- 1Erasmus MC Transplant Institute, Rotterdam, Netherlands
- 2Department of Internal Medicine, Division of Nephrology and Transplantation, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
- 3Department of Internal Medicine, A. Schweitzer Hospital Dordrecht, Dordrecht, Netherlands
- 4Department of Pathology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
- 5Department of Hospital Pharmacy, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
- 6Department of Biostatistics, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
- 7Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
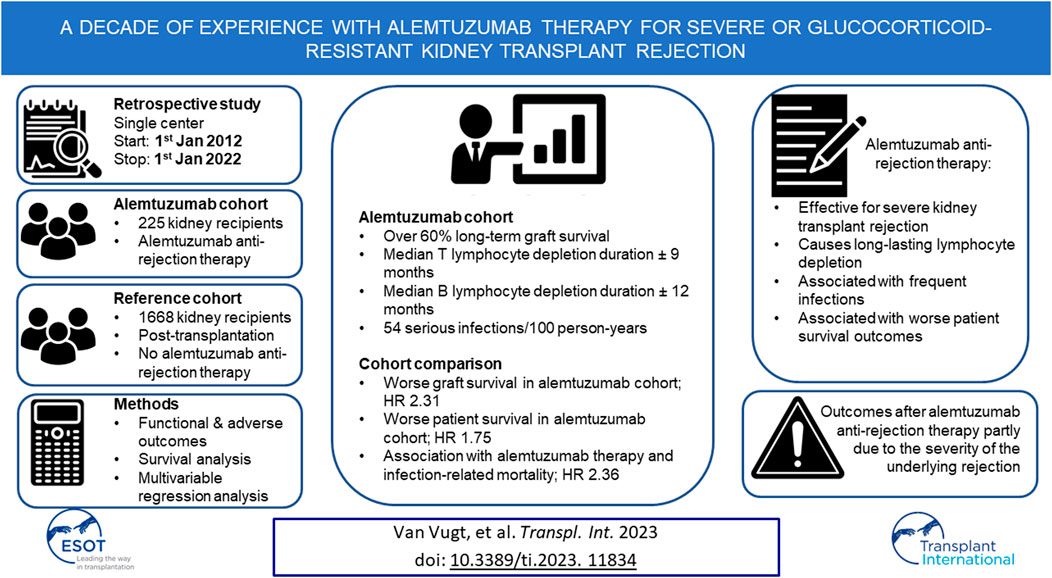
Alemtuzumab is used as lymphocyte-depleting therapy for severe or glucocorticoid-resistant kidney transplant rejection. However, the long-term efficacy and toxicity of alemtuzumab therapy are unclear. Therefore, all cases of alemtuzumab anti-rejection therapy between 2012 and 2022 in our institution were investigated. Graft survival, graft function, lymphocyte depletion, serious infections, malignancies, and patient survival were analyzed and compared with a reference cohort of transplanted patients who did not require alemtuzumab anti-rejection therapy. A total of 225 patients treated with alemtuzumab were identified and compared with a reference cohort of 1,668 patients. Over 60% of grafts was salvaged with alemtuzumab therapy, but graft survival was significantly poorer compared to the reference cohort. The median time of profound T- and B lymphocyte depletion was 272 and 344 days, respectively. Serious infection rate after alemtuzumab therapy was 54.1/100 person-years. The risk of death (hazard ratio 1.75, 95%-CI 1.28–2.39) and infection-related death (hazard ratio 2.36, 95%-CI 1.35–4.11) were higher in the alemtuzumab-treated cohort. In conclusion, alemtuzumab is an effective treatment for severe kidney transplant rejection, but causes long-lasting lymphocyte depletion and is associated with frequent infections and worse patient survival outcomes.
Introduction
Alemtuzumab is a monoclonal antibody directed against CD52 that causes depletion of T- and B lymphocytes, monocytes, and NK cells [1]. Alemtuzumab is prescribed off-label for both the prevention and treatment of acute kidney transplant rejection [2].
Rabbit anti-thymocyte globulin (rATG) is a lymphocyte-depleting antibody registered for the treatment of severe or glucocorticoid-resistant T cell-mediated rejection (TCMR) and may be used for treating severe antibody-mediated rejection (ABMR) [3, 4]. However, the requirement of a high-flow venous access for rATG administration and its associated infusion reactions have instigated the search for alternative therapies [5]. Previous studies demonstrated that alemtuzumab is a safe and efficacious alternative for rATG [5–9]. Notably, alemtuzumab is nearly devoid of infusion-related side effects when administered subcutaneously [10]. Therefore, since 2012, alemtuzumab has been the lymphocyte-depleting antibody of choice for treating severe or glucocorticoid-resistant kidney transplant rejection in our hospital [11].
Despite its efficacy and apparent short-term safety, concerns remain about its long-term adverse effects. Alemtuzumab causes profound and long-lasting lymphocyte depletion, which puts patients at risk for infection and malignancy. Furthermore, rare cases of autoimmunity have been linked to alemtuzumab therapy [12, 13].
Here, the long-term safety and efficacy of alemtuzumab was investigated in a large cohort of patients who received alemtuzumab to treat severe or glucocorticoid-resistant kidney transplant rejection.
Patients and Methods
Study Design
This was a retrospective cohort study that included all consecutive adult kidney transplant recipients who were treated with alemtuzumab for acute kidney transplant rejection (AR) between 1st January 2012, and 1st January 2022, at the Erasmus MC, University Medical Center Rotterdam, the Netherlands. The study was approved by the local medical ethical review board (protocol number MEC-2021-0924). Alemtuzumab-treated patients were identified via the hospital pharmacy records.
To interpret patient survival, graft survival, and the risk of malignancy of alemtuzumab-treated patients, they were compared to a reference cohort that consisted of all adult patients that received a kidney transplant in our hospital between 1st January 2012, and 1st January 2022, but were not treated with alemtuzumab for rejection. This reference cohort was identified through the Dutch Organ Transplant Registry (“Nederlandse Orgaantransplantatie Registratie” (NOTR)) database and included some patients with non-depleting anti-rejection therapy and some who received induction therapy with lymphocyte-depleting agents. To account for the effects of alemtuzumab induction therapy, comparative analyses were repeated after exclusion of reference patients who received alemtuzumab induction therapy.
Data was extracted from the electronic patient files and the NOTR. Data was collected after pseudonymization and stored in a protected hospital database. Collected data included patient and transplantation characteristics, pathology data, medication use, information on kidney outcomes, lymphocyte repopulation, and various clinical outcomes, including serious infections and malignancies. “Graft failure” was defined as return to dialysis, transplantectomy or re-transplantation. “Delayed graft function” was defined as the need for dialysis within the first post-transplant week. Primary non-function was determined at 3 months post-transplantation, unless transplantectomy or re-transplantation occurred earlier. “Insufficient treatment response” was defined as the need to treat the same graft with any additional anti-rejection therapy within 6 months after alemtuzumab therapy. “Serious infections” were defined as infections occurring during hospitalization or an infection that required hospital admission. Malignancies were counted from the year of alemtuzumab therapy in the alemtuzumab cohort and from the year of transplantation for reference patients. If multiple dermatologic malignancies were diagnosed within 1 year, they were counted as a single occurrence.
Outcomes and Follow-Up
Transplant-specific outcomes such as graft survival and function and alemtuzumab-specific outcomes such as post-treatment infections and lymphocyte recovery, were analyzed per kidney transplantation case. Patient-specific outcomes such as patient survival and the occurrence of malignancies were analyzed per patient case.
For transplant-specific outcomes, follow-up started at transplantation until graft loss, death or right censoring by loss to follow-up or treatment with rATG occurred. For patient-specific outcomes, follow-up started at first transplantation in the study period until death or right censoring by loss to follow-up or treatment with rATG occurred. For alemtuzumab-specific outcomes, follow-up started on the day of alemtuzumab treatment until death or right censoring by loss to follow-up, treatment with rATG or re-transplantation occurred.
Pathology
Kidney transplant biopsies of all alemtuzumab-treated patients were reviewed and reclassified according to the Banff 2019 classification by a nephro-pathologist (M.C.C–v.G.). No protocol biopsies were performed and only for-cause biopsies were analyzed. When multiple follow-up biopsies were performed after alemtuzumab therapy, only the first was revised. Rejections were considered biopsy-proven acute rejection (BPAR) if the diagnostic criteria of the Banff 2019 classification were fulfilled. Cases classified as presumed ABMRs demonstrated histologic signs of acute tissue injury (e.g., acute tubular necrosis or thrombotic microangiopathy) without C4d positivity or donor-specific anti-HLA antibodies (DSA). Cases demonstrating microvascular inflammation but without C4d and DSA were primarily classified as ABMR, but the functional outcomes were also analyzed without these cases in anticipation of the upcoming Banff 2021 classification. The presence or absence of DSA was assessed within 3 months before and up to 6 months after AR. Patients who were treated with alemtuzumab for non-BPAR were not included in the analysis of the functional outcomes of different types of BPAR.
Immunosuppressive Therapy
The typical immunosuppressive regimen in our center comprises induction therapy with 20 mg intravenous (IV) basiliximab (days 0 and 4) and 100 mg IV prednisolone (days 0–2) for both recipients of a living and deceased donor kidney, followed by an immunosuppressive maintenance regimen consisting of tacrolimus, mycophenolate mofetil (MMF), and glucocorticoids. Target tacrolimus pre-dose concentrations were 10–15 μg/L (weeks 1 and 2), 8–12 μg/L (weeks 3 and 4), 5–10 μg/L (weeks 5–16), and 5–8 μg/L thereafter [11]. MMF was started at a dose of 1,000 mg twice daily and was subsequently adjusted to target pre-dose concentrations of 1.5–3.0 mg/L. A 20 mg daily dose of prednisolone was started on day 3 and then tapered. Except for immunologically high-risk recipients, prednisolone was completely withdrawn around week 16 [11].
Anti-Rejection Therapy
The first-line treatment for TCMR and empirical treatment for suspected AR consisted of 1,000 mg IV methylprednisolone for three consecutive days. ABMR and mixed-type rejections were treated with methylprednisolone plus two doses of intravenous immunoglobulin (IVIG; 1 g/kg) with or without plasma exchange [14]. Alemtuzumab was prescribed for glucocorticoid-resistant, severe (Banff IIA or worse), or recurrent AR at the discretion of the treating nephrologist. The standard alemtuzumab dose was a single 30 mg dose administered subcutaneously. Premedication consisted of 50 mg IV prednisolone, acetaminophen, and clemastine. Patients received sulfamethoxazole/trimethoprim and valganciclovir prophylaxis until their T lymphocyte count exceeded 200 × 106/L.
Statistical Analysis
Statistical analysis was performed with the R statistical software (v4.3.0) [15], using the cmprsk (v2.2.11), ggeffects (v1.1.4), ggsurvfit (v0.2.1), icenReg (v2.0.15), interval (v1.1.0.8), kidney.epi (v1.2.0), MASS (v7.3.55), nlme (v3.1.155), survival (v3.4.0) and tidycmprsk (v0.2.0) packages. A two-sided p-value <0.05 was considered statistically significant. Continuous variables were expressed as means with standard deviations or medians with interquartile ranges (IQRs) when not normally distributed. Normality was assessed by visual inspection. The Mann-Whitney U and Kruskal–Wallis tests were used to compare continuous variables between groups. Categorical variables were reported as proportions with percentages, and differences between groups were assessed using the Fisher’s exact test.
Graft survival was analyzed with death as a competing risk and the non-parametric estimate of the cumulative incidence was plotted for its visualization. Patient survival was analyzed as a definitive endpoint and infection-free survival was analyzed as the time to first serious infection. Both were visualized with Kaplan-Meier survival curves. To correct for differences between the alemtuzumab and reference cohorts, while accounting for the time-dependent exposure of certain covariates, multivariable time-varying Cox proportional hazard models were used for the analysis of graft and patient survival. When a separation problem occurred, this was resolved with a ridge regression term. Multivariable Cox proportional hazard models were also used to evaluate associations between patient characteristics and survival outcomes from the initiation of therapy in the alemtuzumab cohort solely, and the cumulative incidence function between alemtuzumab-treated rejection subgroups was compared using the Gray’s test [16, 17]. To analyze interval-censored lymphocyte recovery data, the nonparametric maximum likelihood estimators of the survival functions were calculated to construct interval-censored survival curves [18]. Negative binomial regression models, where follow-up time was used as offset, were applied to assess covariate associations with malignancy and infection events.
To compare the median values of paired measurements of estimated glomerular filtration rate (eGFR), lymphocytes and urinary creatinine-protein ratios, the paired Wilcoxon signed rank test was used. To analyze the evolution of eGFR over time, we considered a linear mixed-effects model, with a linear fixed effect of time and an individual-specific random intercept.
Results
Patient, Transplant, and Rejection Characteristics
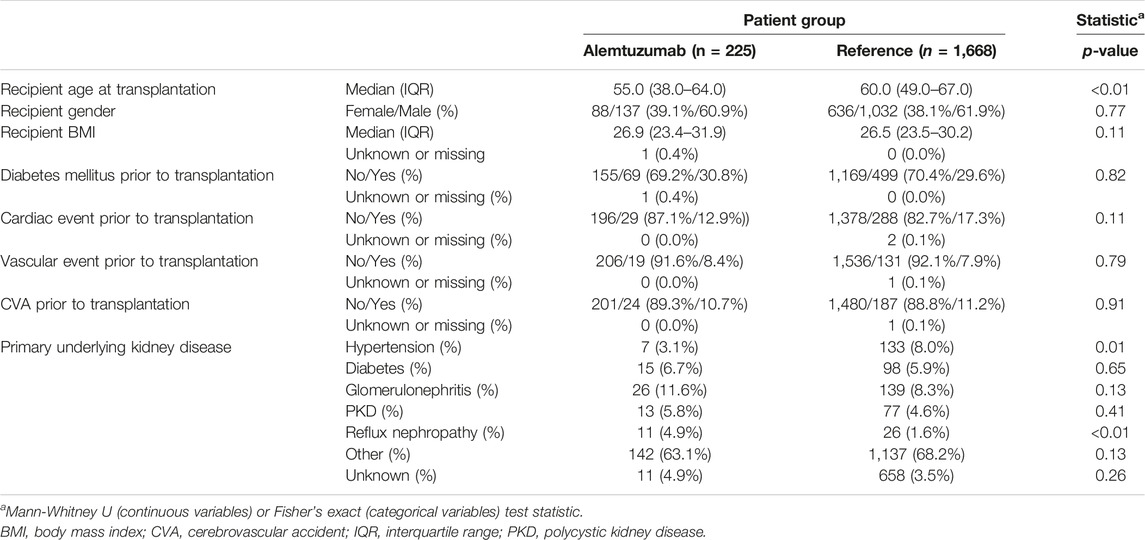
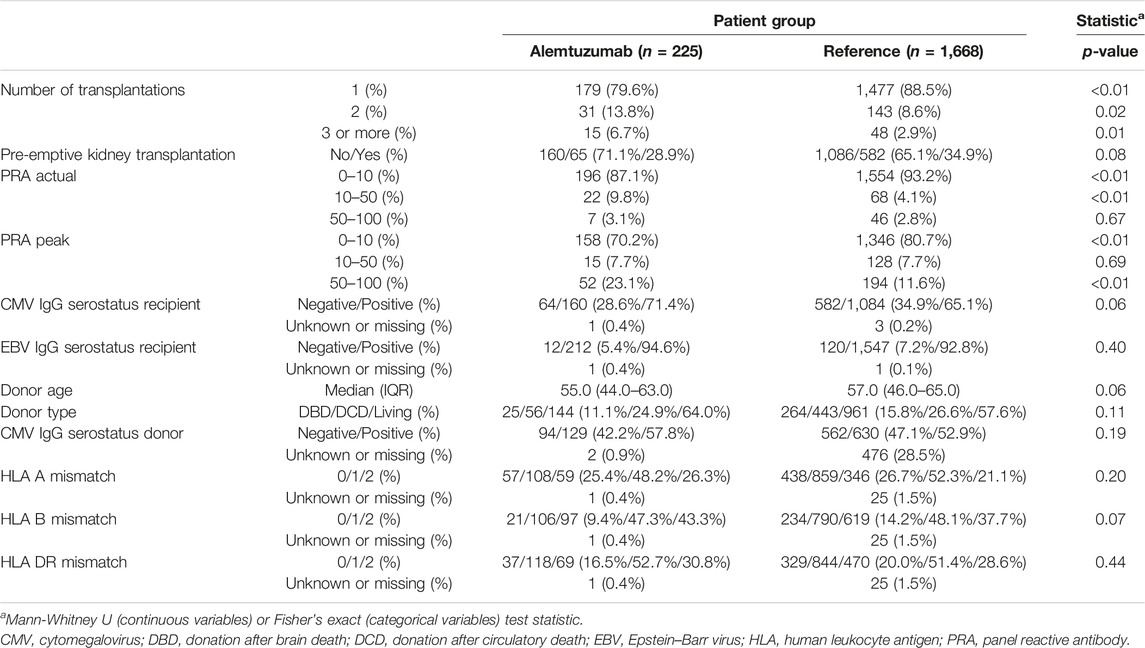
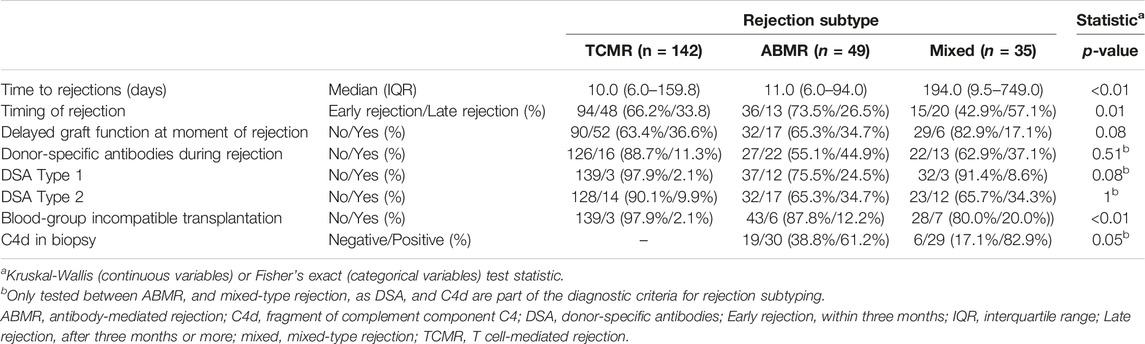
Between 1st January 2012, and 1st January 2022, 236 rejections were treated with alemtuzumab in 225 patients. Alemtuzumab was prescribed as second-line therapy for 174 glucocorticoid-resistant rejections (73.7% of 236 cases), and as first-line therapy for 62 severe rejections (26.3% of 236 cases). The reference cohort consisted of 1,732 kidney transplantations performed in 1,668 patients. This reference cohort included 53 transplantations in 46 patients in whom alemtuzumab was given as induction therapy. Alemtuzumab-treated patients were younger than reference patients (Table 1), had higher panel reactive antibodies (PRA) and were more likely to be repeat transplantations (Table 2). Of the 236 alemtuzumab-treated rejections, 226 were biopsy-proven. Details of these rejection episodes and their treatment are provided in Tables 3, 4.
Functional Outcomes
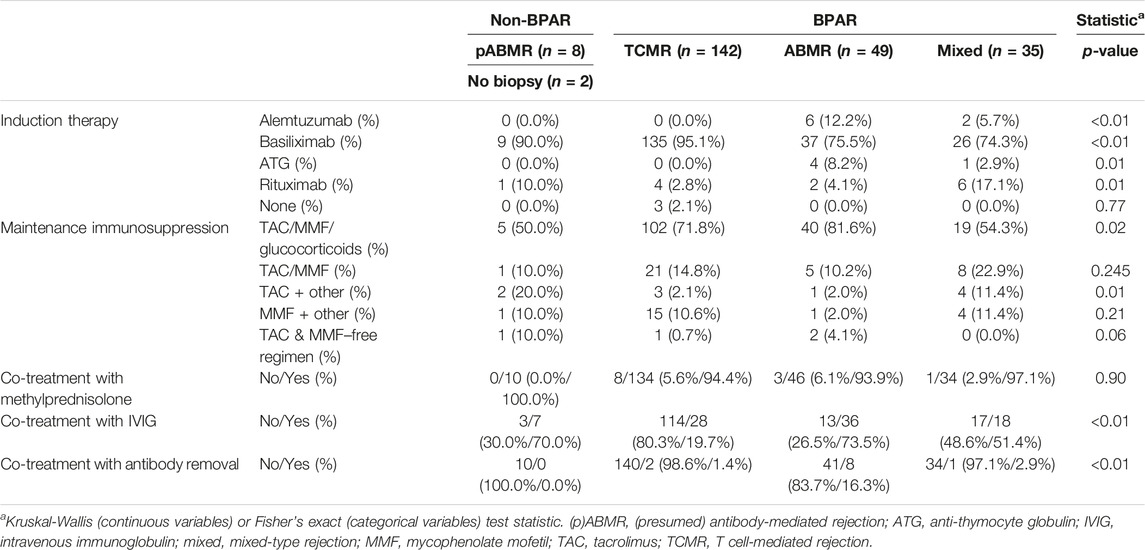
For better estimates of graft loss over time, the cumulative incidence of graft loss with death as competing risk was calculated (Figure 1). The cumulative incidence of graft loss at one, three and five years after alemtuzumab therapy was 21.7% (95%-CI 16.3–27.1), 32.3% (95%-CI 26.2–38.5), and 37.4% (95%-CI 31.1–43.8), respectively. The cumulative incidence of graft loss at one, three and five years after transplantation was 4.1% (95%-CI 3.2–5.0), 5.4% (95%-CI 4.4–6.5), and 7.0% (95%-CI 5.9–8.2), respectively. Alemtuzumab-treated patients also had a higher risk of graft loss after correcting for other covariates, including the start of any rejection treatment (hazard ratio [HR] 2.31, 95%-CI 1.72–3.10, Supplementary Table S1). These conclusions were not altered after exclusion of reference patients who received alemtuzumab induction.
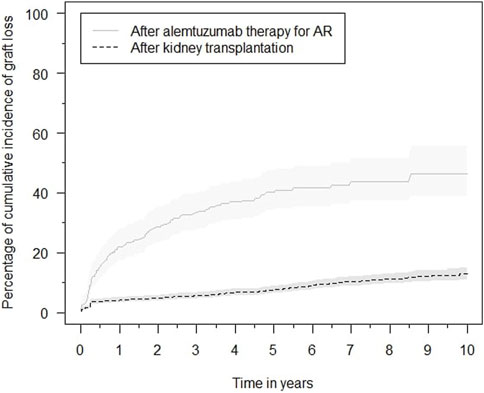
FIGURE 1. Cumulative incidence functions of graft loss in the alemtuzumab and reference groups, with associated 95% confidence intervals.
Graft loss was compared between different BPAR subtypes with a competing risk analysis for death (Supplementary Figure S1). The overall cumulative incidence of graft loss was not significantly different between TCMR, ABMR, and mixed-type rejection (p = 0.12). The cumulative incidence of graft loss associated with TCMR, ABMR and mixed-type rejection at 5 years after alemtuzumab therapy was: 36.1% (95%-CI 27.6–44.7), 44.0% (95%-CI 28.7–59.3) and 56.7% (95%-CI 38.7–74.6), respectively. Rejection type was not significantly associated with an increased risk of graft loss in multivariable analysis (Supplementary Table S2). Exclusion of C4d and DSA-negative rejections did not alter these conclusions.
The eGFR of patients not on dialysis are depicted in Figure 2. Kidney function improved significantly within 2 weeks after treatment with alemtuzumab and remained significantly better compared to baseline at all other time points (p < 0.01), with median values of 25–35 mL/min per 1.73 m2. A linear mixed-effects model was generated to model the trend of eGFR over time (Figure 3). eGFR tended to increase in the first year after alemtuzumab treatment, gradually decreased between 1 to 3 years after treatment, and then stabilized after 3 to 5 years. After 5 years, eGFR gradually declined. No significant differences were modelled for the different rejection subtypes (Supplementary Figure S2). The urinary protein-creatinine ratio was increased at the start of therapy (median 56.7 mg/mmol) and decreased significantly at three, six and twelve months after therapy (Supplementary Figure S3).
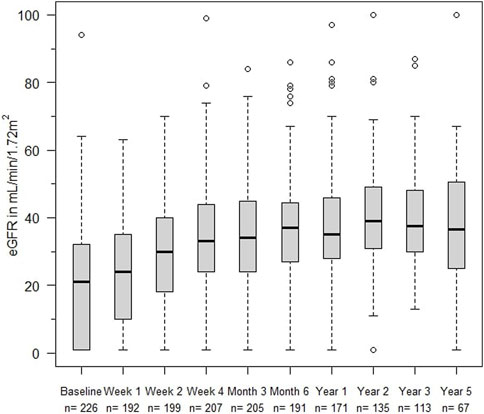
FIGURE 2. eGFR (mL/min/1,72 m2) at alemtuzumab therapy initiation and subsequent time points. Box indicates 25th–75th percentiles with medians. Whiskers indicate the value of 1.5 times the IQR below the 25th percentile or above the 75th percentile respectively. Dots indicate outliers.
FIGURE 3. Averaged estimated effect of time on eGFR (mL/min/1,72 m2) progression after alemtuzumab initiation, with associated 95% confidence intervals.
Follow-Up Biopsies
In 109 cases (46.2% of 236 cases), for-cause follow-up biopsies were obtained. Of these, 50 (45.9% of 109 biopsies) showed no rejection but another diagnosis such as recurrent, primary disease or infection. 59 (54.1% of 109 biopsies) showed TCMR (n = 19), ABMR (n = 21), or mixed-type (n = 19) rejection. Twenty biopsies demonstrated ABMR or mixed-type rejection after an initial diagnosis of TCMR. An overview of rejection type at diagnosis and during the first follow-up biopsy is provided in Supplementary Table S3.
Insufficient Treatment Response
During the first six months after alemtuzumab treatment, additional anti-rejection therapy was prescribed for 25 rejections (10.6%). Methylprednisolone was administered in 18 cases, IVIG in ten cases, a second course of alemtuzumab in ten cases and both tocilizumab and rATG in one case. Fifteen out of these 25 rejections were lost after additional therapy after a median of 419 days (IQR 133–980 days).
Hematologic Effects
Rapid and profound depletion of both T and B lymphocytes occurred after treatment and was not fully restored after 18 months (Figure 4). The baseline median T lymphocyte count was 627 × 106/L, and after 18 months, it was 201 × 106/L (p < 0.01). The baseline median B lymphocyte count was 140 × 106/L, and after 18 months, it was 97.5 × 106/L (p = 0.03). Figure 5 shows the interval-censored survival curve of lymphocyte recovery. The median time of T lymphocyte depletion, defined as a T lymphocyte count below 200 × 106/L, was 272 days. The median time of B lymphocyte depletion, defined as a B lymphocyte count below 100 × 106/L, was 344 days.
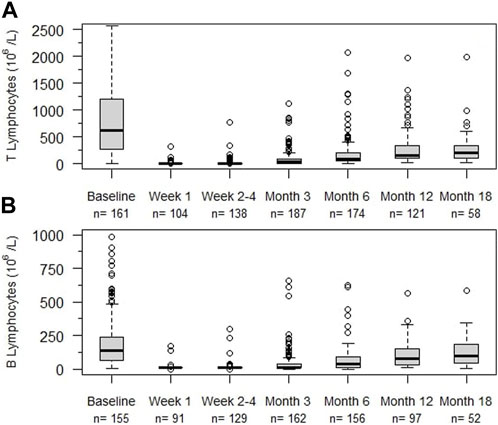
FIGURE 4. Lymphocyte counts (106/L) at different times after alemtuzumab initiation. (A) T lymphocytes, (B) B lymphocytes, n: number of individuals. Box indicates 25th – 75th percentiles with medians. Whiskers indicate the value of 1.5 times the IQR below the 25th percentile or above the 75th percentile respectively. Dots indicate outliers.
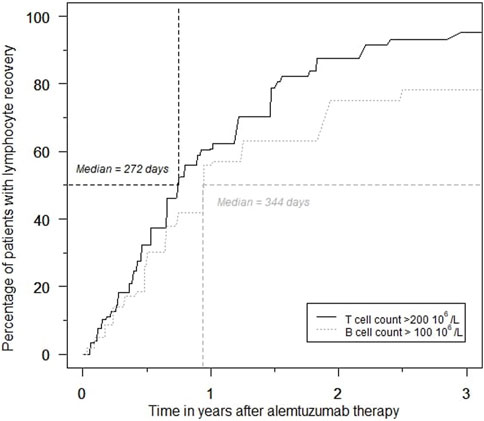
FIGURE 5. Percentage of patients with T- and B lymphocyte recovery over time during the first 3 years after alemtuzumab therapy.
Infections
A total of 512 serious infections occurred in 236 alemtuzumab-treated cases. The overall infection rate was 54.1 infections per 100 person-years (Table 5). Urinary tract infections were the most common (20.7 per 100 person-years), followed by pulmonary infections (12.9 per 100 person-years). The incidence of primo- and reactivation infection with BK virus (BKV), cytomegalovirus (CMV) and Epstein–Barr virus (EBV) was 22.5% (n = 53), 26.7% (n = 63), and 3.0% (n = 7), respectively. Serious infection-free survival is depicted in Supplementary Figure S4. Almost half of the alemtuzumab-treated patients experienced at least one serious infection within the first year after treatment. However, serious infections did not affect all patients to a similar degree. In approximately one-third of alemtuzumab-treated rejections (n = 73), no serious infections occurred. The infection count or time to first infection was not related to the duration of T- and B-cell depletion, but this explorative analysis was limited by missing repopulation data. The infection-free survival decreased and number of infections increased for older age at alemtuzumab initiation and with the presence of cardiovascular disease in medical history (Supplementary Tables S4, S5).
Malignancies
74 malignancies were diagnosed in 42 patients in the alemtuzumab cohort (18.7%), while 460 malignancies were diagnosed in 330 patients in the reference cohort (19.6%). Total malignancy counts and incidence rates are provided in Table 6. The incidence rates of overall, solid, dermatologic and hematologic malignancy counts were higher in the alemtuzumab cohort than the reference cohort, but only the overall malignancy incidence rate differed significantly. In multivariable count regression, however, alemtuzumab was not significantly associated with higher malignancy risk. This finding was not altered after exclusion of reference patients who received alemtuzumab induction.
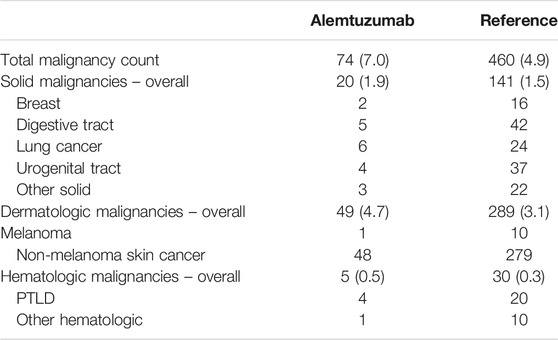
TABLE 6. Overview of malignancies. Data in absolute counts with incidence rates per 100 person-years.
Autoimmunity
Three cases of suspected alemtuzumab-related autoimmunity occurred among 225 patients (1.3%): one case of acquired hemophilia A [12], one case of Guillain-Barré syndrome [13] and one case of chronic inflammatory demyelinating polyradiculoneuropathy [13]. Furthermore, five autoimmune-related phenomena of unknown etiology were observed (2.2%): one case of vitiligo, one case of Raynaud’s phenomenon, one unexplained case of pericarditis, peritonitis and axonal polyneuropathy without demyelination, one case of recurrent pericarditis (which necessitated anakinra treatment), and one case of pulmonary granulomas.
Patient Survival
Patient survival after alemtuzumab treatment was inferior to overall post-transplantation survival (Figure 6). The survival probability one, three and 5 years after transplantation was 96.1%, 91.2%, and 84.7%, respectively. Comparatively, after alemtuzumab treatment, patient survival was 95.4%, 83.1%, and 72.7%, respectively. Alemtuzumab-treated patients had a significantly higher risk of death (HR 1.75, 95%-CI 1.28–2.39). Other baseline variables significantly associated with death were the start of any treatment for rejection, older age, diabetes mellitus, and having a medical history of at least one cardiac, peripheral vascular or cerebrovascular event at the time of transplantation (Supplementary Table S6). Alemtuzumab-treated patients also had a higher risk of infection-related death (HR 2.36, 95%-CI 1.35–4.11; Supplementary Table S7). However, they did not have a higher risk of malignancy-related death (Supplementary Table S8). These conclusions remained unaltered after excluding reference patients who received alemtuzumab induction.
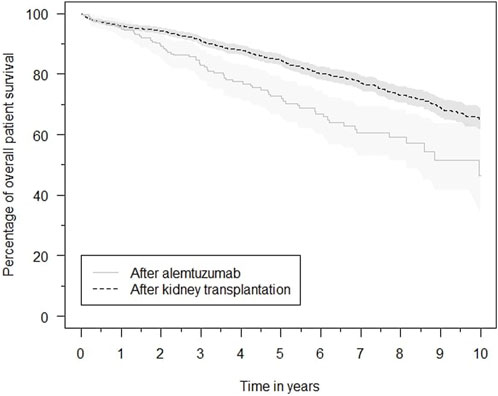
FIGURE 6. Kaplan–Meier estimates of the survival probability after kidney transplantation in general and after initiation of alemtuzumab therapy, with associated 95% confidence intervals.
Discussion
Here the efficacy and safety of alemtuzumab therapy for glucocorticoid-resistant or severe kidney transplant rejection is reported for the largest cohort described in the literature. The present findings demonstrate that alemtuzumab is an effective therapy to counter severe kidney transplant rejection. However, it leads to a profound, long-lasting lymphocyte depletion and is frequently complicated by serious infections. Furthermore, patient survival after alemtuzumab therapy is worse compared to the general post-transplant population.
Limitations
The major limitation of this study is the absence of a control group treated with rATG. A prospective comparison between alemtuzumab and rATG would be ideal for determining the superiority of one therapy over the other. Although we feel it is unlikely that such a head-to-head comparison will be performed anytime soon, the present data may serve as a power calculation basis for such a trial.
The aim of this study was to report the outcomes after alemtuzumab anti-rejection therapy and how these relate to the outcomes in a general transplantation cohort (our “reference” cohort). One should realize that the outcomes after alemtuzumab therapy are not solely dependent of the biological effects of alemtuzumab itself, but also of the effects of the severe rejection that prompted this therapy.
Another limitation was the presence of missing data due to the retrospective study design. Furthermore, bias may have been introduced due to incomplete outcome reporting, especially for the recording of serious infections and malignancies.
Graft Survival
Not surprisingly as AR is associated with a higher risk of graft loss [19], graft prognosis was worse for patients who required alemtuzumab treatment compared to the reference group. However, despite the severity of the rejection, over 60% of kidney transplants functioned for at least 5 years after alemtuzumab. The renal function was acceptable, ranging around 30 mL/min/1.72 m2. Clatworthy et al. reported a higher death-censored graft survival of 75% after 10 years in 15 patients [7], but these patients received alemtuzumab as a first-line treatment. In contrast, here, it was primarily used as a second-line therapy for glucocorticoid-resistant rejections. Our findings are in line with those of Basu et al., who reported a graft survival rate of 73.5% after 453 ± 163 days of follow-up in 40 patients treated with alemtuzumab for glucocorticoid-resistant rejection.
Most of the available data of rATG was published before 1998 [20], which complicates the comparison with a recent cohort. Van der Zwan et al. previously reported a death-censored graft survival of 60% 5 years after rATG therapy in patients treated between 2002 and 2012 in our center, which was comparable to alemtuzumab [11]. They therefore concluded that alemtuzumab and rATG probably have similar efficacy, although they could not correct for all potential confounders that arose from the comparison of two cohorts that were treated during different decades [11]. Without a contemporary cohort of rATG-treated patients as control group however, whether alemtuzumab outperforms rATG remains an unanswered question.
Patient Survival
Overall patient survival was worse in the alemtuzumab cohort. AR is associated with an increased mortality risk [19]. Increased mortality after AR probably stems both from both the loss of transplant function as complications from anti-rejection therapies. To what extend alemtuzumab therapy contributes to the increased mortality in this cohort, cannot be determined. Nevertheless, there is no evidence that alemtuzumab is associated with lower patient survival compared with rATG, as Van der Zwan et al. previously reported equal allograft survival between rATG- and alemtuzumab-treated patients [11].
Infections, Malignancies and Auto-Immunity
Unfortunately, data of serious infections were not available for the reference cohort and therefore the incidence rates could not be compared. Infections seem to occur regularly in other alemtuzumab-treated cohorts as well. Basu et al. and Clathworthy et al. also reported frequent infectious complications and an excess of early infection-related deaths after alemtuzumab therapy [6, 7]. Again, it is unclear if infections occur more frequently after alemtuzumab than rATG. A high incidence of opportunistic infections has also been observed after rATG anti-rejection therapy [20, 21], and van der Zwan et al. reported significantly shorter infection-free survival and a higher number of serious infection after rATG compared to alemtuzumab [11]. If the duration of lymphocyte depletion and excess susceptibility to infection are correlated, could not be determined in the present study because of missing data. Possibly, lower doses of alemtuzumab may result in more rapid recovery of lymphocyte counts and reduce excess infection.
Treatment with T cell-depleting antibodies for AR is a significant risk factor for the development of post-treatment malignancy in general [22]; however, this risk is not specified per type of T cell-depleting antibody. The present study did not find a significantly increased risk of malignancies or malignancy-related death in the alemtuzumab cohort. Nevertheless, we cannot state with any certainty that alemtuzumab does not lead to an increased incidence of malignancies, considering the higher incidence rates of all malignancies in the alemtuzumab cohort and the fact that the present cohort was relatively small from a cancer epidemiology perspective.
The incidence of autoimmunity after alemtuzumab was low, with an incidence of 1.3% of proven cases. Concerns regarding autoimmune complications after alemtuzumab treatment originate from studies in patients who were treated for multiple sclerosis, where thyroid autoimmunity and immune thrombocytopenia occurred frequently [23, 24]. The current study observed neither type of autoimmunity. However, several other cases of suspected autoimmune disease did occur. Differences in autoimmunity risk between the transplant and neurologic populations may be explained by differences in concomitant immunosuppression and baseline risks.
Lymphocyte Repopulation
Lymphocyte repopulation in the present cohort exceeded the recovery times in multiple sclerosis [25] and transplant trials with alemtuzumab induction therapy [26, 27]. This might be due to differences in the concomitant use of other myelosuppressive therapies and comorbid conditions. Nonetheless, the observed long-lasting lymphocyte depletion is unwanted and is likely a sign of alemtuzumab overdosing. In other studies, a lower dose of alemtuzumab has been applied in kidney transplant induction and demonstrated equal efficacy but faster lymphocyte recovery and fewer infection-related side effects [28, 29]. A recent pharmacokinetic study reported supra-therapeutic concentrations and long periods of lymphocytic drug exposure after 30 mg of alemtuzumab induction therapy [30], which delayed lymphocyte repopulation [31], suggesting that a fixed dose of 30 mg is sub-optimal. These findings and the fact that the current dosing strategy of alemtuzumab anti-rejection therapy is not supported by dose-finding studies [2] indicate the need for alternative dosing strategies [32, 33]. We suggest a stepwise dosing strategy, starting with a lower dose of alemtuzumab with the possibility of a repeated dose in case of incomplete lymphocyte depletion or fast lymphocyte recovery.
Without a clear graft and patient survival benefit of alemtuzumab over rATG and considering the possible risks alemtuzumab, the question remains if rATG should be the preferred treatment. Although contemporary reports of rATG-anti-rejection therapy are scarce in terms of number and follow-up, the available data show this therapy also has substantial risks [20]. Alemtuzumab does have benefits over rATG in terms of mode of administration and fewer infusion reactions [11]. We are currently planning future studies to resolve this matter.
In summary, alemtuzumab is an effective therapy to counter severe kidney transplant rejection. However, the current dose leads to a profound, long-lasting depletion of both B and T lymphocytes, frequent serious infections, and is associated with increased patient mortality. Further research is necessary to both determine the additional risks of alemtuzumab over alternative treatment strategies, and to optimize alemtuzumab therapy.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, upon reasonable request.
Ethics Statement
The studies involving humans were approved by the Medical-Ethical Board Erasmus MC, Rotterdam, the Netherlands. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data was obtained from the NOTR database, for which patients consented at the time of kidney transplantation.
Author Contributions
LV participated in the research design, performance of the research, data-analysis and writing of the paper. MC-vG participated in the performance of the research and writing of the paper. PA participated in data analysis and provided statistical consultation. DH participated in the research design, performance of the research and writing of the paper. MZ, MA, DH-P, BW, and MR participated in writing of the paper.
Conflict of Interest
DH received lecture and consulting fees from Astellas Pharma, Astra Zeneca, Chiesi Pharma, Medincell, Novartis Pharma, Sangamo Therapeutics, and Vifor Pharma. He received grant support from Astellas Pharma, Bristol-Myers Squibb and Chiesi Pharma (paid to his institution). DH does not have employment or stock ownership at any of these companies, nor does he have patents or patent applications. MC-vG received consulting honoraria from Sangamo Therapeutics and project support from Astellas Pharma (paid to her institution).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Judith A. Kal-van Gestel for her contribution to this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11834/full#supplementary-material
References
1. Ravandi, F, and O’Brien, S. Alemtuzumab. Expert Rev Anticancer Ther (2005) 5(1):39–51. doi:10.1586/14737140.5.1.39
2. van der Zwan, M, Baan, CC, van Gelder, T, and Hesselink, DA. Review of the Clinical Pharmacokinetics and Pharmacodynamics of Alemtuzumab and its Use in Kidney Transplantation. Clin Pharmacokinet (2018) 57(2):191–207. doi:10.1007/s40262-017-0573-x
3. Kasiske, BL, Zeier, MG, Chapman, JR, Craig, JC, Ekberg, H, Garvey, CA, et al. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients: A Summary. Kidney Int (2010) 77(4):299–311. doi:10.1038/ki.2009.377
4. FDA. Thymoglobulin (2018). Available from: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/thymoglobulin (Accessed February 26, 2018).
5. van den Hoogen, MW, Hesselink, DA, van Son, WJ, Weimar, W, and Hilbrands, LB. Treatment of Steroid-Resistant Acute Renal Allograft Rejection With Alemtuzumab. Am J Transpl (2013) 13(1):192–6. doi:10.1111/j.1600-6143.2012.04328.x
6. Basu, A, Ramkumar, M, Tan, HP, Khan, A, McCauley, J, Marcos, A, et al. Reversal of Acute Cellular Rejection After Renal Transplantation With Campath-1H. Transpl Proc (2005) 37(2):923–6. doi:10.1016/j.transproceed.2004.12.019
7. Clatworthy, MR, Friend, PJ, Calne, RY, Rebello, PR, Hale, G, Waldmann, H, et al. Alemtuzumab (CAMPATH-1H) for the Treatment of Acute Rejection in Kidney Transplant Recipients: Long-Term Follow-Up. Transplantation (2009) 87(7):1092–5. doi:10.1097/TP.0b013e31819d3353
8. Csapo, Z, Benavides-Viveros, C, Podder, H, Pollard, V, and Kahan, BD. Campath-1H as Rescue Therapy for the Treatment of Acute Rejection in Kidney Transplant Patients. Transpl Proc (2005) 37(5):2032–6. doi:10.1016/j.transproceed.2005.03.042
9. Kayler, LK, Mohanka, R, Morgan, C, Basu, A, Shapiro, R, and Randhawa, PS. Clinical Course of Kidney Transplant Patients With Acute Rejection and BK Virus Replication Following Campath Therapy. Clin Transpl (2008) 22(3):348–53. doi:10.1111/j.1399-0012.2008.00791.x
10. Magliocca, JF, and Knechtle, SJ. The Evolving Role of Alemtuzumab (Campath-1H) for Immunosuppressive Therapy in Organ Transplantation. Transpl Int (2006) 19(9):705–14. doi:10.1111/j.1432-2277.2006.00343.x
11. van der Zwan, M, Clahsen-Van Groningen, MC, van den Hoogen, MWF, Kho, MML, Roodnat, JI, Mauff, KAL, et al. Comparison of Alemtuzumab and Anti-Thymocyte Globulin Treatment for Acute Kidney Allograft Rejection. Front Immunol (2020) 11:1332. doi:10.3389/fimmu.2020.01332
12. van der Zwan, M, Leebeek, FWG, Sandberg, Y, Kruip, M, and Hesselink, DA. Acquired Haemophilia A After Alemtuzumab Therapy. Haemophilia (2020) 26(6):e337–e339. doi:10.1111/hae.14107
13. van der Zwan, M, Hesselink, DA, Brusse, E, van Doorn, PA, van den Hoogen, MWF, de Weerd, AE, et al. Guillain-Barré Syndrome and Chronic Inflammatory Demyelinating Polyradiculoneuropathy After Alemtuzumab Therapy in Kidney Transplant Recipients. Neurol Neuroimmunol Neuroinflamm (2020) 7(4):e721. doi:10.1212/NXI.0000000000000721
14. Betjes, MGH, Kho, MML, Litjens, NHR, de Weerd, AE, and Roodnat, JI. Alemtuzumab as Second-Line Treatment for Late Antibody-Mediated Rejection of Transplanted Kidneys. Transplant Proc (2021) 53(7):2206–11. doi:10.1016/j.transproceed.2021.07.005
15. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing (2023).
16. Gray, RJ. A Class of $K$-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat (1988) 16(3):1141–54. doi:10.1214/aos/1176350951
17. Dignam, JJ, and Kocherginsky, MN. Choice and Interpretation of Statistical Tests Used When Competing Risks Are Present. J Clin Oncol (2008) 26(24):4027–34. doi:10.1200/JCO.2007.12.9866
18. Fay, MP, and Shaw, PA. Exact and Asymptotic Weighted Logrank Tests for Interval Censored Data: The Interval R Package. J Stat Softw (2010) 36(2):i02–34. doi:10.18637/jss.v036.i02
19. Clayton, PA, McDonald, SP, Russ, GR, and Chadban, SJ. Long-Term Outcomes After Acute Rejection in Kidney Transplant Recipients: An ANZDATA Analysis. J Am Soc Nephrol (2019) 30(9):1697–707. doi:10.1681/ASN.2018111101
20. Bamoulid, J, Staeck, O, Crépin, T, Halleck, F, Saas, P, Brakemeier, S, et al. Anti-Thymocyte Globulins in Kidney Transplantation: Focus on Current Indications and Long-Term Immunological Side Effects. Nephrol Dial Transplant (2016) 32(10):1601–8. doi:10.1093/ndt/gfw368
21. Colak, T, Sevmiş, S, Karakayali, H, Moray, G, and Haberal, M. One Center's Experience With Antithymocyte Globulin Treatment for Acute Rejection in Renal Transplantation. Transpl Proc (2008) 40(1):123–5. doi:10.1016/j.transproceed.2007.12.008
22. Lim, WH, Turner, RM, Chapman, JR, Ma, MKM, Webster, AC, Craig, JC, et al. Acute Rejection, T-Cell–Depleting Antibodies, and Cancer After Transplantation. Transplantation (2014) 97(8):817–25. doi:10.1097/01.TP.0000442773.38510.32
23. Costelloe, L, Jones, J, and Coles, A. Secondary Autoimmune Diseases Following Alemtuzumab Therapy for Multiple Sclerosis. Expert Rev Neurotherapeutics (2012) 12(3):335–41. doi:10.1586/ern.12.5
24. Willis, MD, Harding, KE, Pickersgill, TP, Wardle, M, Pearson, OR, Scolding, NJ, et al. Alemtuzumab for Multiple Sclerosis: Long Term Follow-Up in a Multi-Centre Cohort. Mult Scler J (2016) 22(9):1215–23. doi:10.1177/1352458515614092
25. Li, Z, Richards, S, Surks, HK, Jacobs, A, and Panzara, MA. Clinical Pharmacology of Alemtuzumab, an Anti-CD52 Immunomodulator, in Multiple Sclerosis. Clin Exp Immunol (2018) 194(3):295–314. doi:10.1111/cei.13208
26. Heidt, S, Hester, J, Shankar, S, Friend, PJ, and Wood, KJ. B Cell Repopulation After Alemtuzumab Induction-Transient Increase in Transitional B Cells and Long-Term Dominance of Naïve B Cells. Am J Transpl (2012) 12(7):1784–92. doi:10.1111/j.1600-6143.2012.04012.x
27. Hanaway, MJ, Woodle, ES, Mulgaonkar, S, Peddi, VR, Kaufman, DB, First, MR, et al. Alemtuzumab Induction in Renal Transplantation. New Engl J Med (2011) 364(20):1909–19. doi:10.1056/NEJMoa1009546
28. Guthoff, M, Berger, K, Althaus, K, Mühlbacher, T, Bakchoul, T, Steurer, W, et al. Low-Dose Alemtuzumab Induction in a Tailored Immunosuppression Protocol for Sensitized Kidney Transplant Recipients. BMC Nephrol (2020) 21(1):178. doi:10.1186/s12882-020-01767-z
29. Willicombe, M, Goodall, D, McLean, AG, and Taube, D. Alemtuzumab Dose Adjusted for Body Weight Is Associated With Earlier Lymphocyte Repletion and Less Infective Episodes in the First Year Post Renal Transplantation - A Retrospective Study. Transpl Int (2017) 30(11):1110–8. doi:10.1111/tri.12978
30. Zwart, TC, Bezstarosti, S, Achini, FR, Reinders, MEJ, Schilham, MW, Heidt, S, et al. Population Pharmacokinetics of Subcutaneous Alemtuzumab in Kidney Transplantation. Br J Clin Pharmacol (2023) 89:1471–85. doi:10.1111/bcp.15608
31. Bezstarosti, S, Zwart, TC, Achini, FR, Schilham, MW, Moes, DJAR, Reinders, MEJ, et al. 246.9: High Alemtuzumab Exposure Is Associated With Delayed Lymphocyte Recovery in Kidney Transplant Recipients. Transplantation (2022) 106(9S):S166. doi:10.1097/01.tp.0000886180.08246.f9
32. Augustine, JJ. Weight-Based Dosing of Alemtuzumab: An Ounce of Prevention? Transpl Int (2017) 30(11):1095–7. doi:10.1111/tri.12992
Keywords: adverse effects, alemtuzumab, kidney transplant rejection, efficacy, kidney transplantation
Citation: van Vugt LK, van der Zwan M, Clahsen-van Groningen MC, van Agteren M, Hullegie-Peelen DM, De Winter BCM, Reinders MEJ, Miranda Afonso P and Hesselink DA (2023) A Decade of Experience With Alemtuzumab Therapy for Severe or Glucocorticoid-Resistant Kidney Transplant Rejection. Transpl Int 36:11834. doi: 10.3389/ti.2023.11834
Received: 27 July 2023; Accepted: 27 October 2023;
Published: 07 November 2023.
Copyright © 2023 van Vugt, van der Zwan, Clahsen-van Groningen, van Agteren, Hullegie-Peelen, De Winter, Reinders, Miranda Afonso and Hesselink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas K. van Vugt, bC5rLnZhbnZ1Z3RAZXJhc211c21jLm5s
†ORCID: Lukas K. van Vugt, 0000-0001-7343-0130; Marieke van der Zwan, 0000-0002-9404-6135; Marian C. Clahsen-van Groningen, 0000-0003-0565-9560; Daphne M. Hullegie-Peelen, 0000-0003-4489-8417; Brenda C. M. De Winter, 0000-0002-4452-8443; Marlies E. J. Reinders, 0000-0001-9543-567X; Pedro Miranda Afonso, 0000-0001-6708-9597; Dennis A. Hesselink, 0000-0003-1871-1962
 Lukas K. van Vugt
Lukas K. van Vugt Marieke van der Zwan3†
Marieke van der Zwan3† Daphne M. Hullegie-Peelen
Daphne M. Hullegie-Peelen Brenda C. M. De Winter
Brenda C. M. De Winter Marlies E. J. Reinders
Marlies E. J. Reinders Pedro Miranda Afonso
Pedro Miranda Afonso Dennis A. Hesselink
Dennis A. Hesselink