1.
Agence de la biomédecine. Organs - Kidney Transplant (2023). Available from: https://rams.agence-biomedecine.fr/greffe-renale-0 (Accessed August 11, 2023).
2.
USRDS Annual Data Report. 2022 Annual Data Report (2022). Available from: https://adr.usrds.org/ (Accessed August 11, 2023).
3.
Eurotransplant. Statistics Report Library (2023). Available from: https://statistics.eurotransplant.org/index.php?search_type=waiting+list&search_organ=kidney&search_region=All+ET&search_period=by+year&search_characteristic=re-registrations&search_text=&search_collection= (Accessed August 11, 2023).
4.
The UK Kidney Association. 23rd Annual Report - Data to 31/12/2019 (2019). Available from: https://ukkidney.org/audit-research/annual-report/23rd-annual-report-data-31122019 (Accessed August 11, 2023).
5.
Agence de la biomédecine. Table R4. Demographic Characteristics of Patients Registered According to Their Future on the Kidney Transplant Waiting List in 2021 (2022). Available from: https://rams.agence-biomedecine.fr/media/2606 (Accessed August 11, 2023).
6.
Nimmo AMSA McIntyre S Turner DM Henderson LK Battle RK . The Impact of Withdrawal of Maintenance Immunosuppression and Graft Nephrectomy on HLA Sensitization and Calculated Chance of Future Transplant. Transpl Direct (2018) 4(12):e409. 10.1097/TXD.0000000000000848
7.
Scornik JC Kriesche HUM . Human Leukocyte Antigen Sensitization After Transplant Loss: Timing of Antibody Detection and Implications for Prevention. Hum Immunol (2011) 72(5):398–401. 10.1016/j.humimm.2011.02.018
8.
Hyun J Park KD Yoo Y Lee B Han BY Song EY et al Effects of Different Sensitization Events on HLA Alloimmunization in Solid Organ Transplantation Patients. Transpl Proc (2012) 44(1):222–5. 10.1016/j.transproceed.2011.12.049
9.
Sypek MP Kausman JY Watson N Wyburn K Holt SG Hughes P et al The Introduction of cPRA and its Impact on Access to Deceased Donor Kidney Transplantation for Highly Sensitized Patients in Australia. Transplantation (2021) 105(6):1317–25. 10.1097/TP.0000000000003410
10.
Bostock IC Alberú J Arvizu A Hernández-Mendez EA De-Santiago A González-Tableros N et al Probability of Deceased Donor Kidney Transplantation Based on % PRA. Transpl Immunol (2013) 28(4):154–8. 10.1016/j.trim.2013.05.002
11.
Wong G Chua S Chadban SJ Clayton P Pilmore H Hughes PD et al Waiting Time Between Failure of First Graft and Second Kidney Transplant and Graft and Patient Survival. Transplantation (2016) 100(8):1767–75. 10.1097/TP.0000000000000953
12.
Arnol M Prather JC Mittalhenkle A Barry JM Norman DJ . Long-Term Kidney Regraft Survival From Deceased Donors: Risk Factors and Outcomes in A Single Center. Transplantation (2008) 86(8):1084–9. 10.1097/TP.0b013e318187ba5c
13.
Lefaucheur C Loupy A Hill GS Andrade J Nochy D Antoine C et al Preexisting Donor-Specific HLA Antibodies Predict Outcome in Kidney Transplantation. J Am Soc Nephrol (2010) 21(8):1398–406. 10.1681/ASN.2009101065
14.
Heaphy ELG Poggio ED Flechner SM Goldfarb DA Askar M Fatica R et al Risk Factors for Retransplant Kidney Recipients: Relisting and Outcomes From Patients' Primary Transplant. Am J Transplant (2014) 14(6):1356–67. 10.1111/ajt.12690
15.
Kainz A Kammer M Reindl-Schwaighofer R Strohmaier S Petr V Viklicky O et al Waiting Time for Second Kidney Transplantation and Mortality. Clin J Am Soc Nephrol (2022) 17(1):90–7. 10.2215/CJN.07620621
16.
Sapir-Pichhadze R Tinckam KJ Laupacis A Logan AG Beyene J Kim SJ . Immune Sensitization and Mortality in Wait-Listed Kidney Transplant Candidates. J Am Soc Nephrol (2016) 27(2):570–8. 10.1681/ASN.2014090894
17.
KDIGO. Controversies Conference on Challenges in Management of the Kidney Allograft: From Decline to Failure – KDIGO (2022). Available from: https://kdigo.org/conferences/challenging-allograft/ (Accessed April 09, 2022).
18.
Marinaki S Skalioti C Boletis J . Patients After Kidney Allograft Failure: Immunologic and Nonimmunologic Considerations. Transpl Proc (2015) 47(9):2677–82. 10.1016/j.transproceed.2015.09.054
19.
Morales A Gavela E Kanter J Beltrán S Sancho A Escudero V et al Treatment of Renal Transplant Failure. Transpl Proc (2008) 40(9):2909–11. 10.1016/j.transproceed.2008.09.047
20.
Pham PT Pham PC . Immunosuppressive Management of Dialysis Patients With Recently Failed Transplants. Semin Dial (2011) 24(3):307–13. 10.1111/j.1525-139X.2011.00864.x
21.
Lubetzky M Tantisattamo E Molnar MZ Lentine KL Basu A Parsons RF et al The Failing Kidney Allograft: A Review and Recommendations for the Care and Management of a Complex Group of Patients. Am J Transpl (2021) 21(9):2937–49. 10.1111/ajt.16717
22.
Woodside KJ Schirm ZW Noon KA Huml AM Padiyar A Sanchez EQ et al Fever, Infection, and Rejection After Kidney Transplant Failure. Transplantation (2014) 97(6):648–53. 10.1097/01.TP.0000437558.75574.9c
23.
Smak Gregoor PJ Zietse R van Saase JL op de Hoek CT Ijzermans JN Lavrijssen AT et al Immunosuppression Should Be Stopped in Patients With Renal Allograft Failure. Clin Transpl (2001) 15(6):397–401. 10.1034/j.1399-0012.2001.150606.x
24.
van Leeuwen MT Webster AC McCredie MRE Stewart JH McDonald SP Amin J et al Effect of Reduced Immunosuppression After Kidney Transplant Failure on Risk of Cancer: Population Based Retrospective Cohort Study. BMJ (2010) 340:c570. 10.1136/bmj.c570
25.
Andrews PA, Standards Committee of the British Transplantation Society. Summary of the British Transplantation Society Guidelines for Management of the Failing Kidney Transplant. Transpl Transplant (2014) 98(11):1130–3. 10.1097/TP.0000000000000426
26.
Augustine JJ Woodside KJ Padiyar A Sanchez EQ Hricik DE Schulak JA . Independent of Nephrectomy, Weaning Immunosuppression Leads to Late Sensitization After Kidney Transplant Failure. Transplantation (2012) 94(7):738–43. 10.1097/TP.0b013e3182612921
27.
López del Moral Cuesta C Guiral Foz S Gómez Pereda D Pérez Canga JL de Cos Gómez M Mazón Ruiz J et al Immunosuppression With Calcineurin Inhibitor After Renal Transplant Failure Inhibits Allosensitization. Biomedicines (2020) 8(4):72. 10.3390/biomedicines8040072
28.
Knoll G Campbell P Chassé M Fergusson D Ramsay T Karnabi P et al Immunosuppressant Medication Use in Patients With Kidney Allograft Failure: A Prospective Multicenter Canadian Cohort Study. J Am Soc Nephrol (2022) 33(6):1182–92. 10.1681/ASN.2021121642
29.
Schachtner T Otto NM Stein M Reinke P . Transplantectomy Is Associated With Presensitization With Donor-Reactive T Cells and Graft Failure After Kidney Retransplantation: A Cohort Study. Nephrol Dial Transpl (2018) 33(5):889–96. 10.1093/ndt/gfy002
30.
Fishman JA . Opportunistic Infections--Coming to the Limits of Immunosuppression?Cold Spring Harbor Perspect Med (2013) 3(10):a015669. 10.1101/cshperspect.a015669
31.
Fishman JA . Infection in Solid-Organ Transplant Recipients. N Engl J Med (2007) 357(25):2601–14. 10.1056/nejmra064928
32.
Metzger RA Delmonico FL Feng S Port FK Wynn JJ Merion RM . Expanded Criteria Donors for Kidney Transplantation. Am J Transpl (2003) 3(4):114–25. 10.1034/j.1600-6143.3.s4.11.x
33.
Mallon DH Summers DM Bradley JA Pettigrew GJ . Defining Delayed Graft Function After Renal Transplantation: Simplest Is Best. Transplantation (2013) 96(10):885–9. 10.1097/TP.0b013e3182a19348
34.
Loupy A Haas M Roufosse C Naesens M Adam B Afrouzian M et al The Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T Cell– And Antibody‐mediated Rejection. Am J Transpl (2020) 20(9):2318–31. 10.1111/ajt.15898
35.
Bertrand D Farce F Laurent C Hamelin F François A Guerrot D et al Comparison of Two Luminex Single-Antigen Bead Flow Cytometry Assays for Detection of Donor-Specific Antibodies After Renal Transplantation. Transplantation (2019) 103(3):597–603. 10.1097/TP.0000000000002351
36.
France – Health Research and Data Protection. Research Standard MR-004 (2023). Available from: https://www.cnil.fr/fr/declaration/mr-004-recherches-nimpliquant-pas-la-personne-humaine-etudes-et-evaluations-dans-le (Accessed July 16, 2023).
37.
Rosembaum P Rubin D . The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika (1983) 70(1):41–55. 10.1093/biomet/70.1.41
38.
Robins JM Hernán MÁ Brumback B . Marginal Structural Models and Causal Inference in Epidemiology: Epidemiology. Epidemiology (2000) 11(5):550–60. 10.1097/00001648-200009000-00011
39.
Larkins NG Wong G Johnson DW Hawley C Teixeira-Pinto A Pleass H et al Early Graft Loss Following Transplantation From Expanded Criteria Donors. Transplant Direct (2021) 7(11):e783. 10.1097/TXD.0000000000001235
40.
Ma MKM Lim WH Craig JC Russ GR Chapman JR Wong G . Mortality Among Younger and Older Recipients of Kidney Transplants From Expanded Criteria Donors Compared With Standard Criteria Donors. Clin J Am Soc Nephrol (2016) 11(1):128–36. 10.2215/CJN.03760415
41.
Aubert O Kamar N Vernerey D Viglietti D Martinez F Duong-Van-Huyen JP et al Long Term Outcomes of Transplantation Using Kidneys From Expanded Criteria Donors: Prospective, Population Based Cohort Study. BMJ (2015) 351:h3557. 10.1136/bmj.h3557
42.
Montero N Farouk S Gandolfini I Crespo E Jarque M Meneghini M et al Pretransplant Donor-Specific IFNγ ELISPOT as a Predictor of Graft Rejection: A Diagnostic Test Accuracy Meta-Analysis. Transplant Direct (2019) 5(5):e451. 10.1097/TXD.0000000000000886
43.
Duneton C Winterberg PD Ford ML . Activation and Regulation of Alloreactive T Cell Immunity in Solid Organ Transplantation. Nat Rev Nephrol (2022) 18(10):663–76. 10.1038/s41581-022-00600-0
44.
Freist M Bertrand D Bailly E Lambert C Rouzaire PO Lemal R et al Management of Immunosuppression After Kidney Transplant Failure: Effect on Patient Sensitization. Transpl Proc (2021) 53(3):962–9. 10.1016/j.transproceed.2020.10.009
45.
Casey MJ Wen X Kayler LK Aiyer R Scornik JC Meier-Kriesche HU . Prolonged Immunosuppression Preserves Nonsensitization Status After Kidney Transplant Failure. Transplantation (2014) 98(3):306–11. 10.1097/TP.0000000000000057
46.
Schold JD Augustine JJ Huml AM O’Toole J Sedor JR Poggio ED . Modest Rates and Wide Variation in Timely Access to Repeat Kidney Transplantation in the United States. Am J Transpl (2020) 20(3):769–78. 10.1111/ajt.15646
47.
Fiorentino M Gallo P Giliberti M Colucci V Schena A Stallone G et al Management of Patients With A Failed Kidney Transplant: What Should We Do? Clin Kidney J (2021) 14(1):98–106. 10.1093/ckj/sfaa094
48.
Alhamad T Lubetzky M Lentine KL Edusei E Parsons R Pavlakis M et al Kidney Recipients With Allograft Failure, Transition of Kidney Care (KRAFT): A Survey of Contemporary Practices of Transplant Providers. Am J Transpl (2021) 21(9):3034–42. 10.1111/ajt.16523
49.
Udayaraj U Ben-Shlomo Y Roderick P Casula A Dudley C Johnson R et al Social Deprivation, Ethnicity, and Access to the Deceased Donor Kidney Transplant Waiting List in England and Wales. Transplantation (2010) 90(3):279–85. 10.1097/TP.0b013e3181e346e3
50.
Schold JD Sehgal AR Srinivas TR Poggio ED Navaneethan SD Kaplan B . Marked Variation of the Association of ESRD Duration Before and After Wait Listing on Kidney Transplant Outcomes. Am J Transpl (2010) 10(9):2008–16. 10.1111/j.1600-6143.2010.03213.x
51.
Haugen CE Agoons D Chu NM Liyanage L Long J Desai NM et al Physical Impairment and Access to Kidney Transplantation. Transplantation (2020) 104(2):367–73. 10.1097/TP.0000000000002778
52.
Bayliss GP Gohh RY Morrissey PE Rodrigue JR Mandelbrot DA . Immunosuppression After Renal Allograft Failure: A Survey of Us Practices. Clin Transpl (2013) 27(6):895–900. 10.1111/ctr.12254
53.
Garg N Viney K Burger J Hidalgo L Parajuli S Aziz F et al Factors Affecting Sensitization Following Kidney Allograft Failure. Clin Transpl (2022) 36(3):e14558. 10.1111/ctr.14558
54.
Lucisano G Brookes P Santos-Nunez E Firmin N Gunby N Hassan S et al Allosensitization After Transplant Failure: The Role of Graft Nephrectomy and Immunosuppression - A Retrospective Study. Transpl Int (2019) 32(9):949–59. 10.1111/tri.13442
Summary
Keywords
kidney retransplant, kidney transplant failure, calcineurin inhibitor maintenance, waiting list, immunosuppression
Citation
Noelle J, Mayet V, Lambert C, Couzi L, Chauveau B, Thierry A, Ecotière L, Bertrand D, Laurent C, Lemal R, Grèze C, Freist M, Heng A-E, Rouzaire P-O and Garrouste C (2023) Impact of Calcineurin Inhibitor-Based Immunosuppression Maintenance During the Dialysis Period After Kidney Transplant Failure on the Next Kidney Graft Outcome: A Retrospective Multicenter Study With Propensity Score Analysis. Transpl Int 36:11775. doi: 10.3389/ti.2023.11775
Received
06 July 2023
Accepted
25 August 2023
Published
15 September 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Noelle, Mayet, Lambert, Couzi, Chauveau, Thierry, Ecotière, Bertrand, Laurent, Lemal, Grèze, Freist, Heng, Rouzaire and Garrouste.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cyril Garrouste, cgarrouste@chu-clermontferrand.fr
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.