- 1Merck & Co., Inc., Kenilworth, NJ, United States
- 2Evidera, Bethesda, MD, United States
- 3Rush Medical College, Rush University, Chicago, IL, United States
by Raval AD, Ganz ML, Fraeman K, Lorden AL, Saravanan S, Tang Y and Santos CAQ (2022). Transpl Int. 35:10528. doi: 10.3389/ti.2022.10528
by Transplant International Editorial Office (2023) Transpl Int. 36:12367. doi: 10.3389/ti.2023.12367
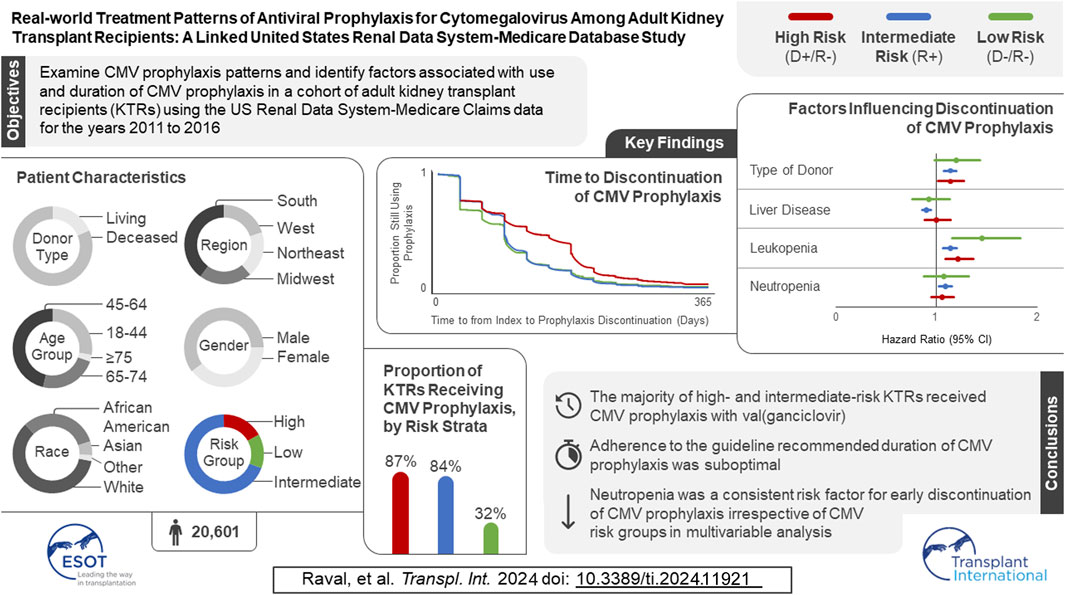
In the original article, there was a mistake in the Graphical Abstract as published. The number of participants in the study sample has changed, along with minor changes to the proportion of KTRs receiving CMV prophylaxis by risk strata, time to discontinuation of CMV prophylaxis, and the factors influencing discontinuation of CMV prophylaxis. The corrected Graphical Abstract appears below.
In the original article, there was a mistake in Figure 1 as published. Programing errors in the cohort selection led to differences in the cohort selected along with minor changes to the patient attrition related to inclusion and exclusion criteria. The corrected Figure 1 appears below.
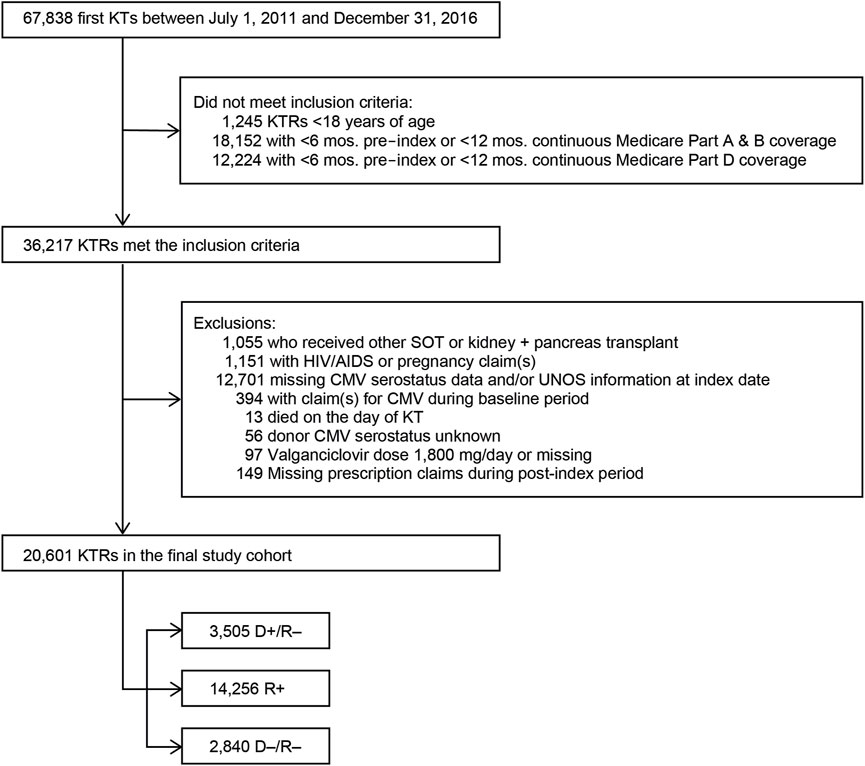
FIGURE 1. Study sample selection. Abbreviations: AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; D+, seropositive donor; D–, seronegative donor; HIV, human immunodeficiency virus; KT, kidney transplant; KTRs, kidney transplant recipients; mos., months; R+, seropositive recipient; R–, seronegative recipient; SOT, solid organ transplant.
In the original article, there was a mistake in Figure 2 as published. Changes in the composition of the study cohort due to programming changes resulted in slightly different KM curves. The corrected Figure 2 appears below.
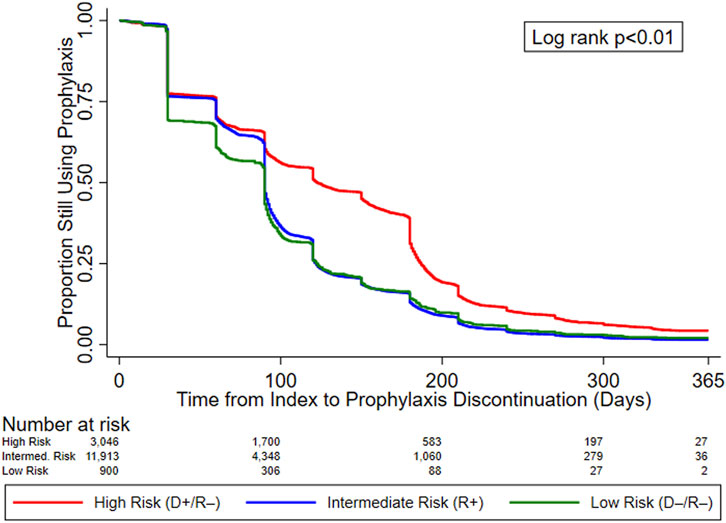
FIGURE 2. KM curves for time to prophylaxis discontinuation, by serostatus (CMV Risk Group). Abbreviations: D+, seropositive donor; D–, seronegative donor; R+, seropositive recipient; R–, seronegative recipient.
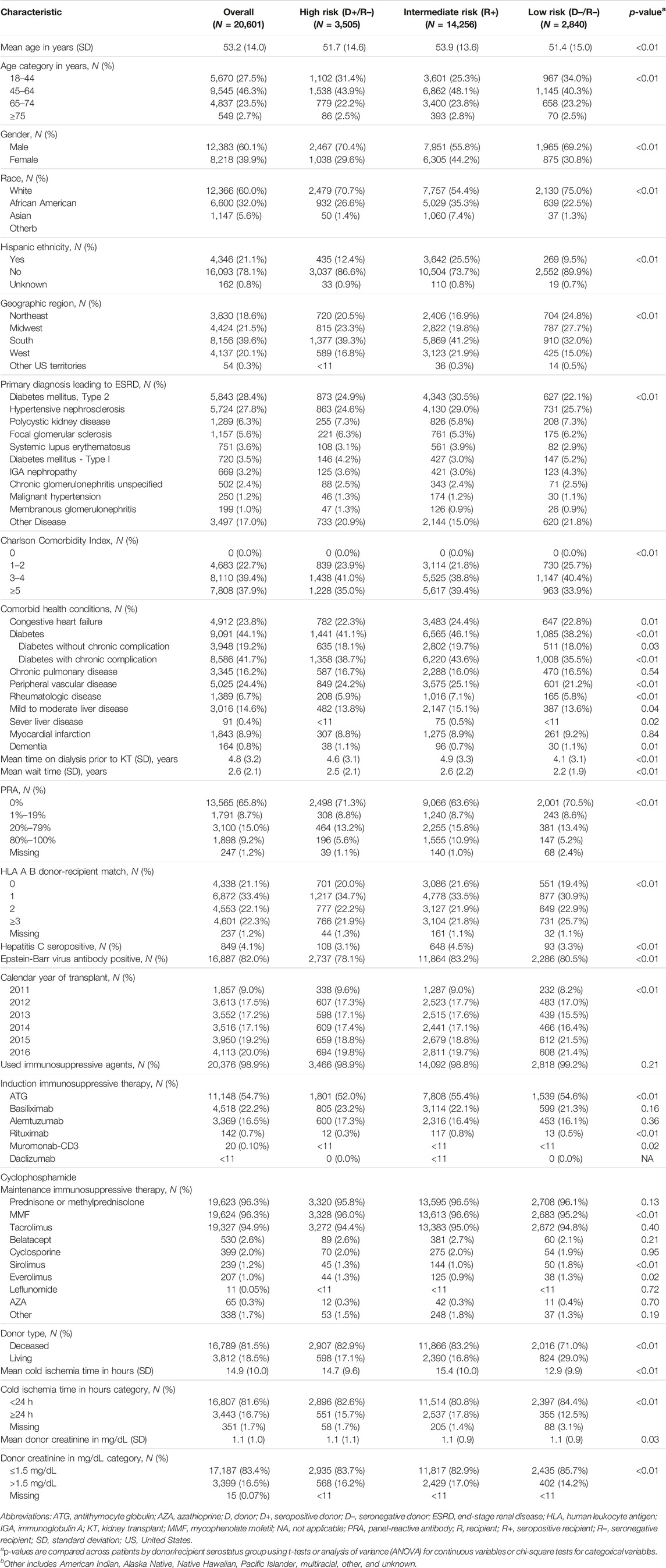
In the original article, there was a mistake in Table 1 as published. Changes in the composition of the study cohort due to programming errors that were corrected resulted in different numbers of patients reported throughout the table and slight differences in the proportions of patients in the various subgroups reported. The corrected Table 1 appears below.
In the original article, there was a mistake in Table 2 as published. Changes in the composition of the study cohort due to programming errors that were corrected resulted in different numbers of patients reported throughout the table and slight differences in the proportions of patients in the various subgroups reported. The corrected Table 2 appears below.
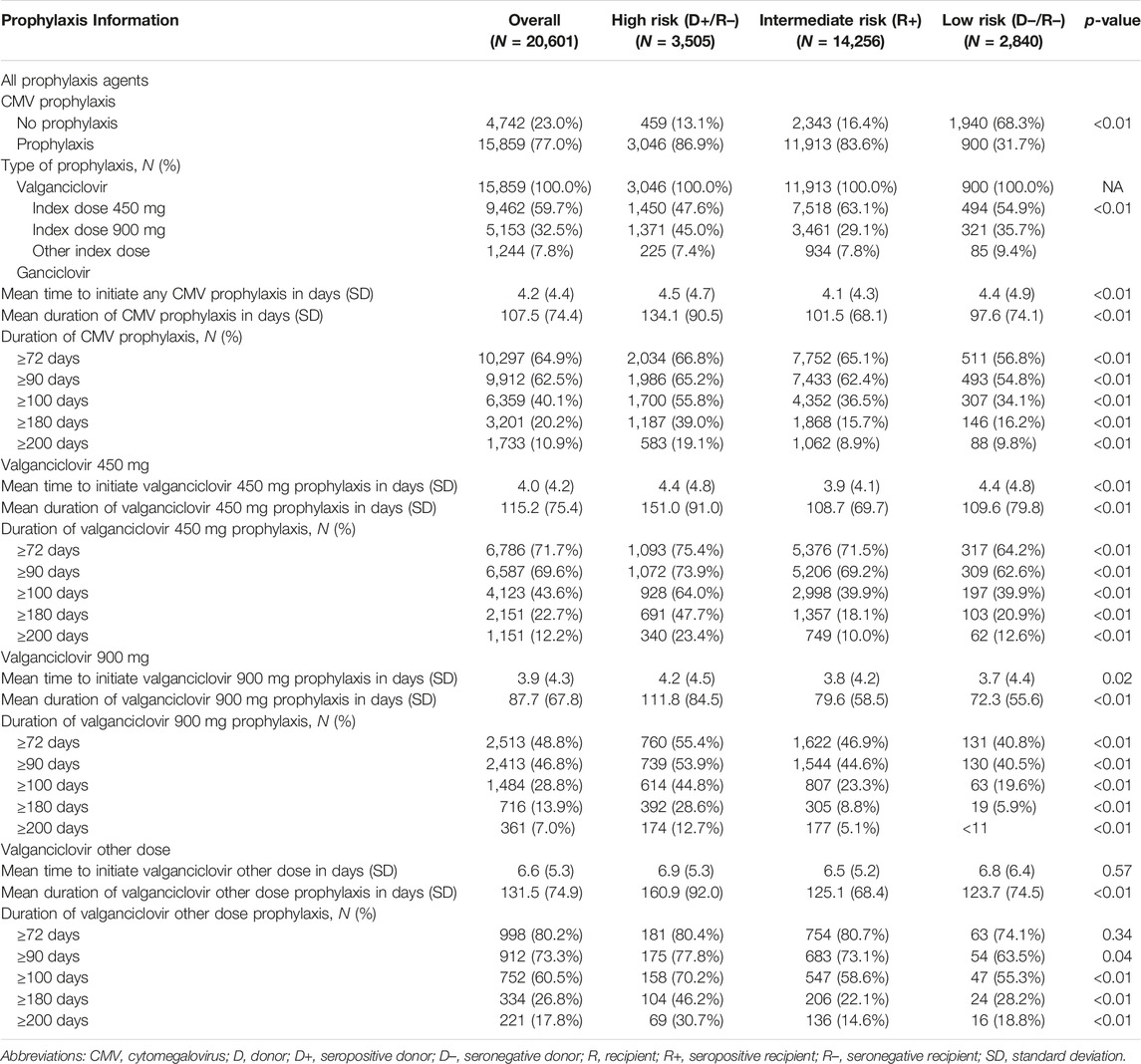
TABLE 2. Characteristics of CMV prophylaxis among adults undergoing first kidney transplant by serostatus.
In the original article, there was a mistake in Table 3 as published. Changes in the composition of the study cohort due to programming errors that were corrected resulted in different coefficients and confidence intervals for the variables included in the regression. While a few relationships changed, those that did change did not influence or create a need to revise the conclusions of the study. The corrected Table 3 appears below.
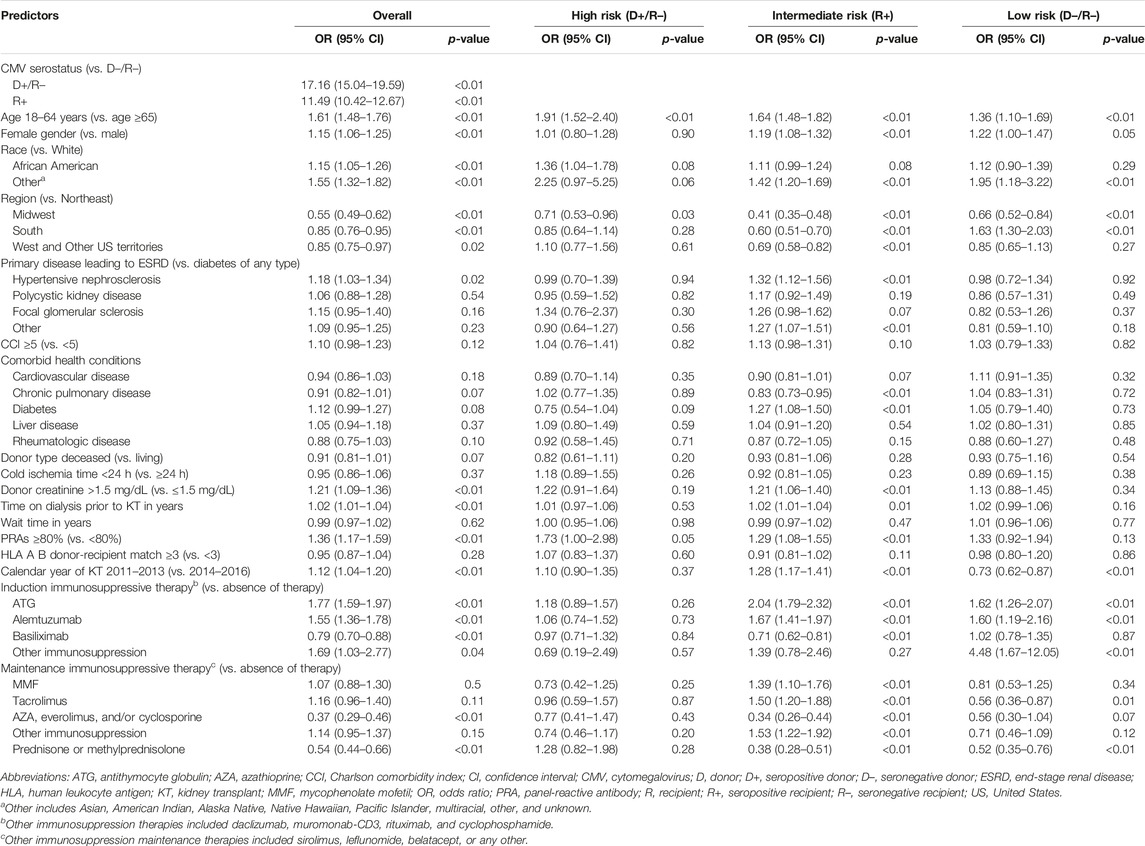
TABLE 3. Logistic regression for probability of starting CMV prophylaxis among adults undergoing a first kidney transplant.
In the original article, there was a mistake in Table 4 as published. Changes in the composition of the study cohort due to programming errors that were corrected resulted in different coefficients and confidence intervals for the variables included in the regression. While a few relationships changed, those that did change did not influence or create a need to revise the conclusions of the study. The corrected Table 4 appears below.
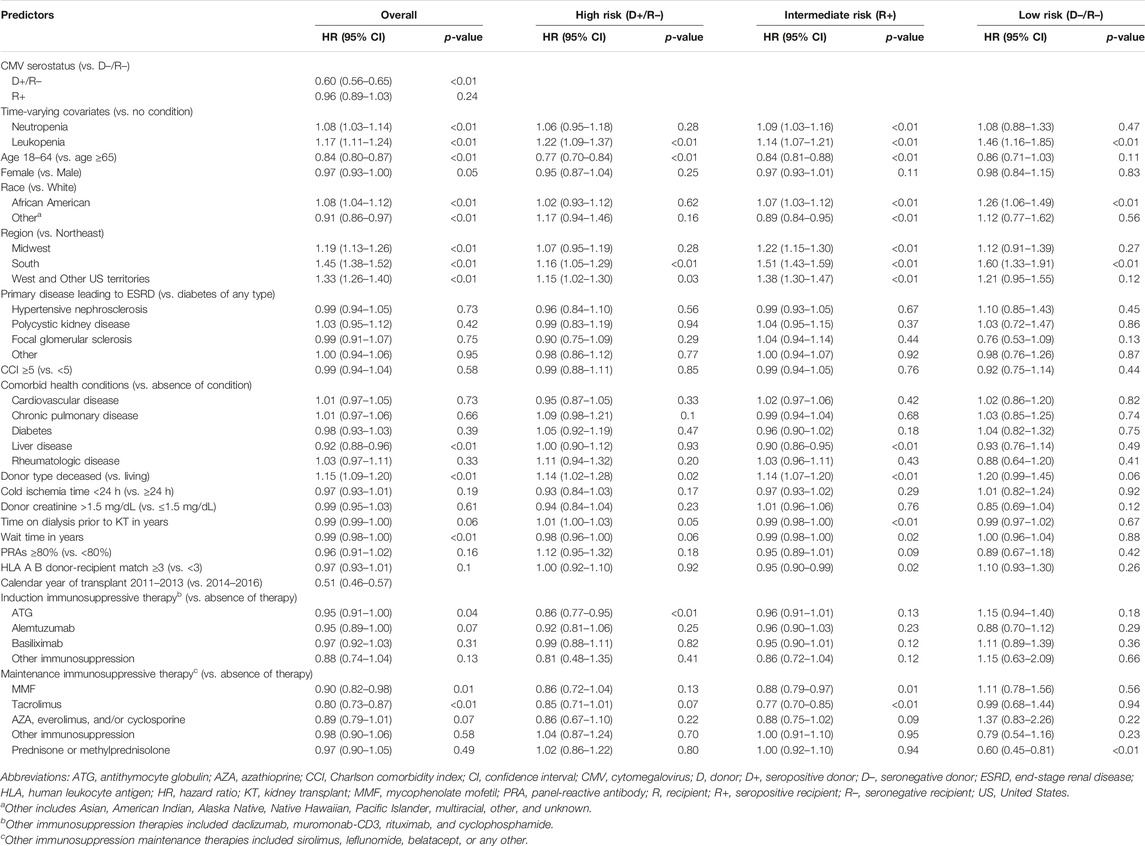
TABLE 4. Cox proportional hazard regression for time to CMV prophylaxis discontinuation among adults undergoing a first kidney transplant.
In the original article, there was a mistake in Supplementary Table 1 as published. Changes in the composition of the study cohort due to programming errors that were corrected resulted in different numbers of patients reported throughout the table and slight differences in the proportions of patients in the various subgroups reported. The corrected Supplementary Table 1 is available at the Supplementary Material link of the original paper.
In the original article, there were several errors. Changes in the composition of the study cohort due to programming errors that were corrected resulted in changes to all numeric results. However, conclusions drawn from the corrected analysis have not changed from those originally presented. Below are the changes necessary to correct all paragraphs reporting numeric values from the analysis.
A correction has been made to the Abstract:
“Using United States Renal Database System registry data and Medicare claims (1 January 2011–31 December 2017), we examined CMV antiviral use in 20,601 KTRs who received their first KT from 2011 to 2016. Three-quarters of KTRs started CMV prophylaxis (86.9% of high-, 83.6% of intermediate-, and 31.7% of low-risk KTRs). Median time to prophylaxis discontinuation was 121, 90, and 90 days for high-, intermediate-, and low-risk KTRs, respectively. Factors associated with receiving CMV prophylaxis were high-risk status, diabetes, receipt of a well-functioning kidney graft, greater time on dialysis before KT, panel reactive antibodies ≥80%, and use of antithymocyte globulin, alemtuzumab, and tacrolimus. KTRs were more likely to discontinue CMV prophylaxis if they developed leukopenia/neutropenia, had liver disease, or had a deceased donor.”
A correction has been made to Results, Baseline Characteristics, paragraph 1:
“We identified 67,838 individuals who received their first KT from 2011 to 2016, of whom 20,601 satisfied all inclusion and exclusion criteria (Figure 1). Table 1 summarizes the characteristics of our sample. Most (69.2%) KTRs were at intermediate risk of CMV infection, while 17.0% and 13.8% were at high and low risk, respectively. KTRs were, on average, 53.2 years of age at their initial KT. Most KTRs were male (60.1%) and White (60.0%); one-third were African American. Diabetes (28.4%), hypertensive nephrosclerosis (27.8%), polycystic kidney disease (6.3%), focal glomerular sclerosis (5.6%), and systemic lupus erythematosus (3.6%) were the five most frequent primary diseases leading to ESRD. More than one-third (37.9%) of the KTRs had a CCI score ≥5, and nearly one-quarter of KTRs also had congestive heart failure (23.8%). KTRs spent, on average, 4.8 years on dialysis prior to their KT and 2.6 years on the transplant waiting list. Large proportions of KTRs received their kidney grafts from a deceased donor (81.5%) and were positive for Epstein-Barr virus (82.0%). Most donor kidneys experienced <24 h of cold ischemia time (81.6%) and were well-functioning (donor creatinine clearance ≤1.5 mg/dL). Approximately 22% had HLA A B donor-recipient match scores ≥3, and 9.2% of KTRs had PRA ≥80%. ATG was the most used induction immunosuppressive agent (54.7%), followed by basiliximab (22.2%) and alemtuzumab (16.5%). Almost all KTRs used prednisone and/or methylprednisolone (96.3%), MMF (96.3%), and tacrolimus (94.9%) as maintenance immunosuppressive agents. High-risk KTRs were more likely to have had PRA equal to zero, and high- and intermediate-risk KTRs were less likely to have had three or more HLA A B matches than other KTRs. Intermediate-risk KTRs were slightly older and more likely to be female, African American or Asian, Hispanic, reside in the South or West regions, have diabetes or hypertensive nephrosclerosis as the primary cause of ESRD, have a CCI score ≥5, and PRA ≥80% than KTRs in the other groups. Low-risk KTRs were more likely to reside in the Northeast or Midwest, and they were less likely to have had comorbid diabetes and to have used basiliximab as an induction immunosuppressive agent than other KTRs.”
A correction has been made to Results, Use and Factors Associated with the Use of CMV Antiviral Prophylaxis, paragraph 1:
“Table 2 displays, and compares across risk groups, the CMV prophylaxis characteristics of KTRs who started CMV prophylaxis. Slightly over three-quarters (77.0%) of KTRs started CMV prophylaxis (86.9% of high-, 83.6% of intermediate-, and 31.7% of low-risk KTRs). Overall, 59.7% and 32.5% of KTRs who started CMV prophylaxis used valganciclovir 450 mg and 900 mg, respectively, while 7.8% used other doses of valganciclovir; no patients used ganciclovir. Overall, KTRs who started prophylaxis did so, on average, 4.2 days after receiving their KTs; time to starting prophylaxis did not vary substantially across risk groups (4.1–4.5 days).”
A correction has been made to Results, Use and Factors Associated with the Use of CMV Antiviral Prophylaxis, paragraph 2:
“Table 3 displays the results of the logistic regression models for use of CMV prophylaxis (descriptive statistics stratified by CMV prophylaxis status within risk group are available in Supplementary Table 1). In general, CMV risk status was the factor most strongly associated with the use of CMV prophylaxis. KTRs who were younger, female, African American or of other races, resided in the Northeast, as well as those whose donor creatinine levels were >1.5 mg/dL, who spent more time on dialysis prior to KT, had PRA ≥80%, and who used ATG, and alemtuzumab were more likely to receive CMV prophylaxis (all and intermediate-risk KTRs). KTRs whose kidney graft experienced cold ischemia time <24 h, used basiliximab, AZA, everolimus, or cyclosporine, or prednisone and/or methylprednisolone were less likely to receive CMV prophylaxis (all and intermediate-risk KTRs). Additionally, high-risk KTRs who had PRA ≥80% were more likely to receive CMV prophylaxis; whereas those with comorbid diabetes, and who used AZA, everolimus, or cyclosporine, MMF or other maintenance immunosuppressive agents were less likely to receive CMV prophylaxis. Low-risk KTRs who were female, resided in the South, and used ATG and alemtuzumab or other immunosuppression as induction immunosuppressive agents were more likely to receive CMV prophylaxis.”
A correction has been made to Results, Duration of Prophylaxis and Factors Associated with Risk of CMV Prophylaxis Discontinuation, Paragraph 1:
“Figure 2 displays the KM curves for time to prophylaxis discontinuation. The median time to prophylaxis discontinuation (i.e., prophylaxis duration), derived from the KM curves, for the high-risk group of KTRs was longer (121 days) than for intermediate- (90 days) and low-risk (90 days) KTRs. Regardless of type of antiviral agent used, 10.9% of KTRs who used CMV prophylaxis did so for ≥200 days (23.4% and 12.7% of high-risk KTRs who used valganciclovir 450 mg and 900 mg, respectively, did so for ≥200 days) and more than half (55.8%) of high-risk KTRs used CMV prophylaxis for ≥100 days (64.0% and 44.8% of high-risk KTRs who used valganciclovir 450 mg and 900 mg, respectively, did so for ≥100 days). Over one-third (36.5%) of intermediate-risk KTRs used CMV prophylaxis for ≥100 days (39.4% and 23.3% of intermediate-risk KTRs who used valganciclovir 450 mg and 900 mg, respectively, did so for ≥100 days).”
A correction has been made to Results, Duration of Prophylaxis and Factors Associated with Risk of CMV Prophylaxis Discontinuation, Paragraph 2:
“Table 4 displays the results of the PH Cox regression models for time to CMV prophylaxis discontinuation. We found that, regardless of risk group, KTRs who resided in the South and who developed leukopenia were more likely to discontinue CMV prophylaxis; all KTRs, as well as intermediate-risk group KTRs who developed neutropenia were also more likely to discontinue. Additionally, overall and intermediate-risk KTRs with comorbid liver disease, who experienced a longer wait time, lived in the Midwest, or received MMF or tacrolimus were more likely to discontinue CMV prophylaxis. Among the overall, high-, and intermediate-risk KTRs, those who were younger, received kidney grafts from deceased donors, or lived in the West or other US territories were more likely to discontinue prophylaxis. Finally, overall, intermediate-, and low-risk KTRs who identified as African American were more likely to discontinue CMV prophylaxis, as were overall and intermediate-risk KTRs of other races.”
A correction has been made to Discussion, paragraph 1:
“CMV prophylaxis was more common among high- (86.9%) than intermediate- (83.6%) and low-risk (31.7%) KTRs, with all those KTRs using valganciclovir and almost 60% of valganciclovir users using 450 mg per day.”
A correction has been made to Discussion, paragraph 2:
“Furthermore, we found that the mean duration of CMV prophylaxis was also longer in our study; however, still only approximately one in five high-risk KTRs completed 200 days of CMV prophylaxis and just over one in three intermediate-risk KTRs completed 100 days of CMV prophylaxis.”
In the original article, there was a mistake in the Data Availability Statement as published. The original statement incorrectly stated that the USRDS-Medicare data was publicly available. The corrected Data Availability statement is as follows:
“This study used data from the USRDS-Medicare database, which was provided to the study team subject to the terms of data use agreement (DUA) 2020-41f. The data are not publicly available due to privacy laws and cannot be shared by the authors. However, data obtained from the USRDS-Medicare database for this study may be accessed by applying to USRDS/NIDDK/CMS at dXNyZHNAbmlkZGsubmloLmdvdg==. Upon request, the corresponding author will provide the original data request and the programs used to derive this study’s analytic cohort.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article. The original article has been updated.
Keywords: kidney transplantation, antiviral, cytomegalovirus, prophylaxis, pharmacoepidemiology
Citation: Raval AD, Ganz ML, Fraeman K, Lorden AL, Saravanan S, Tang Y and Santos CAQ (2024) Corrigendum: Real-World Treatment Patterns of Antiviral Prophylaxis for Cytomegalovirus Among Adult Kidney Transplant Recipients: A Linked USRDS-Medicare Database Study. Transpl Int 37:11921. doi: 10.3389/ti.2024.11921
Received: 14 August 2023; Accepted: 16 January 2024;
Published: 14 February 2024.
Copyright © 2024 Raval, Ganz, Fraeman, Lorden, Saravanan, Tang and Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit D. Raval, YW1pdGt1bWFyLmQucmF2YWxAZ21haWwuY29t; Michael L. Ganz, bWljaGFlbC5nYW56QGV2aWRlcmEuY29t
 Amit D. Raval
Amit D. Raval Michael L. Ganz
Michael L. Ganz Kathy Fraeman2
Kathy Fraeman2 Andrea L. Lorden
Andrea L. Lorden Shanmugapriya Saravanan
Shanmugapriya Saravanan