Introduction
This year’s GTI (“Groupe Transplantation and Infection”) annual meeting was held in Paris, France in February 2023. This meeting focused on new approaches to manage infectious complications in solid organ and stem cell transplant recipients.
In this meeting report, we summarize the presentations and discussions from this annual meeting. Covered topics included new anti-infective agents and non-antibiotic approaches to manage infections due to multidrug-resistant Gram-negative bacteria, staphylococci, and fungal infections, as well as new approaches to manage symptomatic urinary tract infections and asymptomatic bacteriuria in kidney transplant recipients. Innovative approaches are needed to manage infectious complications in transplant recipients, who are at high risk of difficult-to-treat infections and side effects associated with the use of anti-infective agents.
Management of Post-Transplant Bacterial Infections
Multidrug Resistant Enterobacterales Infections in Solid Organ Transplantation: Current Situation and New Non-Antibiotic Approaches
Solid-organ transplantation (SOT) is the treatment of choice for patients diagnosed with end-stage organ disease, and the median survival of both recipients and grafts has significantly increased in the last years [1]. While the incidence of infections (including opportunistic ones such as cytomegalovirus [CMV]) is decreasing due to better prevention, the burden of “classical” infections linked to multidrug-resistant (MDR) bacteria especially related to Gram-negative bacilli (GNB) is increasing [2, 3]. Multidrug resistant Enterobacterales are involved in one-third of bacterial infections in SOT recipients [4]. Prior intestinal colonization with ESBL (extended spectrum beta-lactamase)-producing Enterobacterales is an essential prerequisite for the onset of infection among SOT recipients [5]. Furthermore, among patients with intestinal colonisation with MDR (multidrug resistance) Enterobacterales, prior exposure to anti-infectives appears to be a major risk factor for subsequent infection due to the colonizing strain [5]. This can be explained by an increase in intestinal density of resistant Gram-negative bacilli (commonly referred as relative fecal abundance) during antibiotic administration [6]. Antimicrobial stewardship (AMS) programs are designed to improve the quality of prescribing practices in terms of choice of antibiotic, dosage, duration, route of administration and de-escalation. Benoit Pilmis presented innovative AMS strategies aimed at limiting antibiotic-induced dysbiosis, decolonizing patients colonized by MDR Enterobacterales, and restoring a healthy microbiota [7]. The efficacy of oral colistin-neomycin in preventing multidrug-resistant Enterobacterales (MDR-E) infections in solid organ transplant (SOT) recipients have been evaluated previously in a multicentre, randomized, controlled, open-label, parallel-group clinical trial [8] but showed negative results in term of efficacy and tolerance (particularly for colistin).
Among these strategies, the exact benefits of fecal microbiota transplantation (FMT) remain unclear [9]. A multicenter randomized controlled trial (FeCeS study) evaluating the efficacy of FMT in decolonizing carriers of ESBL- or carbapenemase-producing Enterobacterales will provide an answer (NCT05035342). This indication of FMT in decolonizing patients has been evaluated in allo-hematopoietic stem cell transplant (allo-HSCT) recipients a systematic review has been recently published [10]. FMT was performed before or after HSCT but each time on a low number of patients. Decolonization was obtained in 40%–60% of cases. The majority of the included studies report FMT as a generally well tolerated procedure, with no serious adverse events. Interestingly, in the case series of Shouval et al. two patients developed bacteremia after the infusion, but targeted metagenomic sequencing demonstrated that the bacterial strains did not originate from the FMT inoculum [11].
Altogether, FMT seems an interesting option for decolonization, but the safety profile and efficacy of the procedure must be determined more strongly to better assess the role of FMT in allo-HSCT recipients.
One-promising way to protect the gut microbiota is to develop molecules to chelate or degrade the non-absorbed part of orally administered antibiotics and the fraction of oral and parenteral antibiotics excreted in the bile that reach the colon, induce dysbiosis and a decrease in richness and diversity of the microbiota. For example, ribaxamase (an orally administered beta-lactamase hydrolyzing β-lactams in the colon appears promising in Phase 2 studies although limited to β-lactam antibiotics) and DAV-132 which is a millimetric beads consisting of a core of a specific activated charcoal surrounded by a polymer coating that is insoluble during transit. The charcoal is activated in the ileum and adsorbs and thereby inactivates antibiotics in the caecum/colon [12–16]. For now, no investigation of this strategy exist in transplant recipients but its evaluation and implementation are of interest in the TOS patients, a population highly exposed to antibiotics.
Multidrug Resistant Enterobacterales Infections in Solid Organ Transplantation: New Antibiotics
Antibiotic-resistant Gram-negative bacterial infections are the leading cause of death attributable to antibiotic resistance in Europe and worldwide. This is linked to the epidemic success of 3rd generation cephalosporins (3GC)- resistant Enterobacteriaceae. The widespread use of carbapenems to treat 3GC-resistant strains has led to the emergence of carbapenem-resistant isolates, in particular those secreting carbapenemases, with very limited therapeutic options. New molecules have recently been developed to combat carbapenem-resistant bacteria. Victoire de Lastours summarized the updated antimicrobial management of carbapenem-resistant bacteria related infection.
These include ceftazidime-avibactam, a combination of a 3GC with a new betalactamase inhibitor, avibactam. This combination is effective on strains carrying OXA 48 or KPC, but not metallobetalactamases. This molecule was granted authorization in Europe and the USA following 3 phase 3 trials in complicated intra-abdominal infections versus meropenem, as well as two trials in complicated urinary tract infections yielding non-inferiority. In a retrospective cohort study of 210 SOT recipients with carbapenemase-producing Klebsiella pneumoniae blood stream infections, ceftazidime-avibactam significantly increased the probability of 14 and 30 days clinical success, as compared to the best available therapy [17].
A second compound, meropenem-varbobactam, is also active against class A betalactamases (KPC) and cephalosporinases, but inactive against metallobetalactamases and oxacillinases, which limits its interest in some European coutries such as France, where KPCs are rare. Non-inferiority has been demonstrated in several trials against optimized treatment. A third molecule, imipenem-relebactam, is also active against KPCs but not against oxacillinases or metallobetalactamases. Imipenem-relebactam is also effective against carbapenem-resistant strains of Pseudomonas aeruginosa, but not against carbapenem-resistant Acinetobacter baumanii (CRAB). The molecule has been approved in France only as a last resort for the treatment of patients with no other possible therapeutic alternative, and in particular if KPC-type carbapenemase are produced.
Altogether, several choices are now available to treat KPC and OXA-48 oxacillinases which are approved in France and Europe. For carbapenem-resistant P. aeruginosa, ceftolozane-tazobactam is generally effective. Tolerance is generally good (as with beta-lactams), and these molecules are bactericidal. However, these molecules are not effective against metallobetalactamases nor against most CRAB, which poses major therapeutic problems. Its use was reported in a multicenter cohort study of 69 immunocompromised patients including 47 SOT, with multi-drug resistant P. aeruginosa infections, mostly respiratory and wound. Clinical cure was achieved in 68% and mortality was 19% [18].
A recently approved molecule, cefiderocol, is a siderophore cephalosporin which uses the bacterial iron entry machinery to achieve high concentrations inside the bacteria. It is unaffected by betalactamases, even metallobetalactamases, and acts as a Trojan horse. In pivotal trials, cefiderocol showed non-inferiority to high-dose meropenem in the treatment of gram-negative nosocomial pneumonia, except for A. baumanii infections, a result that remains unexplained. Cefiderocol has been marketed in Europe and the USA only as a last resort for infections caused by multi-resistant gram-negative bacteria, notably in cases of KPC and metallo-betalactamases.
This molecule therefore represents an important therapeutic hope, although it appears to have a relatively significant inoculum effect, which needs to be better studied. Finally, some cefiderocol-resistant strains have been described, combining several resistance mechanisms. To date, very few data are available in specific immunocompromised settings including solid organ transplantation [19], hematological malignancies [20, 21]. Most Cefiderocol prescriptions have primarily targeted multi-resistant severe P. aeruginosa infections, but its use has broadened to other difficult-to-treat non-fermentative gram negative bacteria, especially S. maltophilia for which its complex virulence and resistance profile drastically limit available antibiotics. Updated clinical and safety outcome data are needed in highly susceptible immunocompromised settings.
Another interesting combination in this context is ceftazidime-avibactam + aztreonam for strains carrying metallo-betalactamases. Several studies have demonstrated the efficacy of the avibactam + aztreonam combination, which is currently being developed by the manufacturer. An inoculum effect could also have an impact on the efficacy of this combination. This combination proved effective and safe in a serie of 4 SOT recipients with metallo-β-lactamase carbapenemase-producing Enterobacteriaceae [22].
Lastly, plazomicin, an aminoglycoside developed for the treatment of carbapenem-resistant Enterobacteriaceae infections, had shown interesting results in the United States, but was not developed in Europe due to its low commercial potential.
Treatment recommendations for carbapenem-resistant infections are summarized in the 2022 ESCMID guidelines [23]. Several new molecules are under development and could be of interest for the treatment of these infections, particularly those due to organisms producing a metallobetalactamase, such as cefepime-taniborbactam and meropenem-nacubactam. Studies are currently underway.
Finally, in the face of this type of infection, optimizing the use of available molecules is a crucial point, including rapid diagnosis of resistance, determination of MICs (minimal inhibitory concentration) for the different molecules and combinations available, and optimization of dosages with the use of high doses and prolonged infusions. Last but not least, multidisciplinary discussions between microbiologists and clinicians and the reduction of bacterial inoculum through drainage are essential. A summary of antibiotics efficiency regarding resistance mutation has been made in Table 1.
TABLE 1
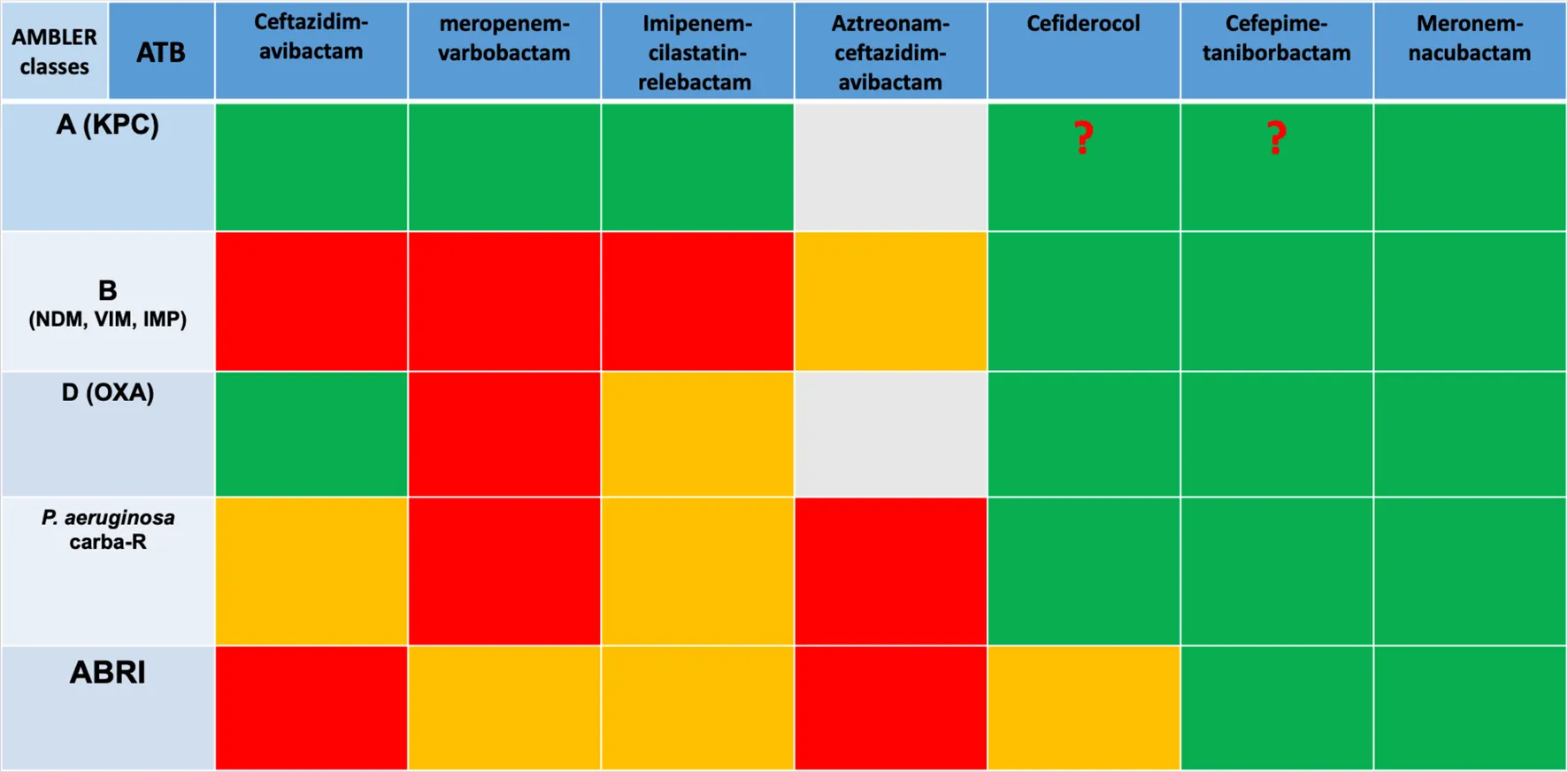
|
Spectrum of new antibiotics regarding the type of resistance.
Abbreviations: ABRI, Acinetobacter baumani mutli resistant; ATB, antibiotic; carba-R, carbapenem-resistant.
New Approaches to Manage Urinary Tract Infections in Kidney Transplant Recipients
The management of urinary tract infections (UTIs) in kidney transplant recipients represents a major opportunity for antimicrobial stewardship because kidney transplantation is the most common type of organ transplant worldwide, and because UTI is the most common infection in this population [3, 24]. Julien Coussement summarized the most recent evidence about the management of post-transplant symptomatic UTI and asymptomatic bacteriuria, and identified gaps of knowledge and clinical scenarios that remain understudied.
Asymptomatic bacteriuria, which is generally defined as significant bacteriuria (≥100.000 CFU/mL) without signs or symptoms of UTI (e.g., fever, chills, kidney pain, or symptoms of bladder inflammation), is relatively common after kidney transplantation [24].
Recent randomized trials have shown that the historical practice of screening for and treating asymptomatic bacteriuria is not beneficial in stable kidney transplant recipients [25–28]. A limited-size trial even suggested that asymptomatic bacteriuria might be left untreated in patients who are in the first 2 months post-transplant and have a ureteral stent [29]. Additional opportunities probably exist to improve the care of kidney transplant recipients with pyelonephritis. First, research is needed to determine the benefits and harms associated with the empiric use of very broad-spectrum antibiotics in kidney transplant recipients admitted for presumed pyelonephritis [24]. Second, a randomized trial is starting to determine whether 7 days of antibiotic therapy can be sufficient to treat non-severe episodes of pyelonephritis in kidney transplant recipients who are beyond the first month post-transplant and do not have a urinary catheter [30–32].
Besides, innovative non-antibiotic-based approaches are needed to better prevent symptomatic UTIs, which remain prevalent and detrimental after kidney transplantation. Julien Coussement discussed the potential benefits, harms and applicability of emerging approaches, including anti-adhesion therapies (which aim at preventing bacterial adhesion to host tissues, and therefore decreasing the risk of UTI) [33], intravesical instillation of a low-virulence organism (which aims at promoting bacterial interference) [34], and FMT (which aims at repopulating the gut with a “healthy” microbiome that could outcompete uropathogens) [35–38]. Vaccine candidates that are in development against extra-intestinal pathogenic Escherichia coli are also promising [39]. Many challenges, however, exist, including the fact that transplant recipients generally have an impaired immune response to vaccines, and the fact that around half of the UTI episodes which occur after kidney transplantation are due to microorganisms other than E. coli.
New Antibiotics to Treat Infections Due to Gram-Positive Cocci
Aurélien Dinh reminded the drawbacks of vancomycin and daptomycin, before presenting new antibiotics targeting gram-positive cocci.
Vancomycin is a relatively old and difficult-to-manage glycopeptide. Several new antibiotics with activity against methicillin-resistant Staphylococci are now available.
Daptomycin is bactericidal and as effective as penicillin M against methicillin-susceptible Staphylococcus aureus and vancomycin for methicillin-resistant S. aureus, according to a randomized controlled trial (RCT) on bloodstream infections (BSI) [40]. Nevertheless, some treatment failures due to inoculum effect have been observed, and bacterial resistance is described, even among patients without previous exposure to this drug, which could be due to in vivo exposure to endogenous cationic peptides [41]. In liver transplant recipients, such resistance was indeed associated with prior daptomycin use and increased mortality [42]. In kidney transplant recipients, combinations of daptomycin and other antibiotics have also been suggested for resistant enterococcal infections [43, 44].
Dalbavancin is a new long acting glycolipopeptide, with a half-life of 14 days. MIC of dalbavancin against S. aureus and resistant coagulase-negative staphylococci are low. One retrospective cohort compared dalbavancin versus standard of care in patients with S. aureus bacteremia and found no significant difference [45]. Two RCTs are currently underway to better determine the effectiveness of dalbavancin in patients with S. aureus bacteremia [46, 47]. Dalbavancin is of particular interest for patients requiring prolonged antibiotic therapy, such as those with endocarditis or bone and joint infection (BJI) such as prosthetic joint infections. Several cohorts and literature reviews found dalbavancin to be safe, with nearly 80% cure rate in these indications and high level of patient satisfaction, mostly due to early discharge [48].
Ceftaroline and ceftobiprole are new generation cephalosporins with excellent activity against methicillin-resistant staphylococci according to bacterial killing curves [49]. Clinical efficacy during BJI and endocarditis are promising according to cohort studies [50, 51]. The ERADICATE trial comparing ceftobiprole versus daptomycin in S. aureus bacteremia showed non-inferiority [52].
So far, to our knowledge, no data exist regarding the use of dalvabancin, ceftaroline and ceftobiprole in SOT recipients.
Finally, oritavancin is a recently available lipopeptide, with a semi long-life activity (7 days) and important intra-cellular activity, which could be of interest for device-associated infection with biofilm [53].
These new antibiotics may allow new management and innovative approaches to treat patients with infections due to resistant Staphylococci.
Management of Fungal Infections
Because of the toxicities of the available drugs and the emergence of resistance caused by an increased use of antifungal agents in the growing population at risk of invasive fungal diseases and in agriculture, there is a pressing need for more antifungal drug options. Recently, several new antifungal drugs have reached late-stage clinical development and obtained a temporary use authorization, as depicted by Alexandra Serris.
Olorofim is the only member of a novel class named orotomide. It inhibits fungal growth through inhibition of the fungal dihydroorotate dehydrogenase enzyme involved in pyrimidine synthesis. It has a good tissue distribution, notably in the kidney, liver, lung, and the brain (although at lower levels) [54]. It is metabolized by several CYP450 enzymes including CYP3A4 and is thus susceptible to strong CYP3A4 inhibitors and inducers. Olorofim exhibits activity in vitro against azole-resistant Aspergillus, Scedosporium, Lomentospora, Rasamsonia, dimorphic fungi (notably Histoplasma), dermatophytes, but has no activity against yeasts, Mucorales and Alternaria alternata [55, 56].
Olorofim is currently evaluated in two clinical studies: one open-label, single-arm study including patients with invasive fungal infections due to Lomentospora prolificans, Scedosporium spp., Aspergillus spp., and other resistant fungi with limited treatment options (ClinicalTrials.gov identifier: NCT03583164) and one phase III, randomized study to evaluate the efficacy and safety of olorofim versus liposomal amphotericin B in patients with invasive aspergillosis (ClinicalTrials.gov Identifier: NCT05101187). Published experience is currently limited to case reports (abstracts).
Ibrexafugerp is a first-in-class oral glucan synthase inhibitor, whose mechanism of action is close to the one of echinocandins (but with a different binding site). It is fungicidal against most wild-type, echinocandin or azole-resistant Candida spp., including C. auris, and fungistatic against Aspergillus spp [57]. Based on animal models, ibrexafungerp shows a high tissue penetration in the spleen, liver, lungs, kidney, vaginal tissue, and muscles, but not in the brain [58].
An interim analysis of the phase III FURI study evaluating the efficacy and safety of ibrexafungerp in patients with severe mucocutaneous candidiasis, invasive candidiasis, chronic or invasive aspergillosis reported complete or partial response in 58% of the patients [59]. Inclusion criteria were further expanded to include histoplasmosis, coccidioidomycosis and blastomycosis.
Rezafungin is the first member of second-generation echinocandins with enhanced pharmacokinetic/pharmacodynamic parameters, allowing for a weekly administration and potential less hepatic toxicity [60]. It has potent in vitro activity against most Candida spp., including C. auris, and common dermatophytes [58].
Moreover, rezafungin has shown promising results as prophylactic and curative treatment of pneumocystis in vivo by eradicating both the cyst and trophic forms of the fungus [61, 62]. A case report of the successful eradication of a refractory intra-abdominal candidiasis with rezafungin in a liver transplant recipient was published in 2022 [63] and rezafungin was recently found non-inferior to caspofungine in a Phase 3 trial (ReSTORE) for the treatment of candidemia/invasive candidiasis [64].
These antifungal treatments offer significant improvement in terms of spectrum of activity, tolerability, drug interactions and/or route of administration. Further clinical studies will be needed to evaluate their optimal place in the therapeutic arsenal in the solid organ transplant recipient population, taking into account the emergence of drug-resistant fungi and the problem of drug-drug interactions with immunosuppressants. Table 2 summarize the Spectrum of activity, tissue diffusion and drug-drug interactions (DDIs) with immunosuppressive drugs of olorofim, ibrexafungerp and rezafungin.
TABLE 2
| Molecule | Spectrum of activity | Diffusion | DDIs with immunosuppressive drugs | Potential advantages |
|---|---|---|---|---|
| Olorofim | Aspergillus spp. Scedosporium spp. Lomentospora prolificans Fusarium spp. Histoplasma capsulatum Blastomyces dermatitidis Coccidioides spp. | • Good diffusion in kidney, liver, and lung | • Substrate of several CYP450 enzymes: anticipate dose reduction if given with a strong 3A4 inhibitor (or a moderate dual 3A4+2C9 inhibitor) | Active against highly resistant molds |
| • Low levels in CNS [54] | • Weak inhibitor of CYP3A4: small reductions of tacrolimus and sirolimus might be needed (guided by standard monitoring) | |||
| ibrexafungerp | Candida spp. including echinocandin resistant C. glabrata and C. auris Aspergillus spp. Paecilomyces variotii Pneumocystis jirovecii | • Good diffusion in liver, spleen, lungs, bone marrow, kidney, skin and uvea | • Substrate of CYP3A and P-glycoprotein: avoid coadministration of strong CYP3A inducers | • Active against resistant Candida species |
| • Low levels in CNS [65] | • Reversible inhibitor of CYP2C8 and CYP3A4 | • First orally bioavailable inhibitor of [1(3)- β-D-glucan synthase] | ||
| • interaction with tacrolimus: 1.4-fold increase in AUC; no change in tacrolimus Cmax [66] | ||||
| Rezafungin | Candida spp. Aspergillus spp. Pneumocystis jirovecii | Improved drug penetration in liver and kidney abscesses (mouse model of intra-abdominal candidiasis) in comparison with micafungin [67] | Minimal inhibition of CYP450 enzymes [68]: Limited reduction (10%–19%) of the AUC or Cmax of tacrolimus, ciclosporine and mycophenolic acid (probably not clinically meaningful) [69] | • Long half-life allows once weekly dosing |
| • Less hepatotoxicity | ||||
| • May prevent Pneumocystis pneumonia [61, 62] |
Spectrum of activity, tissue diffusion and drug-drug interactions (DDIs) with immunosuppressive drugs of olorofim, ibrexafungerp and rezafungin.
Conclusion
During the well-attended “
Infection and Transplantation Group” day, the major advances in the field of
new anti-infective therapies in transplantationwere presented and discussed. New direct and indirect anti-infective approaches in transplantation are devoted to several improvements:
- decrease antibiotics pressure in our high risk multidrug resistant bacteria population with a better use of already known antibiotics and new original non-antibiotic approaches that have promising usages.
- improve efficacy of bacterial and fungal treatment with antibiotics or antifungal therapy that have a good inoculum effect and a good broadcast
- improve the tolerance of antimicrobial drugs in our polymedicated population with high risk of drugs interactions.
Altogether, those new approaches are likely to feature alternative anti-infective therapies that promise to change patient management.
Statements
Author contributions
AlS, JC, BP, VD, AD, and HK wrote the manuscript. AD, AS, FA, OL, EM, NK, EF, DL, JD, FC, AL, and HK revised the manuscript. AS, FA, OL, EM, NK, EF, DL, JD, FC, AL, and HK conceived the manuscript.
Acknowledgments
The 2023 GTI (“Infection and Transplantation Group”) annual meeting was sponsored by Biotest AG. The program was developed by a scientific committee (François Parquin, Eric Epailly, Anne Scemla, Florence Ader, Olivier Lortholary, Emmanuel Morelon, Nassim Kamar, Edouard Forcade, David Lebeaux, Jérôme Dumortier, Filomena Conti, Agnes Lefort and Hannah Kaminski). The scientific committee worked with the faculty to set the agenda and prepare the presentations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Goldfarb SB Levvey BJ Cherikh WS Chambers DC Khush K Kucheryavaya AY et al Registry of the International Society for Heart and Lung Transplantation: Twentieth Pediatric Lung and Heart-Lung Transplantation Report—2017; Focus Theme: Allograft Ischemic Time. J Heart Lung Transplant (2017) 36(10):1070–9. 10.1016/j.healun.2017.07.017
2.
Oriol I Sabé N Simonetti AF Lladó L Manonelles A González J et al Changing Trends in the Aetiology, Treatment and Outcomes of Bloodstream Infection Occurring in the First Year After Solid Organ Transplantation: A Single-Centre Prospective Cohort Study. Transpl Int (2017) 30(9):903–13. 10.1111/tri.12984
3.
Van Delden C Stampf S Hirsch HH Manuel O Meylan P Cusini A et al Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis (2020) 71(7):e159–e169. 10.1093/cid/ciz1113
4.
Bodro M Sabé N Tubau F Lladó L Baliellas C Roca J et al Risk Factors and Outcomes of Bacteremia Caused by Drug-Resistant ESKAPE Pathogens in Solid-Organ Transplant Recipients. Transplantation (2013) 96(9):843–9. 10.1097/TP.0b013e3182a049fd
5.
Anesi JA Lautenbach E Tamma PD Thom KA Blumberg EA Alby K et al Risk Factors for Extended-Spectrum β-Lactamase-Producing Enterobacterales Bloodstream Infection Among Solid-Organ Transplant Recipients. Clin Infect Dis (2021) 72(6):953–60. 10.1093/cid/ciaa190
6.
Ruppé E Lixandru B Cojocaru R Büke Ç Paramythiotou E Angebault C et al Relative Fecal Abundance of Extended-Spectrum-β-Lactamase-Producing Escherichia coli Strains and Their Occurrence in Urinary Tract Infections in Women. Antimicrob Agents Chemother (2013) 57(9):4512–7. 10.1128/AAC.00238-13
7.
Rivard KR Athans V Lam SW Gordon SM Procop GW Richter SS et al Impact of Antimicrobial Stewardship and Rapid Microarray Testing on Patients With Gram-Negative Bacteremia. Eur J Clin Microbiol Infect Dis (2017) 36(10):1879–87. 10.1007/s10096-017-3008-6
8.
Fariñas MC González-Rico C Fernández-Martínez M Fortún J Escudero-Sanchez R Moreno A et al Oral Decontamination With Colistin Plus Neomycin in Solid Organ Transplant Recipients Colonized by Multidrug-Resistant Enterobacterales: A Multicentre, Randomized, Controlled, Open-Label, Parallel-Group Clinical Trial. Clin Microbiol Infect (2021) 27(6):856–63. 10.1016/j.cmi.2020.12.016
9.
Huttner BD De Lastours V Wassenberg M Maharshak N Mauris A Galperine T et al A 5-Day Course of Oral Antibiotics Followed by Faecal Transplantation to Eradicate Carriage of Multidrug-Resistant Enterobacteriaceae: A Randomized Clinical Trial. Clin Microbiol Infect (2019) 25(7):830–8. 10.1016/j.cmi.2018.12.009
10.
Pession A Zama D Muratore E Leardini D Gori D Guaraldi F et al Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J Pers Med (2021) 11(2):100. 10.3390/jpm11020100
11.
Shouval R Youngster I Geva M Eshel A Danylesko I Shimoni A et al Repeated Courses of Orally Administered Fecal Microbiota Transplantation for the Treatment of Steroid Resistant and Steroid Dependent Intestinal Acute Graft vs. Host Disease: A Pilot Study (NCT 03214289). Blood (2018) 132(1):2121. 10.1182/blood-2018-99-110270
12.
De Gunzburg J Ghozlane A Ducher A Le Chatelier E Duval X Ruppé E et al Protection of the Human Gut Microbiome From Antibiotics. J Infect Dis (2018) 217(4):628–36. 10.1093/infdis/jix604
13.
Messaoudene M Saint-Lu N Sablier-Gallis F Ferreira S Bescop CL Loppinet T et al 1306 Prevention of Antibiotic-Induced Dysbiosis in Human Volunteers by DAV132 and Preservation of Responsiveness to Anti-PD-1 Therapy Demonstrated by Transplantation of Human Feces Into Tumor-Bearing Mice. Regular Young Investigator Award Abstr (2022) 10(2):A1354–5. 10.1136/jitc-2022-sitc2022.1306
14.
Kokai-Kun JF Le C Trout K Cope JL Ajami NJ Degar AJ et al Ribaxamase, an Orally Administered β-Lactamase, Diminishes Changes to Acquired Antimicrobial Resistance of the Gut Resistome in Patients Treated With Ceftriaxone. IDR (2020) 13:2521–35. 10.2147/IDR.S260258
15.
Kokai-Kun JF Roberts T Coughlin O Sicard E Rufiange M Fedorak R et al The Oral β-Lactamase SYN-004 (Ribaxamase) Degrades Ceftriaxone Excreted Into the Intestine in Phase 2a Clinical Studies. Antimicrob Agents Chemother (2017) 61(3):e02197. 10.1128/AAC.02197-16
16.
Connelly S Fanelli B Hasan NA Colwell RR Kaleko M . SYN-007, an Orally Administered Beta-Lactamase Enzyme, Protects the Gut Microbiome From Oral Amoxicillin/Clavulanate Without Adversely Affecting Antibiotic Systemic Absorption in Dogs. Microorganisms (2020) 8(2):152. 10.3390/microorganisms8020152
17.
Pérez-Nadales E Fernández-Ruiz M Natera AM Gutiérrez-Gutiérrez B Mularoni A Russelli G et al Efficacy of Ceftazidime-Avibactam in Solid Organ Transplant Recipients With Bloodstream Infections Caused by Carbapenemase-Producing Klebsiella pneumoniae. Am J Transpl (2023) 23(7):1022–34. 10.1016/j.ajt.2023.03.011
18.
Hart DE Gallagher JC Puzniak LA Hirsch EB , C/T Alliance to Deliver Real-World Evidence CARE. A Multicenter Evaluation of Ceftolozane/Tazobactam Treatment Outcomes in Immunocompromised Patients With Multidrug-Resistant Pseudomonas aeruginosa Infections. Open Forum Infect Dis (2021) 8(3):ofab089. 10.1093/ofid/ofab089
19.
Pouch SM . New Drugs for Difficult Bugs: Management of Multidrug-Resistant Gram-Negative Infections in Solid Organ Transplant Recipients. Curr Opin Organ Transpl (2021) 26(4):424–31. 10.1097/MOT.0000000000000890
20.
Zappulo E Grimaldi F Paolillo R Pinchera B Buonomo AR Picardi M et al Successful Treatment of MDR Stenotrophomonas Maltophilia-Associated Pneumonia With Cefiderocol-Based Regimen in a Patient With Hematological Malignancy. Ann Hematol (2022) 101(12):2805–6. 10.1007/s00277-022-05006-3
21.
Lan P Lu Y Chen Z Wu X Hua X Jiang Y et al Emergence of High-Level Cefiderocol Resistance in Carbapenem-Resistant Klebsiella pneumoniae From Bloodstream Infections in Patients With Hematologic Malignancies in China. Microbiol Spectr (2022) 10(2):e0008422. 10.1128/spectrum.00084-22
22.
Cairns KA Hall V Martin GE Griffin DWJ Stewart JD Khan SF et al Treatment of Invasive IMP-4 Enterobacter cloacae Infection in Transplant Recipients Using Ceftazidime/Avibactam With Aztreonam: A Case Series and Literature Review. Transpl Infect Dis (2021) 23(2):e13510. 10.1111/tid.13510
23.
Paul M Carrara E Retamar P Tängdén T Bitterman R Bonomo RA et al European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect (2022) 28(4):521–47. 10.1016/j.cmi.2021.11.025
24.
Coussement J Kaminski H Scemla A Manuel O . Asymptomatic Bacteriuria and Urinary Tract Infections in Kidney Transplant Recipients. Curr Opin Infect Dis (2020) 33(6):419–25. 10.1097/QCO.0000000000000678
25.
Coussement J Kamar N Abramowicz D . New Evidence Shows It Is Time to Stop Unnecessary Use of Antibiotics in Kidney Transplant Recipients With Asymptomatic Bacteriuria. Nephrol Dial Transplant (2021) 36(5):754–6. 10.1093/ndt/gfaa341
26.
Coussement J Kamar N Matignon M Weekers L Scemla A Giral M et al Antibiotics Versus No Therapy in Kidney Transplant Recipients With Asymptomatic Bacteriuria (BiRT): A Pragmatic, Multicentre, Randomized, Controlled Trial. Clin Microbiol Infect (2021) 27(3):398–405. 10.1016/j.cmi.2020.09.005
27.
Origüen J López-Medrano F Fernández-Ruiz M Polanco N Gutiérrez E González E et al Should Asymptomatic Bacteriuria Be Systematically Treated in Kidney Transplant Recipients? Results From a Randomized Controlled Trial. Am J Transpl (2016) 16(10):2943–53. 10.1111/ajt.13829
28.
Sabé N Oriol I Melilli E Manonelles A Bestard O Polo C et al Antibiotic Treatment Versus No Treatment for Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Multicenter Randomized Trial. Open Forum Infect Dis (2019) 6(6):ofz243. 10.1093/ofid/ofz243
29.
Antonio MEE Cassandra BGC Emiliano RJD Guadalupe OLM Lilian REA Teresa TGM et al Treatment of Asymptomatic Bacteriuria in the First 2 Months After Kidney Transplant: A Controlled Clinical Trial. Transpl Infect Dis (2022) 24(6):e13934. 10.1111/tid.13934
30.
Lafaurie M . Efficacy of 7 Days Versus 14 Days of Antibiotic Therapy for Acute Pyelonephritis in Kidney Transplant Recipients, a Multicentre Randomized Non-Inferiority Trial. (SHORTCUT) (2021). Available From: https://www.infectiologie.com/UserFiles/File/jni/2021/com/jni2021-st12-01-lafaurie.pdf (Accessed 2021).
31.
Kumar R Pereira M Taimur S True K Detwiler R Van Duin D . Duration of Antibiotic Treatment for Acute Graft Pyelonephritis: What’s the Standard of Care?Transpl Infect Dis (2023) 25(1):e13996. 10.1111/tid.13996
32.
Coussement J Lafaurie M . Duration of Antibiotics in Kidney Transplant Recipients With Pyelonephritis: Current Practice, Research Gaps, and Future Research. Transpl Infect Dis (2023) 25(1):e13997. 10.1111/tid.13997
33.
Spaulding CN Klein RD Ruer S Kau AL Schreiber HL Cusumano ZT et al Selective Depletion of Uropathogenic E. coli From the Gut by a FimH Antagonist. Nat 22 juin (2017) 546(7659):528–32. 10.1038/nature22972
34.
Toh SL Boswell-Ruys CL Lee BSB Simpson JM Clezy KR . Probiotics for Preventing Urinary Tract Infection in People With Neuropathic Bladder. Cochrane Database Syst Rev (2017) 2017(9). 10.1002/14651858.CD010723
35.
Biehl LM Cruz Aguilar R Farowski F Hahn W Nowag A Wisplinghoff H et al Fecal Microbiota Transplantation in a Kidney Transplant Recipient With Recurrent Urinary Tract Infection. Infection (2018) 46(6):871–4. 10.1007/s15010-018-1190-9
36.
Wang J Li X Wu X Wang Z Wu X Wang S et al Fecal Microbiota Transplantation as an Effective Treatment for Carbapenem-Resistant Klebsiella pneumoniae Infection in a Renal Transplant Patient. IDR (2021) 14:1805–11. 10.2147/IDR.S308308
37.
Magruder M Sholi AN Gong C Zhang L Edusei E Huang J et al Gut Uropathogen Abundance Is a Risk Factor for Development of Bacteriuria and Urinary Tract Infection. Nat Commun (2019) 10(1):5521. 10.1038/s41467-019-13467-w
38.
Lee JR Muthukumar T Dadhania D Toussaint NC Ling L Pamer E et al Gut Microbial Community Structure and Complications After Kidney Transplantation: A Pilot Study. Transplantation (2014) 98(7):697–705. 10.1097/TP.0000000000000370
39.
Frost I Sati H Garcia-Vello P Hasso-Agopsowicz M Lienhardt C Gigante V et al The Role of Bacterial Vaccines in the Fight against Antimicrobial Resistance: An Analysis of the Preclinical and Clinical Development Pipeline. The Lancet Microbe (2023) 4(2):e113–25. 10.1016/S2666-5247(22)00303-2
40.
Fowler VG Boucher HW Corey GR Abrutyn E Karchmer AW Rupp ME et al Daptomycin Versus Standard Therapy for Bacteremia and Endocarditis Caused by Staphylococcus aureus. New Engl J Med (2006) 355(7):653–65. 10.1056/NEJMoa053783
41.
Mishra NN Yang SJ Chen L Muller C Saleh-Mghir A Kuhn S et al Emergence of Daptomycin Resistance in Daptomycin-Naïve Rabbits With Methicillin-Resistant Staphylococcus aureus Prosthetic Joint Infection Is Associated With Resistance to Host Defense Cationic Peptides and mprF Polymorphisms. PLoS One (2013) 8(8):e71151. 10.1371/journal.pone.0071151
42.
Lee RA Goldman J Haidar G Lewis J Arif S Hand J et al Daptomycin-Resistant Enterococcus Bacteremia Is Associated With Prior Daptomycin Use and Increased Mortality After Liver Transplantation. Open Forum Infect Dis (2022) 9(3):ofab659. 10.1093/ofid/ofab659
43.
Castro-Lainez MT Sierra-Hoffman M Valladares V Tillman T Iznaloa-Esquivel OA Howell A et al A Rationale for Combination Ampicillin and Daptomycin in Renal Transplant Patients With Enterococcal Infective Endocarditis. IDCases (2018) 14:e00460. 10.1016/j.idcr.2018.e00460
44.
Descourouez JL Jorgenson MR Wergin JE Rose WE . Fosfomycin Synergy In Vitro With Amoxicillin, Daptomycin, and Linezolid against Vancomycin-Resistant Enterococcus Faecium From Renal Transplant Patients With Infected Urinary Stents. Antimicrob Agents Chemother (2013) 57(3):1518–20. 10.1128/AAC.02099-12
45.
Molina KC Lunowa C Lebin M Segerstrom Nunez A Azimi SF Krsak M et al Comparison of Sequential Dalbavancin With Standard-Of-Care Treatment for Staphylococcus aureus Bloodstream Infections. Open Forum Infect Dis (2022) 9(7):ofac335. 10.1093/ofid/ofac335
46.
Turner NA Zaharoff S King H Evans S Hamasaki T Lodise T et al Dalbavancin as an Option for Treatment of S. aureus Bacteremia (DOTS): Study Protocol for a Phase 2b, Multicenter, Randomized, Open-Label Clinical Trial. Trials (2022) 23(1):407. 10.1186/s13063-022-06370-1
47.
Clinicaltrials. Dalbavancin Versus Standard Antibiotic Therapy for Catheter-Related Bloodstream Infections Due to Staphylococcus Aureus (DALICATH) (2023). ClinicalTrials.gov. Available From: https://clinicaltrials.gov/ct2/show/NCT05117398?titles=dalicath&draw=2&rank=1 (Accessed April 10, 2023).
48.
Matt M Duran C Courjon J Lotte R Moing VL Monnin B et al Dalbavancin Treatment for Prosthetic Joint Infections in Real-Life: A National Cohort Study and Literature Review. J Glob Antimicrob Resist (2021) 25:341–5. 10.1016/j.jgar.2021.03.026
49.
Isnard C Dhalluin A Malandain D Bruey Q Auzou M Michon J et al In Vitro Activity of Novel Anti-MRSA Cephalosporins and Comparator Antimicrobial Agents Against Staphylococci Involved in Prosthetic Joint Infections. J Glob Antimicrob Resist (2018) 13:221–5. 10.1016/j.jgar.2018.01.022
50.
Johnson LB Ramani A Guervil DJ . Use of Ceftaroline Fosamil in Osteomyelitis: CAPTURE Study Experience. BMC Infect Dis (2019) 19(1):183. 10.1186/s12879-019-3791-z
51.
Destache CJ Guervil DJ Kaye KS . Ceftaroline Fosamil for the Treatment of Gram-Positive Endocarditis: CAPTURE Study Experience. Int J Antimicrob Agents (2019) 53(5):644–9. 10.1016/j.ijantimicag.2019.01.014
52.
Hamed K Engelhardt M Jones ME Saulay M Holland TL Seifert H et al Ceftobiprole Versus Daptomycin in Staphylococcus aureus Bacteremia: A Novel Protocol for a Double-Blind, Phase III Trial. Future Microbiol (2020) 15(1):35–48. 10.2217/fmb-2019-0332
53.
Corey GR Kabler H Mehra P Gupta S Overcash JS Porwal A et al Single-Dose Oritavancin in the Treatment of Acute Bacterial Skin Infections. New Engl J Med (2014) 370(23):2180–90. 10.1056/NEJMoa1310422
54.
Oliver JD Sibley GEM Beckmann N Dobb KS Slater MJ McEntee L et al Represents a Novel Class of Antifungal Drug That Inhibits Dihydroorotate Dehydrogenase. Proc Natl Acad Sci U S A (2016) 113(45):12809–14. 10.1073/pnas.1608304113
55.
Kirchhoff L Dittmer S Buer J Rath PM Steinmann J . In Vitro Activity of Olorofim (F901318) Against Fungi of the Genus, Scedosporium and Rasamsonia as Well as Against Lomentospora Prolificans, Exophiala Dermatitidis and Azole-Resistant Aspergillus fumigatus. Int J Antimicrob Agents (2020) 56(3):106105. 10.1016/j.ijantimicag.2020.106105
56.
Mirbzadeh Ardakani E Sharifirad A Pashootan N Nayebhashemi M Zahmatkesh M Enayati S et al Olorofim Effectively Eradicates Dermatophytes In Vitro and In Vivo. Antimicrob Agents Chemother (2021) 65(12):e0138621. 10.1128/AAC.01386-21
57.
Schell WA Jones AM Borroto-Esoda K Alexander BD . Antifungal Activity of SCY-078 and Standard Antifungal Agents Against 178 Clinical Isolates of Resistant and Susceptible Candida Species. Antimicrob Agents Chemother (2017) 61(11):e01102-17–. 10.1128/AAC.01102-17
58.
Rauseo AM Coler-Reilly A Larson L Spec A . Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect Dis (2020) 7(2):ofaa016. 10.1093/ofid/ofaa016
59.
Prattes J King T Azie N Angulo D . P056 Oral Ibrexafungerp Outcomes by Fungal Disease in Patients From an Interim Analysis of a Phase 3 Open-Label Study (FURI). Med Mycol (2022) 60(1):myac072P056. 10.1093/mmy/myac072.p056
60.
Sandison T Ong V Lee J Thye D . Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults. Antimicrob Agents Chemother (2017) 61(2):e01627. 10.1128/AAC.01627-16
61.
Miesel L Cushion MT Ashbaugh A Lopez SR Ong V . Efficacy of Rezafungin in Prophylactic Mouse Models of Invasive Candidiasis, Aspergillosis, and Pneumocystis Pneumonia. Antimicrob Agents Chemother (2021) 65(3):e01992. 10.1128/AAC.01992-20
62.
Cushion MT Ashbaugh A . The Long-Acting Echinocandin, Rezafungin, Prevents Pneumocystis Pneumonia and Eliminates Pneumocystis From the Lungs in Prophylaxis and Murine Treatment Models. J Fungi (Basel) (2021) 7(9):747. 10.3390/jof7090747
63.
Pechacek J Yakubu I Vissichelli NC Bruno D Morales MK . Successful Expanded Access Use of Rezafungin, a Novel Echinocandin, to Eradicate Refractory Invasive Candidiasis in a Liver Transplant Recipient. J Antimicrob Chemother (2022) 77(9):2571–3. 10.1093/jac/dkac206
64.
Thompson GR Soriano A Cornely OA Kullberg BJ Kollef M Vazquez J et al Rezafungin Versus Caspofungin for Treatment of Candidaemia and Invasive Candidiasis (ReSTORE): A Multicentre, Double-Blind, Double-Dummy, Randomised Phase 3 Trial. Lancet (2023) 401(10370):49–59. 10.1016/S0140-6736(22)02324-8
65.
Wring S Borroto-Esoda K Solon E Angulo D . SCY-078, a Novel Fungicidal Agent, Demonstrates Distribution to Tissues Associated With Fungal Infections During Mass Balance Studies With Intravenous and Oral [14C]SCY-078 in Albino and Pigmented Rats. Antimicrob Agents Chemother (2019) 63(2):e02119. 10.1128/AAC.02119-18
66.
Wring S Murphy G Atiee G Corr C Hyman M Willett M et al Clinical Pharmacokinetics and Drug-Drug Interaction Potential for Coadministered SCY-078, an Oral Fungicidal Glucan Synthase Inhibitor, and Tacrolimus. Clin Pharmacol Drug Dev (2019) 8(1):60–9. 10.1002/cpdd.588
67.
Zhao Y Prideaux B Nagasaki Y Lee MH Chen PY Blanc L et al Unraveling Drug Penetration of Echinocandin Antifungals at the Site of Infection in an Intra-abdominal Abscess Model. Antimicrob Agents Chemother (2017) 61(10):e01009. 10.1128/AAC.01009-17
68.
Ong V Hough G Schlosser M Bartizal K Balkovec JM James KD et al Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob Agents Chemother (2016) 60(11):6872–9. 10.1128/AAC.00701-16
69.
Flanagan S Walker H Ong V Sandison T . Absence of Clinically Meaningful Drug-Drug Interactions With Rezafungin: Outcome of Investigations. Microbiol Spectr (2023) 11(3):e0133923. 10.1128/spectrum.01339-23
Summary
Keywords
muli-drug resistant bacteria, antimicrobial resistance, antimicrobial stewardship, antifungal therapy, urinary tract infection
Citation
Serris A, Coussement J, Pilmis B, De Lastours V, Dinh A, Parquin F, Epailly E, Ader F, Lortholary O, Morelon E, Kamar N, Forcade E, Lebeaux D, Dumortier J, Conti F, Lefort A, Scemla A and Kaminski H (2023) New Approaches to Manage Infections in Transplant Recipients: Report From the 2023 GTI (Infection and Transplantation Group) Annual Meeting. Transpl Int 36:11859. doi: 10.3389/ti.2023.11859
Received
27 July 2023
Accepted
24 October 2023
Published
09 November 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Serris, Coussement, Pilmis, De Lastours, Dinh, Parquin, Epailly, Ader, Lortholary, Morelon, Kamar, Forcade, Lebeaux, Dumortier, Conti, Lefort, Scemla and Kaminski.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah Kaminski, hannah.kaminski@chu-bordeaux.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.