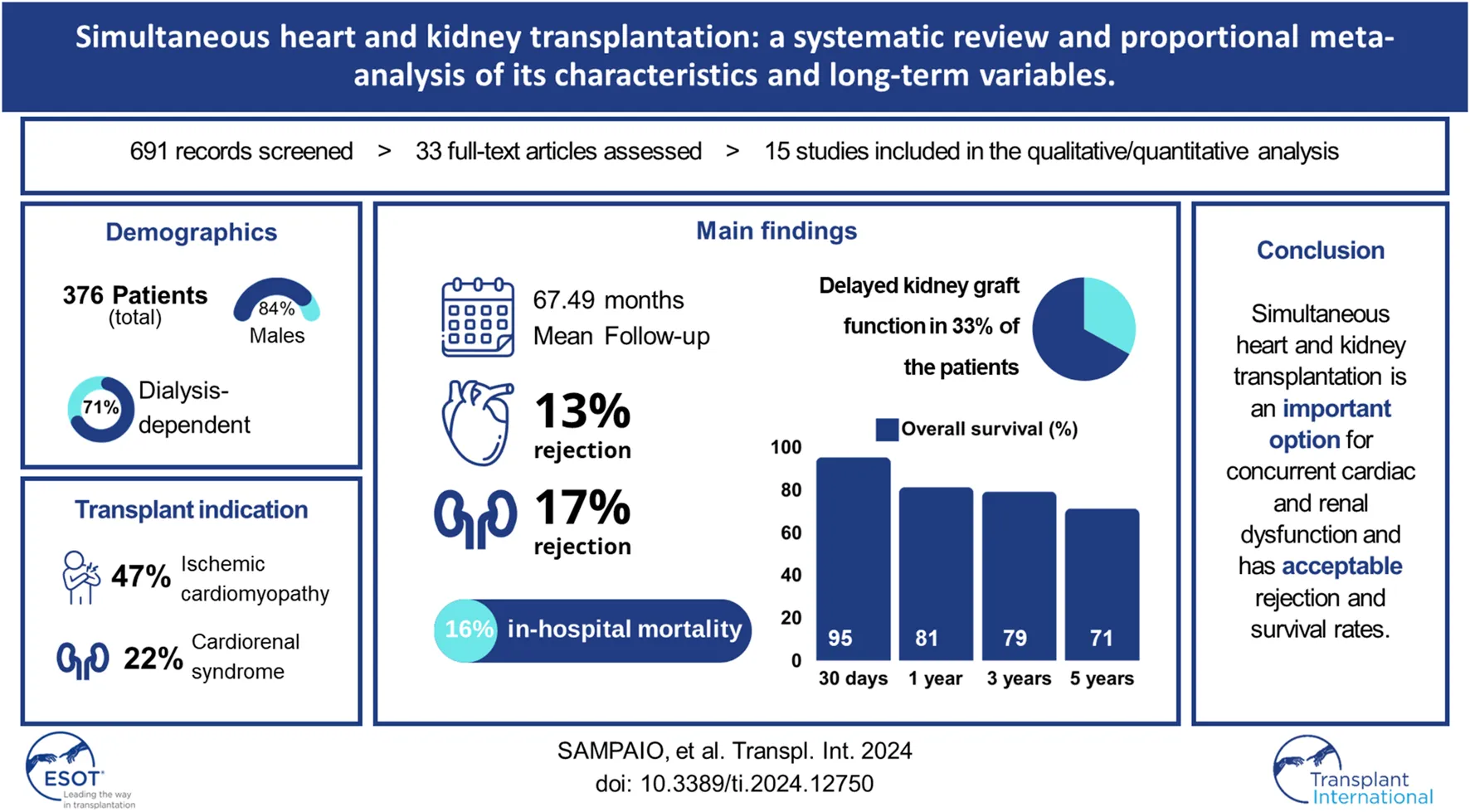
Abstract
Patients with end-stage heart disease who undergo a heart transplant frequently have simultaneous kidney insufficiency, therefore simultaneous heart and kidney transplantation is an option and it is necessary to understand its characteristics and long-term variables. The recipient characteristics and operative and long-term variables were assessed in a meta-analysis. A total of 781 studies were screened, and 33 were thoroughly reviewed. 15 retrospective cohort studies and 376 patients were included. The recipient’s mean age was 51.1 years (95% CI 48.52–53.67) and 84% (95% CI 80–87) were male. 71% (95% CI 59–83) of the recipients were dialysis dependent. The most common indication was ischemic cardiomyopathy [47% (95% CI 41–53)] and cardiorenal syndrome [22% (95% CI 9–35)]. Also, 33% (95% CI 20–46) of the patients presented with delayed graft function. During the mean follow-up period of 67.49 months (95% CI 45.64–89.33), simultaneous rejection episodes of both organ allografts were described in 5 cases only. Overall survival was 95% (95% CI 88–100) at 30 days, 81% (95% CI 76–86) at 1 year, 79% (95% CI 71–87) at 3, and 71% (95% CI 59–83) at 5 years. Simultaneous heart and kidney transplantation is an important option for concurrent cardiac and renal dysfunction and has acceptable rejection and survival rates.
Introduction
Patients with end-stage heart disease who undergo heart transplantation alone (HTx) frequently have simultaneous kidney insufficiency, leading to an outcome of reduced survival [1], as kidney failure is a predictor of morbidity and mortality in patients after HTx [2]. Since simultaneous heart and kidney transplantation (sHKTx) was first described in 1978 by Norman et al [3], it has become a recognized therapy for simultaneous end-stage cardiac and renal failure, with increased numbers since 2010 and representing more than 5% of the total number of HTx performed in the United States currently [4].
Indications for sHKTx are challenged by difficulties in differentiating those patients with cardiorenal syndrome without intrinsic renal disease, who could present renal recovery after HTx, from those with intrinsic advanced kidney disease who would benefit most from sHKTx [4]. Current evidence [1] supports that the simultaneous procedure is strongly recommended for heart transplantation candidates with pre-transplant renal dysfunction that leads to an estimated glomerular filtration rate (eGFR) under 30 mL/min/1.73 m [2]. Although the indications for sHKTx remain unclear, it is known that patients with simultaneous end-stage heart and renal disease who went through sHKTx have similar mortality when compared with HTx [5] and present a lower incidence of cardiac rejection [6].
The limitations of the existing sparse literature on the indications and outcomes of sHKTx highlight the critical need for studies dedicated to filling these gaps. Presently, the primary studies in this domain mainly center on retrospective analyses of the OPTN/UNOS database. However, this approach is limited as it excludes data from international centers performing this procedure. Therefore, this systematic review and meta-analysis was designed to synthesize the global evidence on the indications and outcomes of sHKTx, addressing this particular gap in the literature.
Methods
This systematic review and meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) 2020. The study protocol was registered in AsPredicted (119472).
Literature Search and Study Selection
We searched for relevant studies in PubMed, Embase, Lilacs, Scopus, Ovid Medline, and Web of Science updated to February 03, 2023. Two researchers (author 2 and author 3) searched works independently with a combination of the following terms: “heart kidney transplantation,” “simultaneous heart kidney transplantation” and “combined heart kidney transplantation,” and any discrepancies regarding the selection of studies were resolved by them. The reference lists of all eligible studies were reviewed for further identification of potentially relevant studies. The title and abstract of each identified publication were screened, and only publications that followed the selection criteria were fully read and included in the review.
Selection Criteria
The inclusion criteria were as follows: 1) published clinical studies in English, Portuguese, or Spanish that investigated indications and outcomes of sHKTx patients only; 2) studies including patients that underwent sHKTx as a result of simultaneous end-stage heart disease and concomitant kidney disease; 3) studies that had reported at least one of the outcomes of interest (mean donor age, mean recipient age, recipient body mass index, left ventricular ejection fraction, pre-operative serum creatinine, heart failure etiology, renal failure etiology). The exclusion criteria were: 1) studies with incomplete or unavailable data of interest; 2) studies not involving human subjects; 3) studies that included both the staged procedure and the simultaneous procedure in the same analysis group. If multiple studies were published from the same center, with overlapping patients’ data and follow-up periods, only the most complete reports with the longest follow-up period were included for assessment.
Data Extraction and Outcomes
The data extraction was performed with standardized processes conducted independently by two researchers (MDF, LVSV). Extracted data included study characteristics, such as title, type of study, first author, year of publication, center, study date, and the number of subjects that underwent sHKTx. The baseline demographics of the patients (donor age, recipient age, gender, cardiac and renal failure etiology, BMI, LVEF, pre-operative serum creatinine, inotrope usage, and dialysis dependency), as well as perioperative outcomes (overall allograft ischemic time, cardiac and kidney allograft ischemic times, delayed graft function, and in-hospital mortality) were also included. The assessment of warm allograft time was not feasible due to the lack of reporting of these parameters in the selected studies, although cold ischemic allograft time data was collected. The immunosuppression strategies adopted by each study were assessed and included solely in the qualitative synthesis. Five long-term outcomes (duration of follow-up period, serum creatinine at follow-up, overall, cardiac and renal rejection episodes), were assessed for quantitative synthesis. Cumulative 30-day, 1-, 3- and 5-year overall survival rates were also extracted for assessment for the study. The Newcastle-Ottawa Scale (NOS) - a tool to assess the quality of studies in meta-analyses, with a score from zero to nine, with zero being the worst outcome and nine classifying the paper as the best quality, developed by the Universities of Newcastle, Australia, and Ottawa, Canada [7] -was adopted to evaluate the quality of evidence in each included study by two researchers independently (author 1 and author 4) and they resolved any discrepancies in quality scoring.
Statistical Analysis
A meta-analysis of proportions was conducted for the available recipient demographics and perioperative and postoperative variables with logit transformation. The R software version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all data analysis and visualization. Heterogeneity was evaluated using the I2 test. As a guide, I2 < 25% indicated low, 25%–50% moderate, and >50% high heterogeneity [8]. If there was low or moderate statistical heterogeneity, a fixed-effect model was used. Otherwise, a random-effect model was adopted to evaluate variables with high heterogeneity. The meta-analysis was performed with the metafor package for R. Statistical significance was judged by p values under 0.05. Continuous data were estimated using mean with 95% confidence intervals (CI), and dichotomous data were reported using percentages with 95% CI. For some studies adopting median and range for parameters, estimated mean and standard deviation (SD) were obtained by adopting the formulas proposed by Hozo et al [9].
Results
Characteristics of the Selected Literature
A total of 781 articles were identified based on the literature search criteria and 15 eligible papers were included in qualitative synthesis and meta-analysis [10–24], including 376 patients who underwent the procedure from 1996 to 2019. All articles were single-center retrospective studies from 15 different transplantation centers in Austria, Belgium, the United States of America, the United Kingdom, Germany, Taiwan, Italy, New Zealand, Argentina, Spain, France, and Brazil. The literature search and study characteristics description are reported in Figure 1 and Table 1, respectively. The complete results table, including heterogeneity and the number of studies used to access each pooled variable, is reported in Table A in Supplementary Material.
FIGURE 1
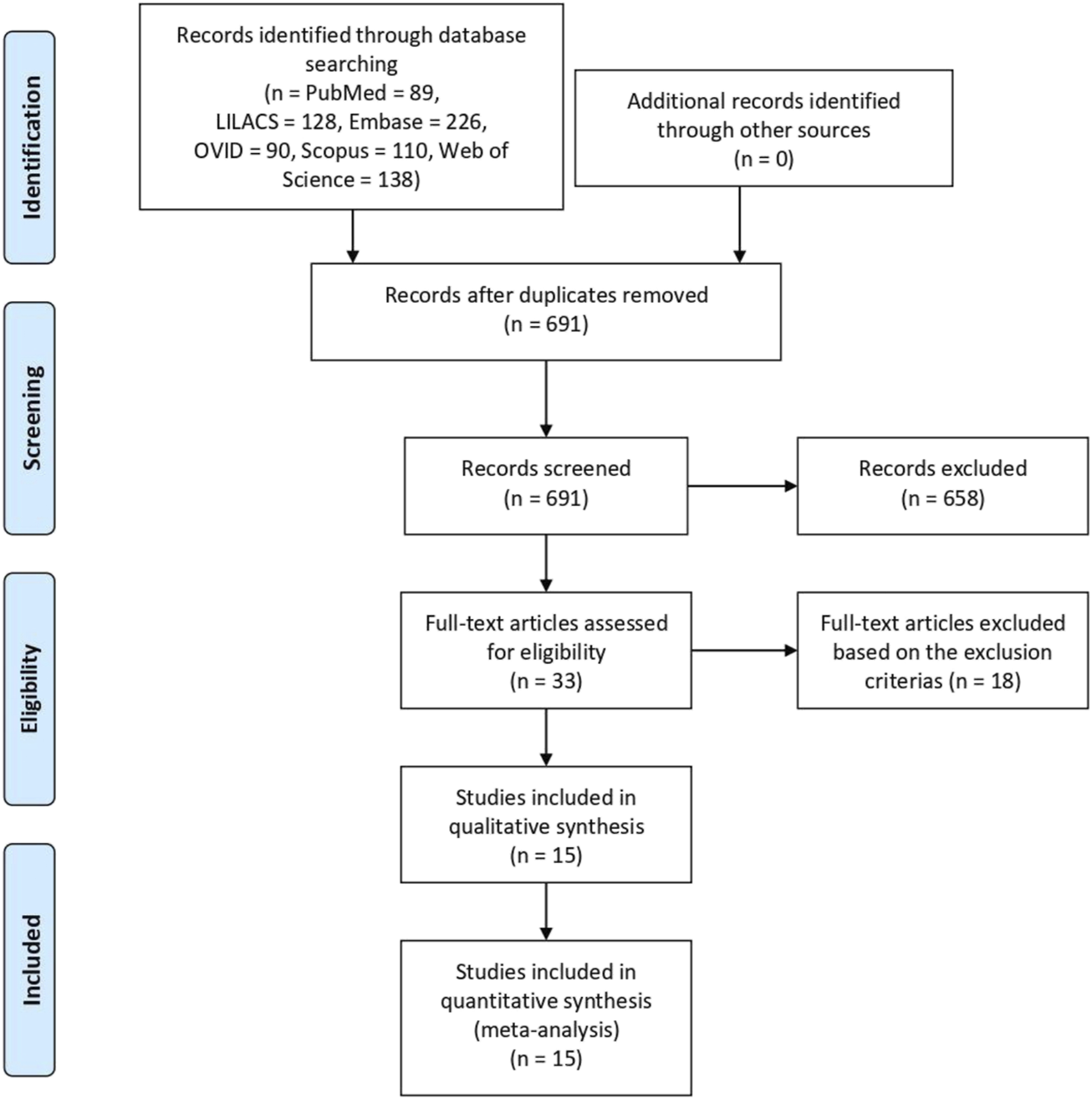
Flow diagram of literature search and study selection.
TABLE 1
| Title | Type of study | Author and year | Institution | Country | Study date | Number of patients | New-Castle Ottawa Score (0–9) |
|---|---|---|---|---|---|---|---|
| Combined heart and kidney transplantation using a single donor: a single left’s experience with nine cases | Retrospective cohort | Kocher et al. 1998 | University of Vienna | Austria | 1990–1997 | 9 | 6 |
| Combined heart-kidney transplantation: report on six cases | Retrospective cohort | Col et al. 1998 | University of Louvain Medical School | Belgium | 1986–1995 | 6 | 8 |
| Simultaneous Heart and Kidney Transplantation in Patients with End-stage Heart and Renal Failure | Retrospective cohort | Leeser et al. 2001 | Temple University Hospital | United States | 1990–1999 | 13 | 8 |
| Short- and long-term outcomes of combined cardiac and renal transplantation with allografts from a single donor | Retrospective cohort | Luckraz et al. 2002 | Papworth Hospital | United Kingdom | 1986–2002 | 13 | 8 |
| Freedom From Graft Vessel Disease in Heart and Combined Heart- and Kidney-transplanted Patients Treated With Tacrolimus-based Immunosuppression | Retrospective cohort | Groetzner et al. 2005 | Ludwig Maximilians University Hospital Grosshadern | Germany | 1995–2003 | 13 | 6 |
| Combined Heart–Kidney Transplantation: The University of Wisconsin Experience | Retrospective cohort | Hermsen et al. 2007 | University of Wisconsin School of Medicine and Public Health | United States | 1999–2006 | 19 | 7 |
| Effect of simultaneous kidney transplantation on heart-transplantation outcome in recipients with preoperative renal dysfunction | Retrospective cohort | Hsu et al. 2009 | National Taiwan University Hospital | Taiwan | 1993–2006 | 13 | 8 |
| Combined Heart and Kidney Transplantation: Long-Term Analysis of Renal Function and Major Adverse Events at 20 Years | Retrospective cohort | Bruschi et al. 2010 | Niguarda Ca’ Granda Hospital | Italy | 1989–2009 | 9 | 8 |
| Outcomes of simultaneous heart–kidney and lung–kidney transplantations: the Australian and New Zealand experience | Retrospective cohort | Ruderman et al. 2015 | 4 centres across Australia and New Zealand | Australia, New Zealand | 1990–2014 | 35 | 7 |
| Combined cardiorenal transplant in heart and advanced renal disease | Retrospective cohort | Lastras et al. 2015 | Hospital Universitario Fundación Favaloro | Argentina | 2006–2014 | 20 | 8 |
| Clinical Characteristics and Long-Term Outcomes of Patients Undergoing Combined Heart-Kidney Transplantation: A Single-Center Experience | Retrospective cohort | López-Sainz et al. 2015 | Complejo Hospitalario Universitario A Coruña | Spain | 1995–2013 | 22 | 8 |
| Combined Heart and Kidney Transplantation: Clinical Experience in 100 Consecutive Patients | Retrospective cohort | Awad et al. 2018 | Cedars-Sinai Medical Center | United States | 1992–2016 | 100 | 8 |
| Renal outcome after simultaneous heart and kidney transplantation | Retrospective cohort | Toinet et al. 2019 | 8 French academic lefts | France | 1998–2017 | 73 | 8 |
| Simultaneous heart-kidney transplantation results in respectable long-term outcome but a high rate of early kidney graft loss in high-risk recipients—a European single left analysis | Retrospective cohort | Beetz et al. 2021 | Hannover Medical School | Germany | 1987–2019 | 27 | 8 |
| Combined Heart and Kidney Transplantation: Initial Clinical Experience | Retrospective cohort | Atik et al. 2022 | Instituto de Cardiologia do Distrito Federal | Brazil | 2007–2019 | 4 | 8 |
Study characteristics and quality assessment.
Baseline Characteristics
This proportional meta-analysis included 376 patients, of whom 84% (95% CI 80–87, p = 0.06) were men. The mean donor age was 32.97 years (95% CI 28.21–37.73, p < 0.01) whereas the mean recipient age was 51.10 years (95% CI 48.52–53.67, p < 0.01). Regarding recipient demographics, the mean BMI was 24.42 kg/m2 (95% CI 23.42–25.41, p = 0.04), mean LVEF was 23.32% (95% CI 16.62–30.02, p < 0.01), and pre-operative serum creatinine was 4.53 mg/dL (95% CI 3.04–6.02, p < 0.01). Overall, 71% (95% CI 59–83, p < 0.01) of the patients were dialysis-dependent and 33% (95% CI 17–50, p = 0.02) were inotrope-dependent before transplantation.
The predominant heart failure etiology was ischemic cardiomyopathy in 47% (95% CI 41–53, p = 0.04) of the patients, followed by dilated cardiomyopathy in 43% of the patients, (95% CI 29–57, p < 0.01), and idiopathic cardiomyopathy in 28% of the patients (95% CI 20–35, p = 0.59). The sum of these percentages results in a value above 100% because of the variability in the assessment of each etiologies in the number of studies included, which can be verified in Table A in Supplementary Material.
Although the diagnosis methods were not registered, renal failure etiology was mostly due to cardiorenal syndrome (22% of the patients, 95% CI 9–35, p < 0.01), followed by glomerulonephritis (16% of the patients, 95% CI 2–30, p < 0.01), nephritis (14% of the patients, 95% CI 3–26, p = 0.01), drug-related toxicity (14% of the patients, 95% CI 9–19, p = 0.17), polycystic kidney disease (7% of the patients, 95% CI 4–11, p = 0.56), and diabetes-related (7% of the patients, 95% CI 4–11, p = 0.13). The sum of these percentages results in a value above 100% for the same reason mentioned before.
Operative Variables
An immunosuppressive regimen based on induction and maintenance therapy was registered in ⅔ of the studies, and the remaining ⅓ only properly reported the use of maintenance therapy. Although it was not possible to associate the specific use of different immunosuppressive regimens with sHKTx outcome, the studies reported, as induction therapy, the use of one or more of the following: thymoglobulin, anti-thymocyte globulin, muromonab-CD3, methylprednisolone, prednisone, and basiliximab. As maintenance therapy, the studies reported the single or combined use of tacrolimus, azathioprine, mycophenolate mofetil, cyclosporine, everolimus, sirolimus, methylprednisolone, belatacept, and prednisone.
The overall cardiac allograft ischemic time was 180.46 min (95% CI 170.48–190.44, p = 0.01), and the overall kidney allograft ischemic time was 11.68 h (95% CI 7.87–15.48, p < 0.01). The studies did not define clearly how ischemic time was assessed and did not specify the difference between cold and warm ischemic time.
After transplantation, the mean ICU length of stay was 14.19 days (95% CI 1.87–26.51, p < 0.01). The rates of infection and sepsis were, respectively, 31% (95% CI 14–48, p < 0.01) and 12% (95% CI 7–17, p = 0.48). Delayed graft function for kidney transplantation (KTx) was presented by 33% of the patients (95% CI 20–46, p = 0.01), and in the hospital, mortality was 16% (95% CI 11–21, p = 0.17). Although not all the studies clarify the definition of how they assessed the early kidney graft function, the delayed graft function definition included requiring more than one hemodialysis. Subsequently, kidney function could be assessed by analyzing serum creatinine, eGFR, and creatinine clearance, and exclusion of any kind of rejection by biopsy. The heart graft function was reported as evaluated with the use of echography.
Long-Term Variables
After a mean follow-up period of 67.49 months (95% CI 45.64–89.33, p < 0.01), serum creatinine was 1.50 mg/dL (95% CI 1.37–1.62, p = 0.16). Overall a cardiac allograft rejection episode was reported in 17% (95% CI 12–23, p = 0.21) of the patients (Figure 2), and a renal allograft rejection episode was also reported in 13% (95% CI 7–18, p = 0.38) of the patients (Figure 3). A simultaneous rejection episode of both organs’ allografts was described in 1 case by Col et al [11] and in 4 cases by Groetzner et al [14]. Allograft rejection episodes were defined after performing a renal or endomyocardial biopsy in the majority of the included studies. Overall patient survival rates of 30 days, 1-, 3-, and 5-years were, respectively: 95% (95% CI 88–100, p = 0.78), 81% (95% CI 76–86, p = 0.45), 79% (95% CI 71–87, p = 0.12), and 71% (95% CI 59–83, p < 0.01). The survival analysis is presented in Figure 4. We could not assess the specific allograft survival because of the lack of report of this information in the included studies. The patient and cardiac survival were equivalent, but the data did not describe kidney transplant survival.
FIGURE 2
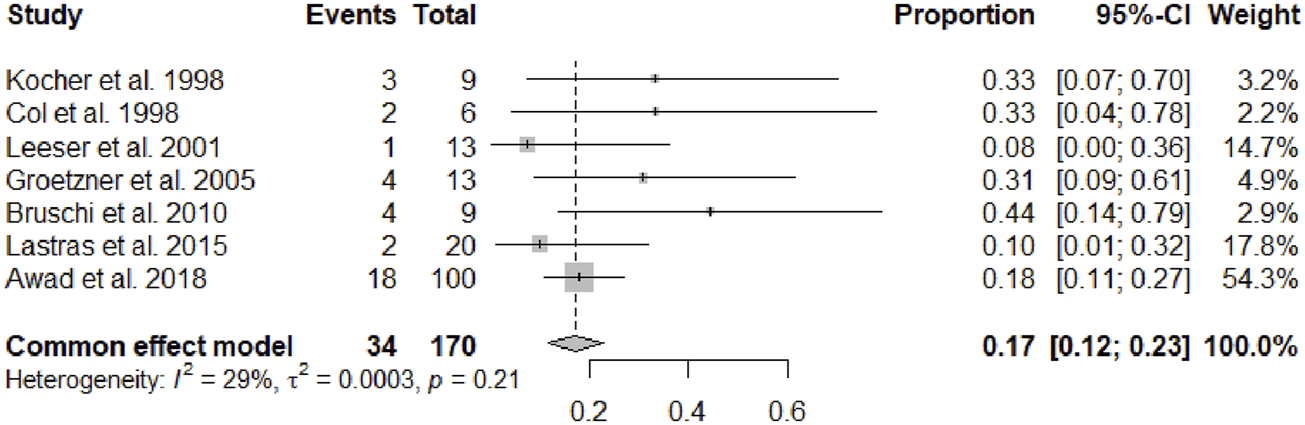
Forest plot representing the pooled occurrence of cardiac allograft rejection episode.
FIGURE 3
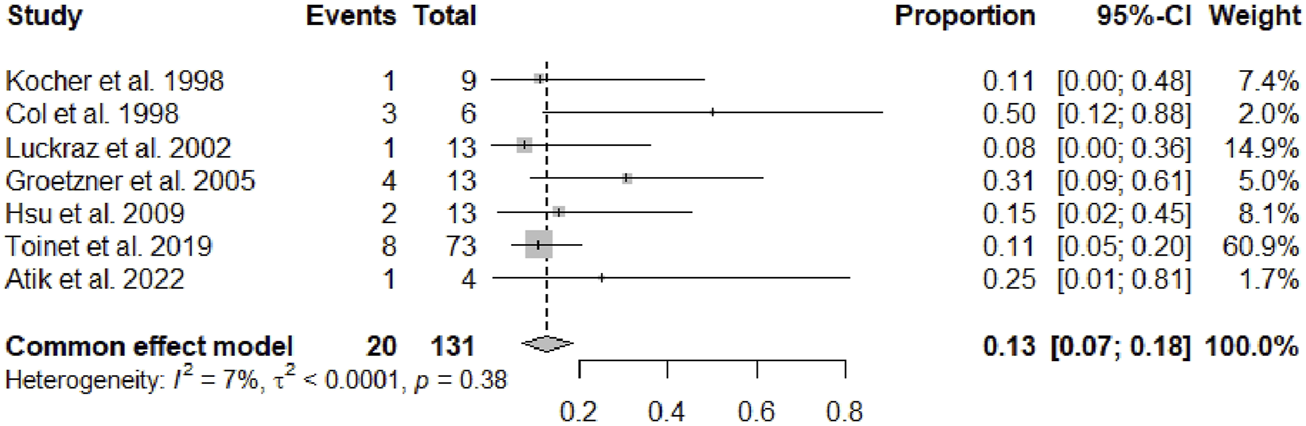
Forest plot representing the pooled occurrence of kidney allograft rejection episode.
FIGURE 4
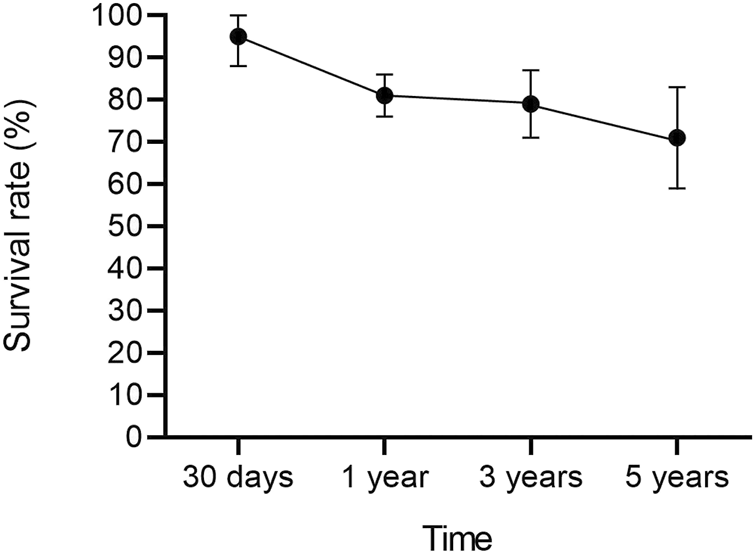
Overall survival analysis after sHKTx with 95% CI.
Discussion
Multi-organ transplantation is a lifesaving surgical procedure for patients with multiple organ failure and is a therapeutic option for select patients who may otherwise not survive [25, 26]. Based on the obtained results and discussion, despite its major technical and logistical challenges, we have formulated the hypothesis that this procedure delivers acceptable mortality and rejection rates. The significance of these findings lies in the fact that patients suffering from end-stage heart disease, who undergo HTx, can experience concurrent kidney insufficiency. As a consequence, their outcomes are generally unfavorable if they only receive an HTx, thereby emphasizing the need to consider KTx for these individuals, which highlights the need for studies focusing on understanding sHKTx indications and outcomes.
Although sHKTx has very scarce data in the literature, United Network for Organ Sharing (UNOS) data has shown that the number of patients on the waiting list for this procedure has progressively increased over the years [27, 28]. Our study met the recommendation of donor age to be younger than 45 years proposed by The International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients in 2022 [29], as the mean donor age was found to be 32.97 years. However, two included studies [12, 23] described no association between donor age and better outcomes after HKTx (p < 0.01). In the consensus conference on sHKTx that took place on June 1, 2019, in Boston, Massachusetts, the discussion of adopting possible age cutoffs on sHKTx donors and recipients was not supported due to ethical principles [4].
Regarding the etiology of organ failure, a previous study based on the UNOS platform [30] reported ischemic cardiomyopathy (35%) and diabetes mellitus (15%) as the leading etiologies of heart and kidney failure, respectively. The results of this previous analysis are comparable with ours concerning heart failure etiology, as the main etiology for this disease was found to be ischemic cardiomyopathy in 47% of the patients included in our analysis. Although diabetes was found to be the main cause of renal failure in this previous study [30], diabetes-related kidney failure accounts for only 7% of kidney failure etiologies in our cohort, and the main etiology for this organ failure was the cardiorenal syndrome. In this syndrome, severe heart failure leads to decreased kidney perfusion and venous congestion, which consequently leads to reduced eGFR and a rise in serum creatinine [4], resulting in a cascade of feedback mechanisms that causes damage to both the organs and is associated with adverse clinical outcomes [31]. Our result is interesting because, although considered reversible, the cardiorenal syndrome was found to be the leading etiology of kidney failure in our cohort. However, regardless of the etiology of organ failure, current evidence supports the need to focus on measuring the kidney and heart function before sHKTx [15, 19, 21, 22, 32].
Pre-transplant dialysis dependence was significantly different between our analysis, which found a high prevalence of pre-transplant dialysis dependence of 71% of the patients, and the UNOS analysis [28] which registered a percentage of 40.2%. In this context, even though the dependence and the time on dialysis could not be fully assessed in the studies due to a lack of data, the pre-transplant dialysis dependence duration may be the best clinical index when determining who should be on the sHKTx transplant list [33, 34]. Patients whose hemodialysis started earlier at a higher eGFR (eGFR >10.5 mL/min per 1.73 m^) are associated with more comorbidities (hypertension and diabetes), malnutrition (serum albumin lower than 3.5 g/dL), and risk of death [31]. According to the Notice of the Organ Procurement and Transplantation Network (OPTN) Policy Change for Heart-Kidney transplant allocation of 2023 [29], candidates for transplant with CKD, eGFR less than or equal to 60 mL/min for more than 90 consecutive days, and regularly administered dialysis are acceptable for sHKTx.
In our perioperative analysis, attention was given to the allograft ischemic time in sHKTx (p = 0.01), as shorter renal cold ischemic times have been previously proposed as a reason for high long-term graft survival after this procedure [35, 36]. The kidney can be safely transplanted as soon as the heart function is restored, and as quickly as possible [11]. In our analysis, the kidney and heart cold ischemic time was documented in seven studies (p < 0.01), with the mean value of heart cold ischemic time of 183.2 min [10, 11, 15–17, 19, 22], and the mean value of kidney cold ischemic time of 11.68 h [10, 11, 15, 17, 19, 22, 23] (p < 0.001). It is known that the heart and kidney have different acceptable cold ischemic times without damage, but the increased time can lead to acute rejection, graft loss, and delayed graft function [37–41]. To avoid this complication, the included studies adopted different strategies to reach the lowest overall ischemic time possible, for example, donors were transferred to the same hospital as recipients to reduce heart ischemic time [16, 24]. However, these single-center studies did not assess the significance of the association between cold or warm ischemic time and sHKTx outcomes.
To prevent injury and improve graft function and surgical long-term outcomes, Machine Perfusion (MP) may be used when longer allograft kidney cold ischemic time is anticipated [42]. In our study, we could not determine the impact of MP on patients who had undergone sHKTx. It is important to recognize the many variables that are related to MP, such as the machine type and perfusion time on the machine, which can affect the transplantation outcome [42–49].
In the postoperative analysis, delayed graft function was significant in 8 studies (n = 167 patients), with approximately 33% occurrence. Although sHKTx has an outcome of acceptable delayed graft function [1], previous literature shows no significant difference in patient survival between HTx and sKHTx associated with delayed graft function [15, 19, 22, 23].
Our rejection analysis is in agreement with previous literature that reported in the early days a reduced rejection rate associated with combined transplantation [50], possibly due to an immunoprotective effect of kidney transplantation on the heart allograft [51]. This finding can be explained by the different immunosuppressive strategies that were adopted [14] and their efficacy. For that, although we were not able to analyze the relationship between the immunosuppressive strategies with sHKTx outcomes in our study, we recommend further investigation of this subject [4].
The sHKTx presented a survival rate of 81% at 1 year and 71% at 5 years in our analysis. These results are only slightly lower than the 1-year and 5-year survival rates of HTx alone, which are 84.5% and 72.5%, according to recent data from the registry of the International Society of Heart and Lung Transplantation [52]. Our 5-year survival analysis of this group of studies shows a significant variation (I2 = 69%), which the increased technical complexity of sHKTx could explain, the lack of standardized multiorgan transplantation eligibility criteria across institutions, and the lack of nationwide policies to regulate this procedure [4], that potentially leads to decreased survival rates in the current transplantation era [53].
The limitations of this study include the impossible calculation of pooled pre-transplant eGFR due to the lack of reporting of this parameter in the included studies of our cohort, which mostly reported pre-operative serum creatinine as a parameter of kidney function. For these patients who are not on dialysis, it represents a limitation because eGFR is the best overall index of kidney function [54], and the adoption of serum creatinine to estimate eGFR is not precise [55], potentially leading to the overdiagnosis of chronic kidney disease. We highlight the need for the report of eGFR in a retrospective cohort study about sHKTx, so future studies could understand how this parameter affects sHKTx outcomes.
This study has other limitations since not all the centers included in this analysis used the same patient and donor selection criteria or adopted the same sHKTx techniques and immunosuppressive regimens, so our results must be interpreted carefully. Despite a lack of granularity due to inconsistent data reports in the literature, this study stands out for its comprehensive synthesis of existing research and potential guidance to future studies and valuable insights after identifying gaps in the literature.
Also, our meta-analysis has challenges with the studies’ heterogeneity and the lack of clarity in variables such as the types of organs used. Regardless of these limitations, this is an important analysis to be conducted in the current literature to discuss HKTx indications and outcomes, which may help to guide future clinical practice. This study includes a 33-year period of literature analysis, with significant temporal and regional policy differences, consisting in limitations and unique strengths for this study, since there are clinical experiences from the 1980s up to the present time and so reflect many of the advances in both the surgical and medical management of these high-risk patients.
Conclusion
sHKTx appears as an effective option for simultaneous end-stage cardiac and renal failure treatment, as it presents acceptable rejection and survival rates. However, further investigation is warranted to ascertain the specific patient population that would benefit the most from this procedure. We encourage future meticulous studies on this theme, with extended data reported. Additionally, global policies should be established to fortify the implementation of sHKTx and improve its outcomes. Nonetheless, it will be important to determine the physiological characteristics that may lead to renal recovery after Acute Kidney Injury (AKI) related to the cardiorenal syndrome.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NS, MF, LV, CT, GL, and SV participated in the design, interpretation of the studies, and analysis of the data and wrote the first manuscript; GB and AM reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.12750/full#supplementary-material
References
1.
Ahsan SA Guha A Gonzalez J Bhimaraj A . Combined Heart-Kidney Transplantation: Indications, Outcomes, and Controversies. Methodist Debakey Cardiovasc J (2022) 18(4):11–8. Published 2022 Sep 6. 10.14797/mdcvj.1139
2.
Karamlou T Welke KF McMullan DM Cohen GA Gelow J Tibayan FA et al Combined Heart-Kidney Transplant Improves Post-Transplant Survival Compared With Isolated Heart Transplant in Recipients With Reduced Glomerular Filtration Rate: Analysis of 593 Combined Heart-Kidney Transplants From the United Network Organ Sharing Database. J Thorac Cardiovasc Surg (2014) 147(1):456–61. 10.1016/j.jtcvs.2013.09.017
3.
Norman JC Brook MI Cooley DA Klima T Kahan BD Frazier OH et al Total Support of the Circulation of a Patient With Post-Cardiotomy Stone-Heart Syndrome by a Partial Artificial Heart (ALVAD) for 5 Days Followed by Heart and Kidney Transplantation. Lancet (1978) 1(8074):1125–7. 10.1016/s0140-6736(78)90301-x
4.
Kobashigawa J Dadhania DM Farr M Tang WHW Bhimaraj A Czer L et al Consensus Conference on Heart-Kidney Transplantation. Am J Transpl (2021) 21(7):2459–67. 10.1111/ajt.16512
5.
Agarwal KA Patel H Agrawal N Cardarelli F Goyal N . Cardiac Outcomes in Isolated Heart and Simultaneous Kidney and Heart Transplants in the United States. Kidney Int Rep (2021) 6(9):2348–57. Published 2021 Jul 14. 10.1016/j.ekir.2021.06.032
6.
Narula J Bennett LE DiSalvo T Hosenpud JD Semigran MJ Dec GW . Outcomes in Recipients of Combined Heart-Kidney Transplantation: Multiorgan, Same-Donor Transplant Study of the International Society of Heart and Lung Transplantation/United Network for Organ Sharing Scientific Registry. Transplantation (1997) 63(6):861–7. 10.1097/00007890-199703270-00012
7.
Cota GF de Sousa MR Fereguetti TO Rabello A . Efficacy of Anti-Leishmania Therapy in Visceral Leishmaniasis Among HIV Infected Patients: A Systematic Review With Indirect Comparison. Plos Negl Trop Dis (2013) 7(5):e2195. Published 2013 May 2. 10.1371/journal.pntd.0002195
8.
Higgins JP Thompson SG . Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. 10.1002/sim.1186
9.
Hozo SP Djulbegovic B Hozo I . Estimating the Mean and Variance From the Median, Range, and the Size of a Sample. BMC Med Res Methodol (2005) 5(1):13. 10.1186/1471-2288-5-13
10.
Kocher AA Schlechta B Kopp CW Ehrlich M Ankersmit J Ofner P et al Combined Heart and Kidney Transplantation Using a Single Donor: A Single Center's Experience With Nine Cases. Transplantation (1998) 66(12):1760–3. 10.1097/00007890-199812270-00033
11.
Col VJ Jacquet L Squifflet JP Goenen M Noirhomme P Goffin E et al Combined Heart-Kidney Transplantation: Report on Six Cases. Nephrol Dial Transpl (1998) 13(3):723–7. 10.1093/ndt/13.3.723
12.
Leeser DB Jeevanandam V Furukawa S Eisen H Mather P Silva P et al Simultaneous Heart and Kidney Transplantation in Patients With End-Stage Heart and Renal Failure. Am J Transpl (2001) 1(1):89–92. 10.1034/j.1600-6143.2001.010116.x
13.
Luckraz H Parameshwar J Charman SC Firth J Wallwork J Large S . Short- and Long-Term Outcomes of Combined Cardiac and Renal Transplantation With Allografts From a Single Donor. J Heart Lung Transpl (2003) 22(12):1318–22. 10.1016/s1053-2498(03)00030-5
14.
Groetzner J Kaczmarek I Mueller M Huber S Deutsch A Daebritz S et al Freedom From Graft Vessel Disease in Heart and Combined Heart- and Kidney-Transplanted Patients Treated With Tacrolimus-Based Immunosuppression. J Heart Lung Transpl (2005) 24(11):1787–92. 10.1016/j.healun.2005.03.012
15.
Hermsen JL Nath DS del Rio AM Eickstaedt JB Wigfield C Lindsey JD et al Combined Heart-Kidney Transplantation: The University of Wisconsin Experience. J Heart Lung Transpl (2007) 26(11):1119–26. 10.1016/j.healun.2007.08.011
16.
Hsu RB Chang CI Tsai MK Lee PH Chou NK Chi NH et al Effect of Simultaneous Kidney Transplantation on Heart-Transplantation Outcome in Recipients With Preoperative Renal Dysfunction. Eur J Cardiothorac Surg (2010) 37(1):68–73. 10.1016/j.ejcts.2009.06.006
17.
Bruschi G Botta L Colombo T Busnach G Pedrazzini G Cannata A et al Combined Heart and Kidney Transplantation: Long-Term Analysis of Renal Function and Major Adverse Events at 20 Years. Transpl Proc (2010) 42(4):1283–5. 10.1016/j.transproceed.2010.03.064
18.
Ruderman I Sevastos J Anthony C Ruygrok P Chan W Javorsky G et al Outcomes of Simultaneous Heart-Kidney and Lung-Kidney Transplantations: The Australian and New Zealand Experience. Intern Med J (2015) 45(12):1236–41. 10.1111/imj.12865
19.
Lastras MP Favaloro LE Fortunato RM Gutiérrez L Rabin GE Absi DO et al Combined Cardiorenal Transplant in Heart and Advanced Renal Disease. Revista de Nefrología, Diálisis y Trasplante (2017) 35(4):188–95. Available from: https://doaj.org/article/baf45d2883bf4bb3850198376d82ce29 (Accessed December 5, 2023).
20.
López-Sainz Á Barge-Caballero E Paniagua-Martin MJ Marzoa Rivas R Sánchez-Fernández G Fernández Arias L et al Clinical Characteristics and Long-Term Outcomes of Patients Undergoing Combined Heart-Kidney Transplantation: A Single-Center Experience. Transpl Proc (2015) 47(1):123–6. 10.1016/j.transproceed.2014.11.009
21.
Awad MA Czer LSC Emerson D Jordan S De Robertis MA Mirocha J et al Combined Heart and Kidney Transplantation: Clinical Experience in 100 Consecutive Patients. J Am Heart Assoc (2019) 8(4):e010570. 10.1161/jaha.118.010570
22.
Toinet T Dominique I Cholley I Vanalderwerelt V Goujon A Paret F et al Renal Outcome After Simultaneous Heart and Kidney Transplantation. Clin Transplant (2019) 33(7):e13615. 10.1111/ctr.13615
23.
Beetz O Thies J Weigle CA Ius F Winkler M Bara C et al Simultaneous Heart-Kidney Transplantation Results in Respectable Long-Term Outcome but a High Rate of Early Kidney Graft Loss in High-Risk Recipients - a European Single Center Analysis. BMC Nephrol (2021) 22(1):258. Published 2021 Jul 9. 10.1186/s12882-021-02430-x
24.
Atik FA Borges CC Ulhoa MB Chaves RB Barzilai VS Biondi RS et al Combined Heart and Kidney Transplantation: Initial Clinical Experience. Braz J Cardiovasc Surg (2022) 37(2):263–7. Published 2022 May 2. 10.21470/1678-9741-2020-0720
25.
Westphal SG Langewisch ED Miles CD . Current State of Multiorgan Transplantation and Implications for Future Practice and Policy. Adv Chronic Kidney Dis (2021) 28(6):561–9. 10.1053/j.ackd.2021.09.012
26.
Orlando G Remuzzi G Williams DF. Kidney Transplantation, Bioengineering, and Regeneration. Academic Press (2017).
27.
Schaffer JM Chiu P Singh SK Oyer PE Reitz BA Mallidi HR . Heart and Combined Heart-Kidney Transplantation in Patients With Concomitant Renal Insufficiency and End-Stage Heart Failure. Am J Transpl (2014) 14(2):384–96. 10.1111/ajt.12522
28.
Parajuli S Karim AS Muth BL Leverson GE Yang Q Dhingra R et al Risk Factors and Outcomes for Delayed Kidney Graft Function in Simultaneous Heart and Kidney Transplant Recipients: A UNOS/OPTN Database Analysis. Am J Transpl (2021) 21(9):3005–13. 10.1111/ajt.16535
29.
Organ Procurement and Transplantation Network. Establish Eligibility Criteria and Safety Net for Heart-Kidney and Lung-Kidney Allocation. Published June 29, 2023 (2023). Available from: https://unos.org/news/policy-changes/safety-net-mot-in-effect-june-29/ (Accessed August 15, 2023).
30.
Velleca A Shullo MA Dhital K Azeka E Colvin M DePasquale E et al The International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients. J Heart Lung Transpl (2023) 42(5):e1–e141. 10.1016/j.healun.2022.10.015
31.
Escoli R Luz I Santos P Vila Lobos A . Glomerular Filtration Rate and Initiation of Dialysis. Ther Apher Dial (2017) 21(6):606–10. 10.1111/1744-9987.12582
32.
Gallo M Trivedi JR Schumer EM Slaughter MS . Combined Heart-Kidney Transplant Versus Sequential Kidney Transplant in Heart Transplant Recipients. J Card Fail (2020) 26(7):574–9. 10.1016/j.cardfail.2020.03.002
33.
FHN Trial Group Chertow GM Levin NW Beck GJ Depner TA Eggers PW et al In-Center Hemodialysis Six Times Per Week Versus Three Times Per Week. N Engl J Med (2010) 363(24):2287–300. 10.1056/NEJMoa1001593
34.
Yamazaki T Shirai H Yashima J Tojimbara T . Successful Cadaveric Kidney Transplantation in an Extended-Hours Hemodialysis Patient With Long-Term Hemodialysis Vintage for 297 Months. Urol Case Rep (2020) 30:101139. Published 2020 Feb 21. 10.1016/j.eucr.2020.101139
35.
Kumar U Wettersten N Garimella PS . Cardiorenal Syndrome: Pathophysiology. Cardiol Clin (2019) 37(3):251–65. 10.1016/j.ccl.2019.04.001
36.
Chadban SJ Ahn C Axelrod DA Foster BJ Kasiske BL Kher V et al KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation (2020) 104(4S1 Suppl. 1):S11–S103. 10.1097/TP.0000000000003136
37.
Castillo-Lugo JA Brinker KR . An Overview of Combined Heart and Kidney Transplantation. Curr Opin Cardiol (1999) 14(2):121–5. 10.1097/00001573-199903000-00008
38.
Liu L Cheng K Huang J . Effect of Long Cold Ischemia Time of Kidneys From Aged Donors on Prognosis of Kidney Transplantation. Ann Transpl (2021) 26:e928735. Published 2021 Oct 19. 10.12659/AOT.928735
39.
Helanterä I Ibrahim HN Lempinen M Finne P . Donor Age, Cold Ischemia Time, and Delayed Graft Function. Clin J Am Soc Nephrol (2020) 15(6):813–21. 10.2215/CJN.13711119
40.
Wu J Liu X Wang M Wang X Luo D Su S . Reduction of Cold Ischemic Injury With the Addition of Compound Glycyrrhizin in HTK Solution in a Mouse Heart Transplantation Model. Int Heart J (2020) 61(3):595–600. 10.1536/ihj.19-321
41.
Jing L Yao L Zhao M Peng L Liu M . Organ Preservation: From the Past to the Future. Acta Pharmacologica Sinica (2018) 39(5):845–57. 10.1038/aps.2017.182
42.
Ciancio G Gaynor JJ Sageshima J Roth D Kupin W Guerra G et al Machine Perfusion Following Static Cold Storage Preservation in Kidney Transplantation: Donor-Matched Pair Analysis of the Prognostic Impact of Longer Pump Time. Transpl Int (2012) 25(1):34–40. 10.1111/j.1432-2277.2011.01364.x
43.
Tatum R O'Malley TJ Bodzin AS Tchantchaleishvili V . Machine Perfusion of Donor Organs for Transplantation. Artif Organs (2021) 45(7):682–95. 10.1111/aor.13894
44.
Hameed AM Pleass HC Wong G Hawthorne WJ . Maximizing Kidneys for Transplantation Using Machine Perfusion: From the Past to the Future: A Comprehensive Systematic Review and Meta-Analysis. Medicine (Baltimore) (2016) 95(40):e5083. 10.1097/MD.0000000000005083
45.
Hosgood SA Callaghan CJ Wilson CH Smith L Mullings J Mehew J et al Normothermic Machine Perfusion Versus Static Cold Storage in Donation After Circulatory Death Kidney Transplantation: A Randomized Controlled Trial. Nat Med (2023) 29(6):1511–9. 10.1038/s41591-023-02376-7
46.
Hosgood SA Brown RJ Nicholson ML . Advances in Kidney Preservation Techniques and Their Application in Clinical Practice. Transplantation (2021) 105(11):e202–e214. 10.1097/TP.0000000000003679
47.
Paloyo S Sageshima J Gaynor JJ Chen L Ciancio G Burke GW . Negative Impact of Prolonged Cold Storage Time Before Machine Perfusion Preservation in Donation After Circulatory Death Kidney Transplantation. Transpl Int (2016) 29(10):1117–25. 10.1111/tri.12818
48.
Ciancio G Gaynor JJ Sageshima J Chen L Roth D Kupin W et al Favorable Outcomes With Machine Perfusion and Longer Pump Times in Kidney Transplantation: A Single-Center, Observational Study. Transplantation (2010) 90(8):882–90. 10.1097/TP.0b013e3181f2c962
49.
Roth D Zucker K Cirocco R Burke G Olson L Esquenazi V et al Transmission of Hepatitis C Virus by Kidney Transplantation: Impact of Perfusion Techniques and Course of Viremia Post Transplant. Pediatr Nephrol (1995) 9(Suppl. l):S29–S34. 10.1007/BF00867680
50.
Blanche C Kamlot A Blanche DA Kearney B Wong AV Czer LS et al Combined Heart-Kidney Transplantation With Single-Donor Allografts. J Thorac Cardiovasc Surg (2001) 122(3):495–500. 10.1067/mtc.2001.115700
51.
Chou AS Habertheuer A Chin AL Sultan I Vallabhajosyula P . Heart-Kidney and Heart-Liver Transplantation Provide Immunoprotection to the Cardiac Allograft. Ann Thorac Surg (2019) 108(2):458–66. 10.1016/j.athoracsur.2019.02.012
52.
Lund LH Edwards LB Kucheryavaya AY Benden C Christie JD Dipchand AI et al The Registry of the International Society for Heart and Lung Transplantation: Thirty-First Official Adult Heart Transplant Report--2014; Focus Theme: Retransplantation. J Heart Lung Transpl (2014) 33(10):996–1008. 10.1016/j.healun.2014.08.003
53.
Javier MFDM Javier Delmo EM Hetzer R . Evolution of Heart Transplantation Since Barnard's First. Cardiovasc Diagn Ther (2021) 11(1):171–82. 10.21037/cdt-20-289
54.
Delgado C Baweja M Crews DC Eneanya ND Gadegbeku CA Inker LA et al A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol (2021) 32(12):2994–3015. 10.1681/ASN.2021070988
55.
Inker LA Schmid CH Tighiouart H Eckfeldt JH Feldman HI Greene T et al Estimating Glomerular Filtration Rate From Serum Creatinine and Cystatin C. N Engl J Med (2012) 367(1):20–9. 10.1056/NEJMoa1114248
Summary
Keywords
transplant, multiorgan transplant, simultaneous heart and kidney transplantation, heart transplantation, kidney transplantation
Citation
Sampaio NZ, Faleiro MD, Vieira LVdS, Lech GE, Viana SW, Tavares CPO, Mattiazzi AD. and Burke GW. (2024) Simultaneous Heart and Kidney Transplantation: A Systematic Review and Proportional Meta-Analysis of Its Characteristics and Long-Term Variables. Transpl Int 37:12750. doi: 10.3389/ti.2024.12750
Received
26 January 2024
Accepted
13 May 2024
Published
31 May 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Sampaio, Faleiro, Vieira, Lech, Viana, Tavares, Mattiazzi and Burke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natália Zaneti Sampaio, nzsampaio@uniara.edu.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.