- 1Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium
- 2Laboratory of Hepatology, Department of Chronic Diseases and Metabolism (CHROMETA), KU Leuven, Leuven, Belgium
- 3Department of Internal Medicine, Division of Gastroenterology and Hepatology, Maastricht University Medical Centre, Maastricht, Netherlands
- 4School of Nutrition and Translational Research in Metabolism (NUTRIM), University Maastricht, Maastricht, Netherlands
- 5Transplantation Research Group, Department of Microbiology, and Transplantation, Laboratory of Abdominal Transplantation, KU Leuven, University of Leuven, Leuven, Belgium
- 6Department of Abdominal Transplant Surgery, University Hospitals Leuven, Leuven, Belgium
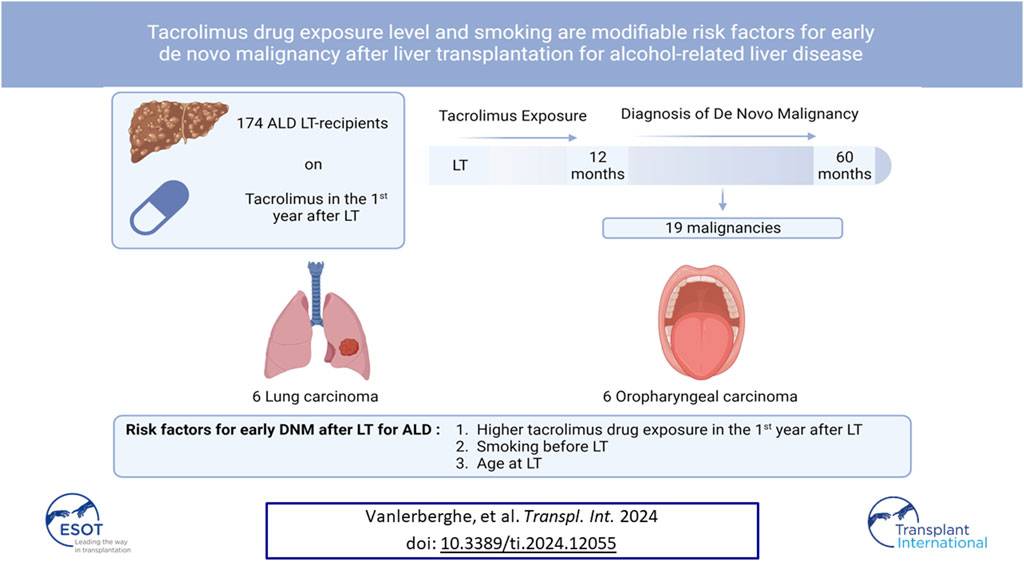
De novo malignancy (DNM) is the primary cause of mortality after liver transplantation (LT) for alcohol-related liver disease (ALD). However, data on risk factors for DNM development after LT are limited, specifically in patients with ALD. Therefore, we retrospectively analyzed all patients transplanted for ALD at our center before October 2016. Patients with a post-LT follow-up of <12 months, DNM within 12 months after LT, patients not on tacrolimus in the 1st year post-LT, and unknown smoking habits were excluded. Tacrolimus drug exposure level (TDEL) was calculated by area under the curve of trough levels in the 1st year post-LT. 174 patients received tacrolimus of which 19 (10.9%) patients developed a DNM between 12 and 60 months post-LT. Multivariate cox regression analysis identified TDEL [HR: 1.710 (1.211–2.414); p = 0.002], age [1.158 (1.076–1.246); p < 0.001], number of pack years pre-LT [HR: 1.021 (1.004–1.038); p = 0.014] and active smoking at LT [HR: 3.056 (1.072–8.715); p = 0.037] as independent risk factors for DNM. Tacrolimus dose minimization in the 1st year after LT and smoking cessation before LT might lower DNM risk in patients transplanted for ALD.
Introduction
Alcohol-related liver disease (ALD) is the primary indication for liver transplantation (LT) in Europe and the United States [1]. De novo malignancies (DNM) are the leading cause of mortality in patients transplanted for ALD [2–4], a population with an increased risk of DNM compared to patients who received a LT for other indications [5]. This might be attributed to the oncogenic effects of long-term alcohol consumption [6, 7] and the high prevalence of tobacco use in patients with ALD [8]. Another risk factor for DNM is the immunosuppressive therapy patients receive after LT [9, 10]. Calcineurin inhibitors (CNIs) are the most frequently used agents for long-term immunosuppression and tacrolimus is currently the most frequently used CNI [1]. Tacrolimus reduces the risk of allograft rejection after LT by inhibiting calcineurin, which leads to decreased cytokine transcription, particularly interleukin-2 (IL-2), and reduces T-cell proliferation [11]. Tacrolimus and its immunosuppressive properties are also associated with the development of DNM due to diminished immunosurveillance [12]. However, studies analyzing the risk of tacrolimus exposure and trough levels on DNM development after LTx are scarce [13–15]. These few studies did not specifically analyze ALD LT recipients [13, 14] and did not examine the effect of lifetime tobacco consumption and alcohol relapse after LT, factors that might have a strong contributive effect on malignancy formation. Furthermore, these studies assessed the lifetime DNM risk after LT based on 1st year of tacrolimus drug exposure. However, the 1st year exposure might only reflect the DNM risk within a shorter time after LT. Therefore, in this study we assessed the risk factors for DNM in the 1st 5-year period after LT for ALD, with specific emphasis on tacrolimus drug level in the 1st year post-LT and other modifiable risk factors such as smoking and alcohol use.
Patients and Methods
Patient Characteristics
We analyzed the Liver Transplantation Cohort from the University Hospitals KU Leuven for adult patients (age ≥ 18 years) transplanted for ALD between 1990 and October 2016. Diagnosis of ALD was made based on the patient’s history of excessive and habitual alcohol consumption, clinical and laboratory findings, histology of the explanted liver, and exclusion of other causes of liver disease (viral hepatitis, autoimmune hepatitis, hereditary hemochromatosis, Wilson’s disease, primary biliary cholangitis, and primary sclerosing cholangitis), and based on the consensus of the multidisciplinary medical team. Patients with less than 12 months follow-up post-LT, a DNM in the 1st year post-LT or patients who did not receive tacrolimus during the 1st 12 months after LT were excluded from the analysis. Patients with unknown smoking habits (no information available on smoking history, i.e., pack years smoked before LT) were also excluded from the analysis. We retrospectively collected data on general patient characteristics, type and timing of DNM, excluding non-melanoma skin cancer and recurrence of hepatocellular carcinoma as events. DNM were reported as events if they occurred between 12 and 60 months of follow-up. Positive smoking history (defined as ≥ 1 pack year pre-LT), absolute number of pack years (PY) smoked before LT and active smoking at LT were extracted from patient’s medical files. Active smoking was defined as smokers who still had an active smoking habit when admitted to the hospital for the LT procedure. Alcohol relapse was defined as relapse with any alcohol use.
In the work-up before LT, each patient is systematically screened for malignancies. Patients receive a gastroscopy, colonoscopy, chest x-ray, liver MRI and/or CT abdomen, abdominal ultrasound, and an assessment by an ear-nose-throat specialist and dermatologist. Female LT candidates also receive a mammography and a gynecological ultrasound. After LT for ALD, patients do not receive additional systematic screening for DNM aside from the Belgian government’s population screening for colon and breast cancer.
Immunosuppressive Regimen
After LT, the standard immunosuppressive protocol consists of tacrolimus, an antimetabolite, and corticosteroids. After 3 months, corticosteroids are discontinued. If possible, antimetabolite is discontinued after 12 months. The used antimetabolites are mycophenolate, and azathioprine in the minority of patients. Deviations from the protocol are only performed on clinical indication. Tacrolimus trough levels are analyzed daily during hospital admission directly following LT. After hospital discharge, trough levels are determined twice a week during the first month, every 2 weeks during the first 3 months, three monthly from 3 months to 1 year post-LT and per 3–6 months thereafter. On clinical indication, tacrolimus trough levels are analyzed more frequently.
Statistical Analysis
Normality was checked by the Shapiro-Wilk test. Qualitative variables were compared using the χ2 test. Normally distributed values are presented as means with 95% confidence intervals (95% CI) or standard deviation (SD) and were compared using an independent t-test. Non-normally distributed values are presented as medians with interquartile range (IQR) and were compared using the Mann-Whitney U and Kruskal-Wallis tests.
Hazard ratios (HR) for the risk of DNM between twelve and 60 months after LT were evaluated by Cox proportional hazards regression. Patients were considered at risk from twelve to 60 months post-LT or until reaching the study endpoints (diagnosis of DNM or death). Patients lost to follow-up were censored at time of loss from data analysis. A proportional hazard model was performed with categorical, continuous, and time-dependent covariates to identify risk factors. Additional covariates were selected by expert opinion and based on literature [13, 14]. The proportional hazard assumption was tested for each covariate by correlation testing of Schoenfeld residuals and rank time; only covariates without significant correlation were included (see Supplementary Table S1). Multivariate analysis was performed by backward elimination with a selection criterion of 0.100. Statistical analysis was performed by SPSS v.28 (SPSS Inc., Chicago, IL, United States). Statistical significance was defined as p ≤ 0.05.
Total tacrolimus drug exposure level (TDEL) was based on the area under the curve of tacrolimus trough levels in µg/L [analyzed in R by PKCNA package (linear)] and corrected by the trapezoidal rule as previously described by Vivarelli et al. [16].
Results
Patient Characteristics
Within the study period, 317 patients were transplanted for ALD at our center, of which 174 were included in the analysis. Patients who had a follow-up of 12 months or less (n = 23; 22 died within the 1st year post-LT and one patient was lost to follow-up at 11 months post-LT), patients who were diagnosed with a DNM within 12 months after LT (n = 11) and patients who did not receive tacrolimus in the 1st year after LT (n = 46) were excluded. 56 patients were excluded because there was insufficient information available on their smoking habits. Seven patients were excluded because of missing data on tacrolimus trough levels in the 1st year post-LT. All included patients were transplanted after December 1998.
During the 1st year after LT, 117 (67.2%) patients received Prograft and 57 (32.8%) Advagraf. 144 (82.8%) patients received mycophenolate and 20 (11.5%) patients received azathioprine as an antimetabolite. The median age at LT was 59.5 years (IQR: 54.0–65.0) (Table 1). Median follow-up was 91 months (IQR: 65.0–143.0).
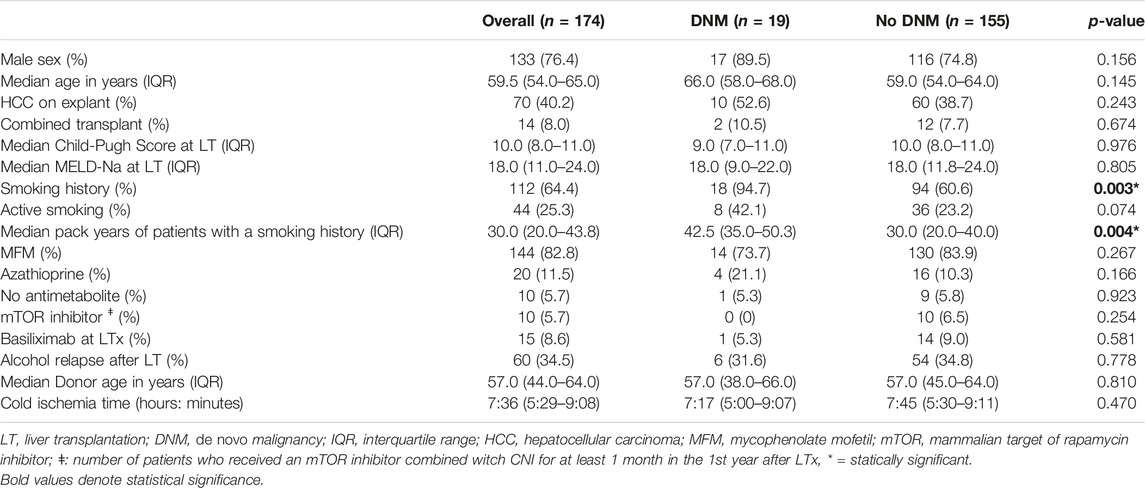
TABLE 1. General characteristics of patients at liver transplantation diagnosed with a de novo malignancy compared to those not diagnosed with a de novo malignancy.
Of the total group, 112 (64.4%) patients had a positive smoking history, of which 44 (25.3% of the total group) actively smoked at the time of transplantation (Table 1). 60 patients (34.5%) had a relapse of any alcohol use after LT. 47 patients (78.3% of the relapsers) relapsed within 5 years after LT, with a median time until relapse of 15.0 months (IQR: 4.0–32.0).
De Novo Malignancy
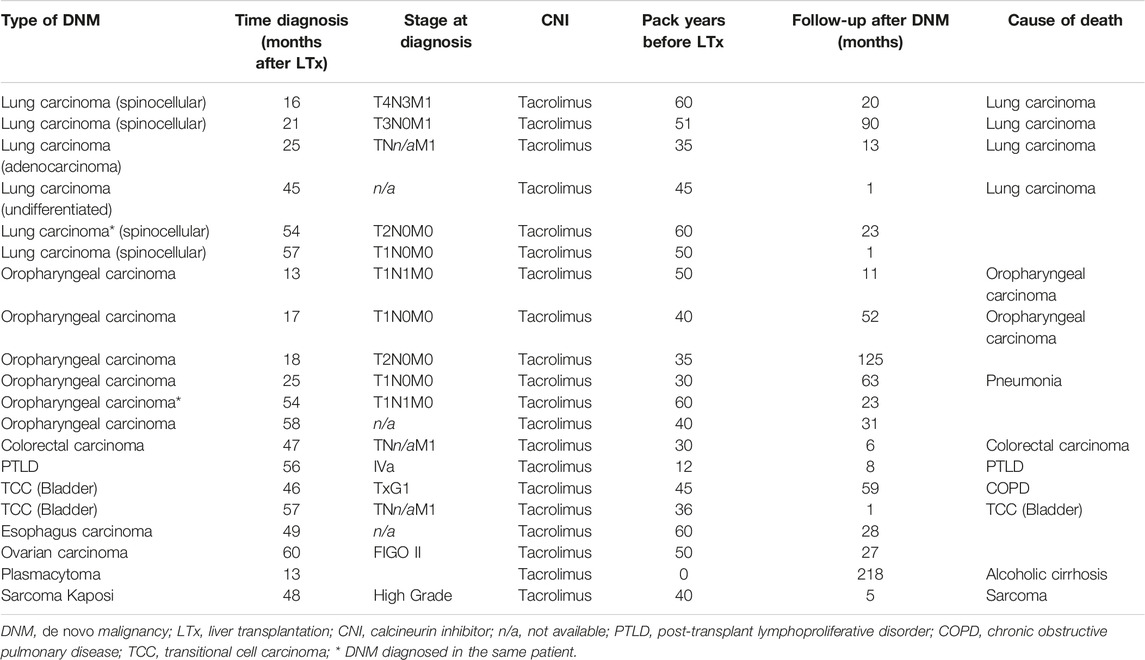
19 (10.9%) patients were diagnosed with a DNM between 12 and 60 months after LT. Characteristics of patients diagnosed with a DNM compared to those who did not can be found in Table 1. Median time until diagnosis of DNM was 46 months (IQR: 18.0–56.0). 17 patients developed a solid organ malignancy with oropharyngeal (n = 6) and lung carcinoma (n = 6) being the most prevalent (Table 2). One patient developed a simultaneous lung and oropharyngeal carcinoma at 54 months post-LT. Of the 17 solid organ DNMs, 5 (29.4%) patients had metastatic disease, 9 (52.9%) had only local disease and in 3 (17.6%) patients staging at the time of diagnosis was not reported. Four of the six patients diagnosed with lung carcinoma died from the malignancy during follow-up. The other two only had local disease at diagnosis and were alive after 1 and 23 months of follow-up. Two out of six patients diagnosed with an oropharyngeal carcinoma died because of the carcinoma, yet all diagnoses were made in a non-metastatic stage. All patients diagnosed with a lung or oropharyngeal malignancy had a smoking history. Two patients have developed hematological malignancies (1.1%) being a post-transplant lymphoproliferative disease and a plasmacytoma. One patient developed a soft tissue tumor (Kaposi sarcoma).
Patients diagnosed with a DNM between 12 and 60 months after LT had a higher mortality than those not diagnosed with a DNM [HR: 2.981 (95% CI: 1.573–5.652); p < 0.001)] (see Figure 1). Of patients diagnosed with a DNM, 13 (68.4%) died during follow-up, of which 10 died due to DNM. The overall 60 months survival was 81.0% (n = 141), and was significantly higher in the group without DNM (83.9%) compared to the group with a DNM (57.9%) (p = 0.006), corresponding with a shorter median follow-up in the DNM group than in the non-DNM group [69.0 months (IQR: 53.0–89.0) vs. 95.0 (IQR: 70.0–145.0); p = 0.020].
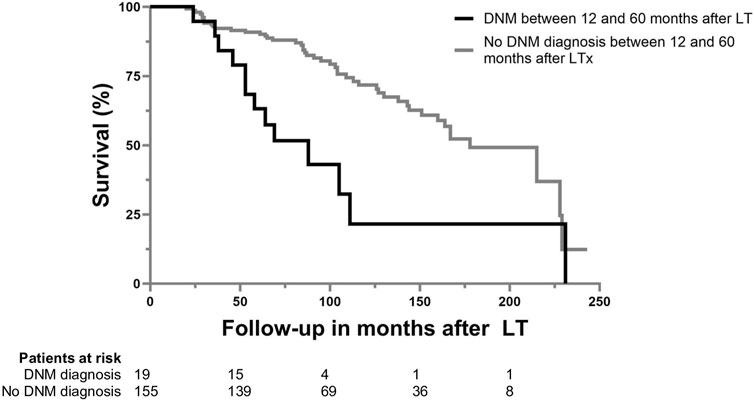
FIGURE 1. Patient survival after LTx for ALD based on the diagnosis of de novo malignancy between 12 and 60 months after LT.
Risk Factors for Developing DNM
Tacrolimus Drug Exposure Level
The mean tacrolimus drug exposure level in the 1st year post-LT was 7.41 μg/L (SD. 1.60) and was higher in patients who developed a DNM within 5 years after LT (8.14 μg/L; SD. 1.56) compared to patients who did not (7.31 μg/L; SD. 1.59) (p = 0.016). Univariate cox regression analysis identified TDEL as a risk factor for DNM between 12 and 60 months after LTx [HR: 1.357 (95% CI: 1.027–1.790); p = 0.031].
When analyzing the ROC of TDEL plotted against DNM prevalence, a TDEL of 6.94 μg/L had the highest discriminative value. 15 out of 98 patients (15.3%) with a TDEL above 6.94 μg/L developed a DNM compared to 4 out 76 patients (5.3%) with a TDEL lower than 6.94 μg/L. Also when implementing TDEL as a categorical value using the cut-off of 6.94 μg/L, TDEL remained a significant risk factor for DNM [HR: 3.009 (95% CI: 1.000–9.067); p = 0.050].
Other Risk Factors for DNM
18 (94.7%) patients with a DNM had a positive smoking history (≥1 pack year pre-LT) compared to 94 (60.6%) of patients who did not develop a DNM (p = 0.003). Patients who smoked and were diagnosed with a DNM had a higher tobacco consumption at LT than those without a DNM diagnosis [median pack years: 42.5 (IQR: 35.0–50.3) vs. 30.0 (IQR: 20.0–40.0); p = 0.004].
Univariate cox regression analysis identified age at LT [HR: 1.115 (95% CI: 1.039–1.197), p = 0.002], smoking history [HR: 11.136 (95% CI: 1.486–83.451); p = 0.019] and number of pack years pre-LT [HR: 1.024 (95% CI: 1.010–1.038); p = 0.001] as risk factors for developing DNM in the 1st 5 years after LT. In multivariate analysis, only pack years and not smoking history (defined as ≥ 1 pack year pre-LT) was included to avoid multicollinearity (Table 3).
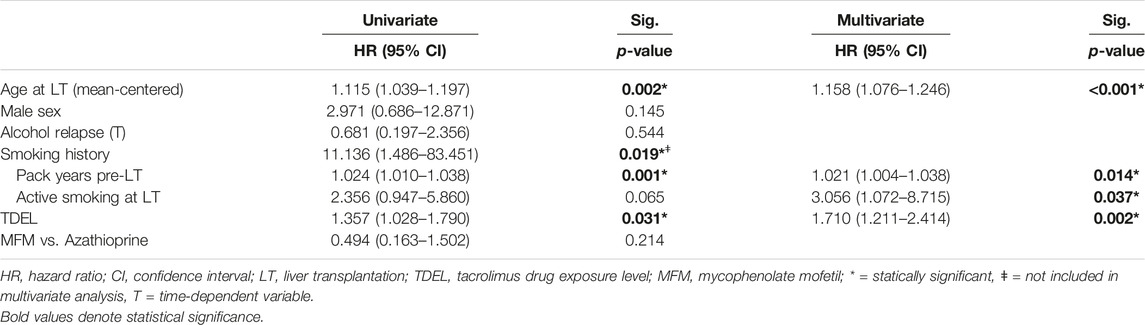
TABLE 3. Univariate and multivariate cox proportional hazard regression for risk on de novo malignancy between 12 and 60 months after liver transplantation (174 patients) in patients under tacrolimus in first-year after liver transplantation.
Multivariate analysis identified age at LT [HR: 1.158 (95% CI: 1.076–1.246); p < 0.001], number of pack years pre-LT [HR: 1.021 (95% CI: 1.004–1.038); p = 0.014], active smoking at LT [HR 3.056 (95% CI: 1.072–8.715); p = 0.037] and TDEL in the 1st year after LT [HR: 1.710 (95% CI: 1.211–2.414); p = 0.002] as independent risk factors for developing DNM in the 1st 5 years after LT (Table 3). The use of mycophenolate mofetil compared to azathioprine, sex, and any alcohol relapse within 5 years after LT were not associated with a higher risk on DNM after LT.
Rate of Liver Graft Rejection
21 (12.1%) patients of the total group developed an acute cellular rejection (ACR) within the first 30 days after LT. Patients with a graft rejection in the first month had a higher TDEL in the first year after LT [mean TDEL: 8.10 μg/L (SD. 1.69) vs. 7.31 μg/L (SD. 1.57); p = 0.034]. This resulted in a trend towards a higher number of DNM in the ACR-group [n = 4 (19.0%)] vs. the non-ACR-group [n = 15 (9.8%)], but this did not reach statistical significance (p = 0.203).
Discussion
Identifying modifiable risk factors for DNM in patients transplanted for ALD is crucial to optimize their outcome. In our study, we found that in patients transplanted for ALD, tacrolimus drug exposure level (TDEL) in the 1st year after LT, number of pack years before LT, active smoking at LT and older age at LT are independent risk factors for the development of early DNM within 12 and 60 months after LT. Hereby, we provide the first evidence on the impact of TDEL in the 1st year after LT for ALD on the occurrence of early DNM, taking into account a detailed analysis on smoking habits.
The development of malignancies after LT is a complex interaction between an individual’s risk on DNM based on genetic predisposition, exposure to carcinogenic viruses, previous and current behavior such as smoking and alcohol use, and immunosuppressive therapy [12]. The observed association of TDEL and DNM is in line with the findings of Carenco et al. [14] and Rodriguez-Perálvarez et al. [13], although these two studies were conducted in LT patients transplanted not merely for ALD, and used DNM at any time point after LT as outcome parameter. Taken together, these observations underline the potential of optimizing tacrolimus drug exposure in clinical care. Prospective studies should focus on identifying protocols aiming for the minimally acceptable tacrolimus through level in order to lower the DNM risk, without increasing allograft rejection. This might be achieved by the use of induction therapy and/or the concurrent use of other immunosuppressive agents such as mammalian target of rapamycin (mTOR) inhibitors. However, there is conflicting evidence on the impact of mTOR inhibitors on DNM and if their underlying effect is associated with their antiproliferative properties or their tacrolimus-saving effect [17]. The development of pharmacometric tools to accurately predict TDEL based on daily tacrolimus dosage, patient characteristics and concomitant medication could be another approach, which we are currently studying in our center [18, 19]. In our study, we observed a higher TDEL in the 1st year after LT in patients that experienced an acute cellular rejection, but could not associate low TDEL with an increased risk for rejection.
Identifying tacrolimus trough level cut-offs that are associated with an increased risk for DNM, yet not with an increased risk on rejection, would be helpful in the follow-up and care of our LT patients. Within our cohort the cut-off over which DNM risk was disproportionately increased was 6.94 μg/L. However given the retrospective nature of our study this cut-off should not be extrapolated to other cohorts. Prospective studies are needed to further establish cut-offs that are usable in clinical care.
Patients with a positive smoking history (≥1 pack year pre-LT) had a substantially higher risk of developing DNM. Accordingly, all patients with lung or oropharyngeal carcinoma, the most frequent DNMs, had a positive smoking history. There seemed to be a dose-related effect since for every pack year smoked before LT there was an increase in DNM risk (HR: 1.021). Furthermore, patients who actively smoked at LT had an increased risk of DNM independent from the number of pack years they smoked before LT or TDEL. Together, these data stress the importance of smoking cessation in the pretransplant period and support the implementation of smoking cessation programs [20]. Since alcohol is a carcinogen [21], we expected that alcohol relapse could lead to a higher incidence of DNM. However, we did not find a higher risk for DNM after any alcohol relapse, in line with other studies [22]. This could be explained by the higher risk of liver-related disease and mortality after alcohol relapse, which could occur before the development of DNM [1]. Age at LT was an independent risk factor for developing DNM. In our cohort and other centers [1], over the last decades, patients have become older at the time of LT due to a switch in focus from chronological to biological age as an eligibility criterium for LT listing [1]. Transplanting older patients will only increase DNM incidence post-LT in the future, underscoring the relevance of our findings and the need for measures to lower DNM risk.
In our study, lung carcinomas were frequently diagnosed in a metastatic setting, whereas oropharyngeal were not. Screening could be implemented in clinical practice to diagnose lung carcinoma in curative stages, which was the focus of several studies. Renaud et al. compared an intensive screening program (yearly chest CT and clinical examination by an otorhinolaryngologist versus chest CT every 5 years) in patients transplanted for ALD who continued to smoke after LT [23]. They found that 63.6% of patients underwent a curative treatment of lung carcinoma in the intensive screening program versus only 26.3% in the standard screening program (p = 0.062) [23]. There was no difference in curative treatment of oropharyngeal tumors between the groups [100% vs. 87.5% (p = 0.498)] [23]. These findings are comparable with our results, where in a clinical setting without systematic post-LT screening, all diagnoses of oropharyngeal tumors were made with a possibility of curative treatment. Other studies analyzing the impact of malignancy screening after LT also showed that screening led to diagnosing DNM in earlier stages with higher rates of curative treatment [24, 25]. However, these studies were not limited to ALD LT recipients, therefore caution is warranted to extrapolate these findings. We propose that future studies assessing the benefit of DNM screening post-LT should primarily focus on ALD patients who have a proportionally higher risk for DNM. Based on our data and those of Renaud et al. a yearly screening with chest CT for lung carcinoma might be beneficial in active smokers [23], and potentially in all patients with a smoking history.
Although we provided a detailed assessment of the effect of TDEL and smoking habits on the risk of DNM in patients transplanted for ALD, the retrospective nature of our study is a limitation. On the other hand, the single-center approach enables assessment of DNM risk in ALD patients that underwent similar work-up, treatment and follow-up regarding LT, yet this also implies that our data need external validation.
In conclusion, our study identified high tacrolimus drug exposure levels in the first year post-LT and smoking as significant risk factors for early DNM after LT for ALD. Therefore, tacrolimus overexposure should be avoided and more efforts for smoking cessation should be initiated in these patients. Future studies are needed to assess the value and cost-benefit of systematical DNM screening after LT.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by Ethische commissie onderzoek UZ/KU Leuven. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the long-term retrospective nature of the study.
Author Contributions
BV and JV participated in research design, writing of the paper and data analysis. HvM, MS-B, IJ, DC, DM, SvM, JP, and FN critically revised the manuscript. All authors contributed to draft finalization and approved the final version of the manuscript.
Funding
The study was supported by the Maag Lever Darm Stichting (Dutch Digestive Foundation)—Grant number D 18-19.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Graphical abstract was made using BioRender.com.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.12055/full#supplementary-material
References
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver Transplantation. J Hepatol (2016) 64(2):433–85. doi:10.1016/j.jhep.2015.10.006
2. Dumortier, J, Guillaud, O, Adham, M, Boucaud, C, Delafosse, B, Bouffard, Y, et al. Negative Impact of De Novo Malignancies Rather Than Alcohol Relapse on Survival After Liver Transplantation for Alcoholic Cirrhosis: A Retrospective Analysis of 305 Patients in a Single Center. Am J Gastroenterol (2007) 102(5):1032–41. doi:10.1111/j.1572-0241.2007.01079.x
3. Verbeek, J, Cassiman, D, and Nevens, F. De Novo Malignancy and Recurrent Alcoholic Cirrhosis Account for 70% of Deaths in Patients Transplanted for End-Stage Alcoholic Liver Disease. Am J Gastroenterol (2016) 111(3):436–7. doi:10.1038/ajg.2016.10
4. Dumortier, J, Dharancy, S, Cannesson, A, Lassailly, G, Rolland, B, Pruvot, FR, et al. Recurrent Alcoholic Cirrhosis in Severe Alcoholic Relapse After Liver Transplantation: A Frequent and Serious Complication. Am J Gastroenterol (2015) 110(8):1160–6. doi:10.1038/ajg.2015.204
5. Chandok, N, and Watt, KD. Burden of De Novo Malignancy in the Liver Transplant Recipient. Liver Transpl (2012) 18(11):1277–89. doi:10.1002/lt.23531
6. Boffetta, P, and Hashibe, M. Alcohol and Cancer. Lancet Oncol (2006) 7(2):149–56. doi:10.1016/s1470-2045(06)70577-0
7. Pasala, S, Barr, T, and Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res (2015) 37(2):185–97.
8. MacLean, RR, Sofuoglu, M, and Rosenheck, R. Tobacco and Alcohol Use Disorders: Evaluating Multimorbidity. Addict Behav (2018) 78:59–66. doi:10.1016/j.addbeh.2017.11.006
9. Dantal, J, and Soulillou, JP. Immunosuppressive Drugs and the Risk of Cancer After Organ Transplantation. N Engl J Med (2005) 352(13):1371–3. doi:10.1056/NEJMe058018
10. Geissler, EK. Post-Transplantation Malignancies: Here Today, Gone Tomorrow? Nat Rev Clin Oncol (2015) 12(12):705–17. doi:10.1038/nrclinonc.2015.186
11. Scalea, JR, Levi, ST, Ally, W, and Brayman, KL. Tacrolimus for the Prevention and Treatment of Rejection of Solid Organ Transplants. Expert Rev Clin Immunol (2016) 12(3):333–42. doi:10.1586/1744666x.2016.1123093
12. Buell, JF, Gross, TG, and Woodle, ES. Malignancy After Transplantation. Transplantation (2005) 80(2 Suppl. l):S254–64. doi:10.1097/01.tp.0000186382.81130.ba
13. Rodríguez-Perálvarez, M, Colmenero, J, González, A, Gastaca, M, Curell, A, Caballero-Marcos, A, et al. Cumulative Exposure to Tacrolimus and Incidence of Cancer After Liver Transplantation. Am J Transpl (2022) 22(6):1671–82. doi:10.1111/ajt.17021
14. Carenco, C, Assenat, E, Faure, S, Duny, Y, Danan, G, Bismuth, M, et al. Tacrolimus and the Risk of Solid Cancers After Liver Transplant: A Dose Effect Relationship. Am J Transpl (2015) 15(3):678–86. doi:10.1111/ajt.13018
15. Bardou, FN, Guillaud, O, Erard-Poinsot, D, Chambon-Augoyard, C, Thimonier, E, Vallin, M, et al. Tacrolimus Exposure After Liver Transplantation for Alcohol-Related Liver Disease: Impact on Complications. Transpl Immunol (2019) 56:101227. doi:10.1016/j.trim.2019.101227
16. Vivarelli, M, Cucchetti, A, La Barba, G, Ravaioli, M, Del Gaudio, M, Lauro, A, et al. Liver Transplantation for Hepatocellular Carcinoma Under Calcineurin Inhibitors: Reassessment of Risk Factors for Tumor Recurrence. Ann Surg (2008) 248(5):857–62. doi:10.1097/SLA.0b013e3181896278
17. de Fijter, JW. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation (2017) 101(1):45–55. doi:10.1097/tp.0000000000001447
18. Kirubakaran, R, Stocker, SL, Hennig, S, Day, RO, and Carland, JE. Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin Pharmacokinet (2020) 59(11):1357–92. doi:10.1007/s40262-020-00922-x
19. Woillard, JB, Labriffe, M, Debord, J, and Marquet, P. Tacrolimus Exposure Prediction Using Machine Learning. Clin Pharmacol Ther (2021) 110(2):361–9. doi:10.1002/cpt.2123
20. Ursic-Bedoya, J, Donnadieu-Rigole, H, Faure, S, and Pageaux, GP. Alcohol Use and Smoking After Liver Transplantation; Complications and Prevention. Best Pract Res Clin Gastroenterol (2017) 31(2):181–5. doi:10.1016/j.bpg.2017.03.005
21. Bagnardi, V, Rota, M, Botteri, E, Tramacere, I, Islami, F, Fedirko, V, et al. Alcohol Consumption and Site-Specific Cancer Risk: A Comprehensive Dose-Response Meta-Analysis. Br J Cancer (2015) 112(3):580–93. doi:10.1038/bjc.2014.579
22. Dumortier, J, Maucort-Boulch, D, Poinsot, D, Thimonier, E, Chambon-Augoyard, C, Ducroux, E, et al. Immunosuppressive Regimen and Risk for De Novo Malignancies After Liver Transplantation for Alcoholic Liver Disease. Clin Res Hepatol Gastroenterol (2018) 42(5):427–35. doi:10.1016/j.clinre.2018.04.011
23. Renaud, L, Hilleret, MN, Thimonier, E, Guillaud, O, Arbib, F, Ferretti, G, et al. De Novo Malignancies Screening After Liver Transplantation for Alcoholic Liver Disease: A Comparative Opportunistic Study. Liver Transpl (2018) 24(12):1690–8. doi:10.1002/lt.25336
24. Herrero, JI, Alegre, F, Quiroga, J, Pardo, F, Iñarrairaegui, M, Sangro, B, et al. Usefulness of a Program of Neoplasia Surveillance in Liver Transplantation. A Preliminary Report. Clin Transpl (2009) 23(4):532–6. doi:10.1111/j.1399-0012.2008.00927.x
Keywords: alcohol-related liver disease, de novo malignancy, calcineurin inhibitor, tacrolimus drug exposure level, liver transplantation
Citation: Vanlerberghe BTK, van Malenstein H, Sainz-Barriga M, Jochmans I, Cassiman D, Monbaliu D, van der Merwe S, Pirenne J, Nevens F and Verbeek J (2024) Tacrolimus Drug Exposure Level and Smoking Are Modifiable Risk Factors for Early De Novo Malignancy After Liver Transplantation for Alcohol-Related Liver Disease. Transpl Int 37:12055. doi: 10.3389/ti.2024.12055
Received: 15 September 2023; Accepted: 07 February 2024;
Published: 19 February 2024.
Copyright © 2024 Vanlerberghe, van Malenstein, Sainz-Barriga, Jochmans, Cassiman, Monbaliu, van der Merwe, Pirenne, Nevens and Verbeek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedict T. K. Vanlerberghe, amVmLnZlcmJlZWtAdXpsZXV2ZW4uYmU=
†ORCID: Benedict T. K. Vanlerberghe, orcid.org/0000-0002-4665-2042; Hannah van Malenstein, orcid.org/0000-0003-0673-0939; Mauricio Sainz-Barriga, orcid.org/0000-0002-9981-4340; Ina Jochmans, orcid.org/0000-0003-4592-2810; David Cassiman, orcid.org/0000-0002-6154-0970; Diethard Monbaliu, orcid.org/0000-0002-0506-1609; Schalk van der Merwe, orcid.org/0000-0002-9891-2686; Frederik Nevens, orcid.org/0000-0003-2905-5112; Jef Verbeek, orcid.org/0000-0002-1549-8003
 Benedict T. K. Vanlerberghe
Benedict T. K. Vanlerberghe Hannah van Malenstein1,2†
Hannah van Malenstein1,2† Mauricio Sainz-Barriga
Mauricio Sainz-Barriga Ina Jochmans
Ina Jochmans Diethard Monbaliu
Diethard Monbaliu Schalk van der Merwe
Schalk van der Merwe Jacques Pirenne
Jacques Pirenne