- 1Department of Public Health, Institute of Nursing Science, University of Basel, Basel, Switzerland
- 2Department of Public Health and Primary Care, Academic Centre for Nursing and Midwifery, KU Leuven, Leuven, Belgium
- 3School of Nursing and Health Studies, University of Missouri-Kansas City, Kansas, MO, United States
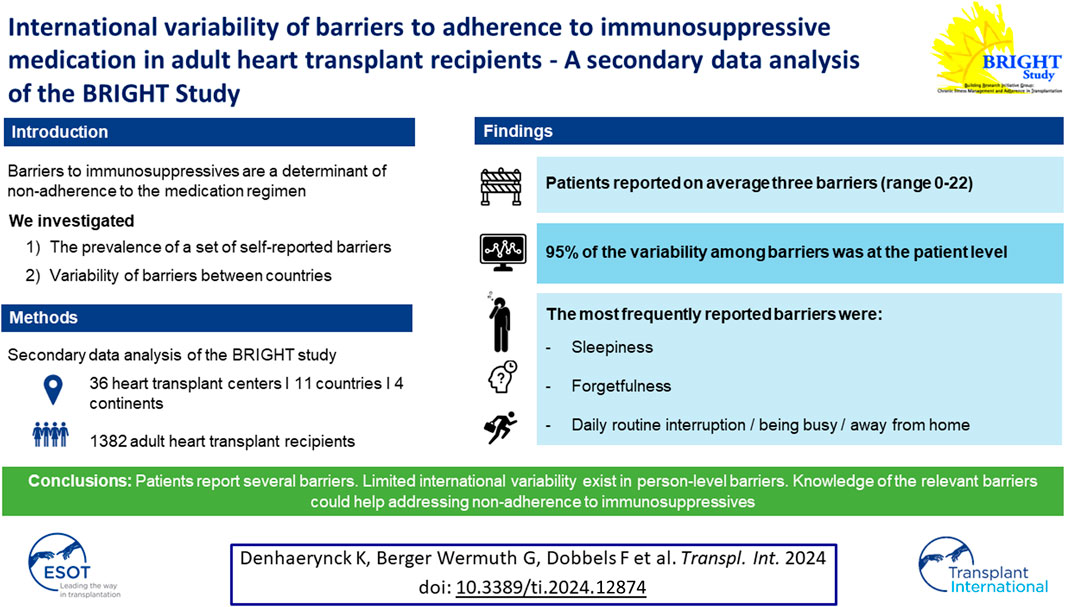
Non-adherence to immunosuppressive medication among transplant patients is associated with poor clinical outcomes and higher economic costs. Barriers to immunosuppressives are a proximal determinant of non-adherence. So far, international variability of barriers to adherence in transplantation has not been studied. As part of the cross-sectional multi-country and multi-center BRIGHT study, barriers to adherence were measured in 1,382 adult heart transplant recipients of 11 countries using the 28-item self-report questionnaire “Identifying Medication Adherence Barriers” (IMAB). Barriers were ranked by their frequency of occurrence for the total sample and by country. Countries were also ranked the by recipients’ total number of barriers. Intra-class correlations were calculated at country and center level. The five most frequently mentioned barriers were sleepiness (27.1%), being away from home (25.2%), forgetfulness (24.5%), interruptions to daily routine (23.6%) and being busy (22.8%), fairly consistently across countries. The participants reported on average three barriers, ranging from zero up to 22 barriers. The majority of the variability among reported barriers frequency was situated at the recipient level (94.8%). We found limited international variability in primarily person-level barriers in our study. Understanding of barriers in variable contexts guides intervention development to support adherence to the immunosuppressive regimen in real-world settings.
Introduction
Transplant recipients are required to adhere to a complex medical regimen of lifelong immunosuppressives (IS), supplemented by medications that prevent or treat co-morbidities [1, 2]. Optimal clinical outcome [3–5], and lower costs [6, 7] can be achieved by adhering well to the medication, which implies that the regimen 1) is initiated promptly, 2) implemented correctly as prescribed and 3) persistently continued over time [8]. In order to identify the patients at risk of nonadherence and to know who to support in enhancing their medication taking behavior, knowledge of the determinants of (non)adherence is essential.
Appropriate theoretical models can guide the identification of relevant determinants. The Integrated Model of Behavioral Prediction integrates insights of the most prominent behavioral theories (i.e., the health belief model, theory of reasoned action, theory of planned behavior and the social cognitive theory) [9] into a limited number of determinants of behavior. The model assumes that intentions drive behavior, while their execution can be hindered by a number of non-intentional barriers. Barriers are defined as “a person’s estimation of the level of challenge of social, personal, environmental, and economic obstacles to a specified behavior or their desired goal status on that behavior” [10]. Barriers frequently reported in the transplant literature are forgetfulness [11–21], interruptions to daily routine (e.g., being away from home) [12–17, 19, 22, 23], or having complex medication regimens (e.g., a high number of pills; several intakes per day; medication or dose changes) [14, 20–22, 24]. Barriers to medication taking are an undervalued problem within the transplant population [12, 25], because they are often not recognized strongly associated with non-adherence to immunosuppressives [20, 26], and are predictive of occurrence of acute rejections [27]. So far research has not explored variability in barriers in diverse transplant contexts and healthcare systems.
To overcome the limitations of the hitherto published studies on barriers, which included mostly limited numbers of patients, and limited cultural perspectives and care systems [28–30], the aim of our study was to assess a comprehensive set of barriers to medication adherence using a large multi-center sample of adult heart transplant (HTx) recipients participating in the BRIGHT study [1, 26], to rate the occurrence of the different barriers and assess its variability internationally.
Materials and Methods
Design, Setting and Sample
This study is a secondary data analysis of the international multi-center cross-sectional Building research initiative group: chronic illness management and adherence in transplantation (BRIGHT) study [1, 26]. The purpose of BRIGHT was to study variability in health behaviors among HTx recipients internationally, to assess risk-factors for non-adherence at different levels in the healthcare system and to describe and compare practice patterns of chronic illness management. Detailed information on the study methods and procedures has previously been published [1, 26]. In summary, multi-staged sampling of HTx recipients occurred in 11 countries and 36 HTx centers. At least two transplant centers per country were included across four continents: Europe: N = 19 (Belgium, n = 2: France, n = 3; Germany, n = 2; Italy, n = 2; Spain, n = 5; Switzerland, n = 2; UK, n = 3); North America: N = 12 (Canada, n = 4; United States, n = 8); Australia, N = 2; South America, N = 3 (Brazil). Further inclusion criteria for the HTx centers were: a) having performed ≥50 HTx in the 12–60 months prior to inclusion and procuring a formal support letter from the HTx center’s transplant director. HTx recipients were recruited using a proportional random sampling method based on size of transplant center using ISHLT criteria as a basis (i.e., small center: 50–74 HTx/last 5 years; medium center: 75–100 HTx/last 5 years; large center: >100 HTx/last 5 years) [1, 31]. Inclusion criteria of HTx recipients were a) being a ≥18-year-old HTx recipient at inclusion time; b) first single-organ transplant; c) being between one and 5 years post-transplant; and d) managing the taking of medication independently (i.e., without any professional support). All patients gave written informed consent for participation in the study, and approval for the BRIGHT study was obtained by all local ethical committees [1].
Variables and Measurement
Measurement of variables collected in this study was done using established or investigator-developed instruments by self-report, structured patient interviews as well as medical chart reviews (completed by a nurse or a clinician) [1, 26]. The questionnaires and instruments were pilot tested in diverse settings and translated into the study languages using established protocols.
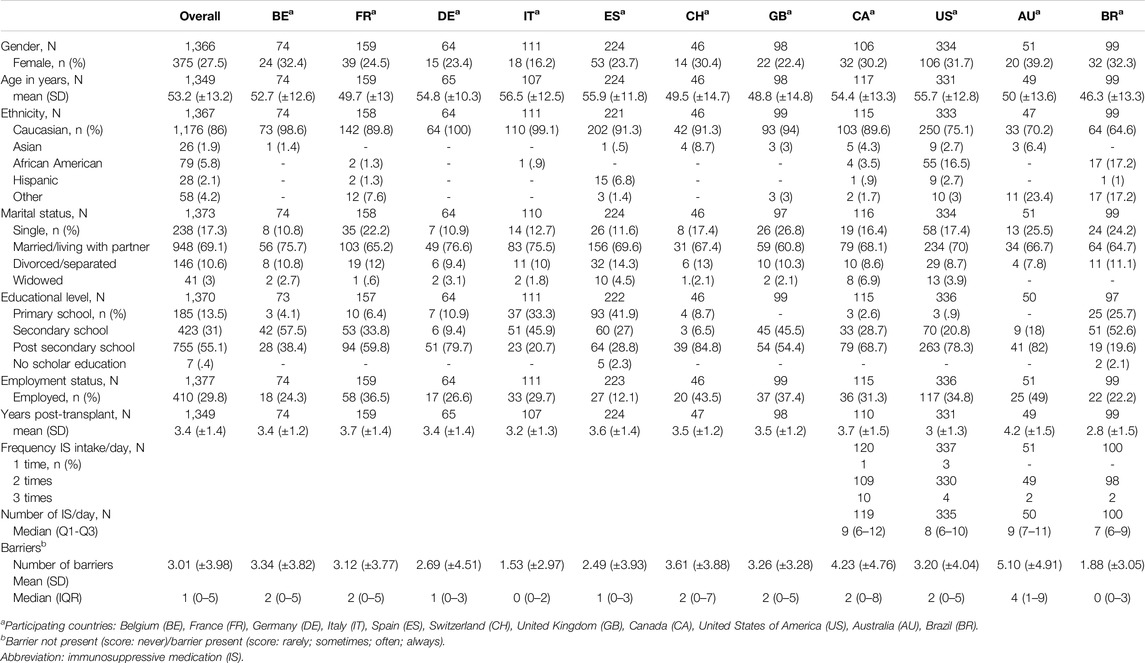
Sociodemographic and clinical variables were age in years, sex, marital status, ethnicity, educational level, employment status, years post-transplant, daily frequency of IS and number of IS per day (see Table 1 for answer categories).
Barriers to IS adherence were assessed by written self-report using the 28-item Identifying Medication Adherence Barriers (IMAB) self-report questionnaire [32]. The IMAB was specifically designed for the transplant population, the item generation was based on a systematic review of existing instruments, investigating barriers to medication adherence, published in the chronic illness literature (e.g., forgetfulness; poor health literacy; frequency, number, taste, or shape of IS; costs of IS; see Table 2). To enhance understandability by the patients, IMAB items were slightly adapted by changing the term “anti-rejection medication” into “immunosuppressant medications.” The content validity of IMAB was tested during the Transplant360 project [32], and its internal consistency as part of this study [26].
Patients rated each of the 28 barrier items on a five point scale (never = 1/rarely = 2/sometimes = 3/often = 4/always = 5). Since answer patterns showed a skewed distribution in favor of the lower frequencies, scores were dichotomized into absence of the barrier (never) versus presence of the barriers (rarely, sometimes, often or always). Next to analyzing the barriers individually, we also calculated the total number of barriers per patient.
Data Analysis
Analyses were of descriptive nature, using the appropriate measures given measurement levels and distributions of the respective variables. Calculation of the intracluster correlation indicated the percentages of variability of the number of barriers per patient, that could be attributed to the different healthcare system levels (i.e., country, center, patient). Analyses were executed in SAS 9.4 (SAS Institute, Cary, NC).
Results
Sample Characteristics
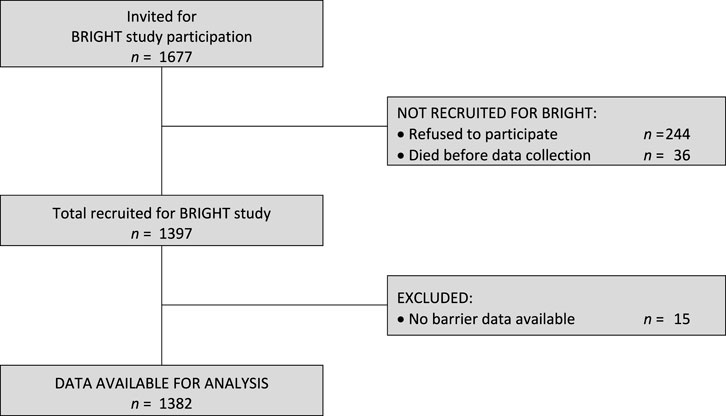
Of the 1,397 HTx recipients recruited in BRIGHT study (from an eligible 1,677), 15 (1.1%) did not provide any barrier data and were thus excluded from further analysis for this study (see Figure 1). The remaining 1,382 participants had a mean age of 53.2 years (SD ±13.2) and were, on average, 3.4 years (SD ± 1.4) post-transplant. The majority were male (72.5%), Caucasian (86%), educated at least post-secondary level (55.1%) and married or living with a partner (69.1%). Detailed information on the sample composition is provided in Table 1.
Number of Barriers Per Patient
The median number of reported barriers was 1 (mean 3.0; SD ± 4.0), with an interquartile range of 5, and ranging from zero (37% patients) to 22 barriers (0.1% of patients). The number of mentioned barriers per participant was diverging too, ranging from an average of 1.5 barriers in Italy to 5.1 barriers in Australia.
Variability of Barrier Prevalence Between Countries
Calculation of the intracluster correlation showed that 4% of variability of the total number of reported barriers was situated at the level of the country, 1.2% at the level of the centers nested within countries, while the remainder (94.8%) was intra-patient variability.
Prevalence of Individual Barriers
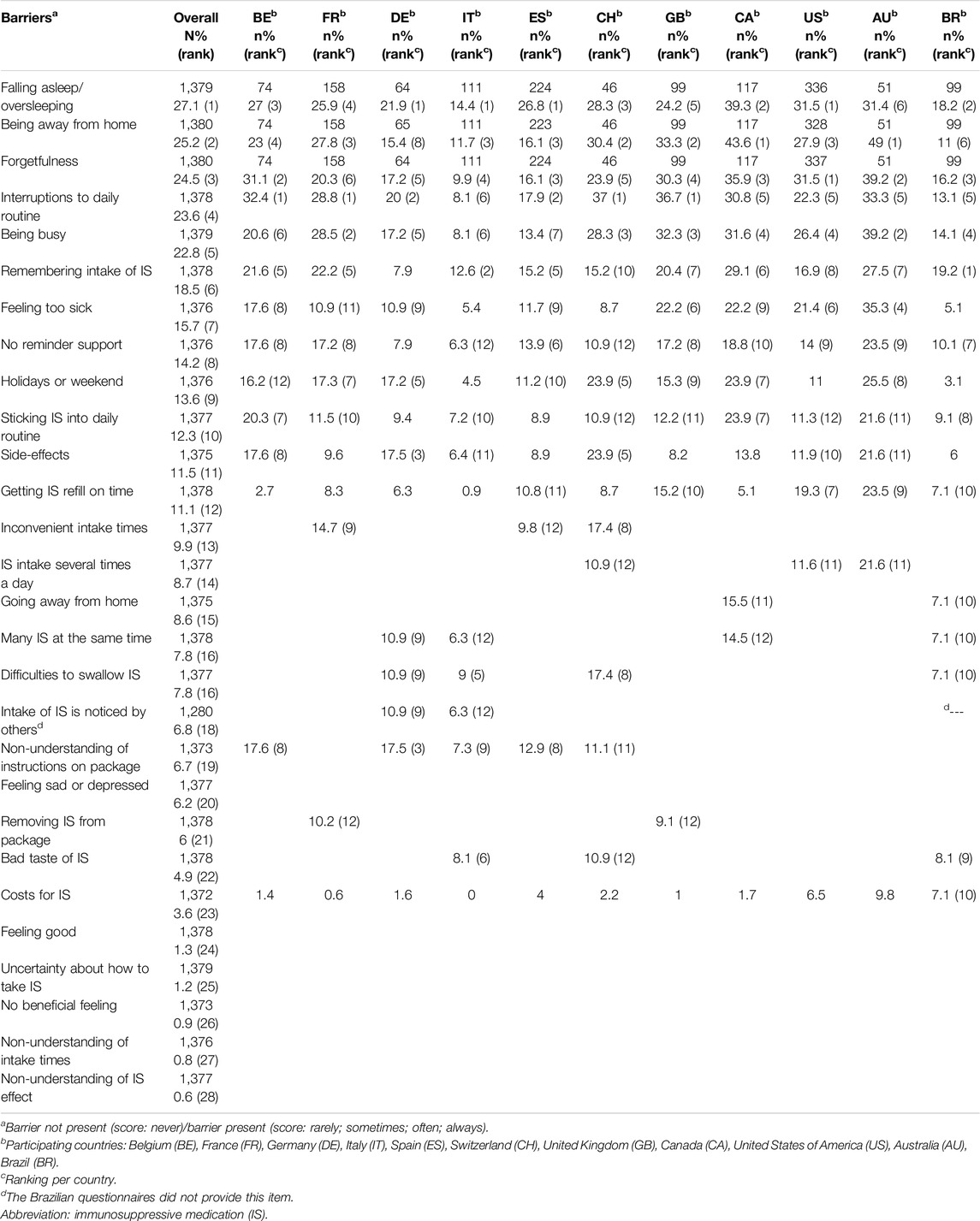
The prevalence of individual barriers ranged from 0.6% (i.e., non-understanding of IS effects) to 27.1% (i.e., falling asleep/oversleeping). Twelve of the 28 barriers were reported by more than 10% of all participants (Table 2). Five barriers were mentioned by more than 20% of the participants, i.e., falling asleep/oversleeping (27.1%), being away from home (25.2%), forgetfulness (24.5%), interruptions to the daily routine (23.6%) and being busy (22.8%). The percentage of patients who reported at least one of these top five barriers was 50.5%, while 5.3% reported them all at once.
Differences of Individual Barrier Prevalence Between Countries
Within the different countries, the ranking of individual barriers is similar to the prevalence ranking of barriers overall. The top-12 of barriers differed between countries, with Brazil having the lowest (all 12 barriers <20%) and Australia having the highest prevalence (all 12 barriers >20%). The overall most frequent barrier of falling asleep/oversleeping, appears in the top 6 of all of the countries. The barrier of being away from home, the 2nd most frequently mentioned overall, is equally ranked in the top 6 of barriers in the different countries, except for Germany (where it appeared as rank number 8). Forgetfulness, ranked as 3rd most prevalent barrier overall, is in all countries again in the 6 most prominent barriers. Some barriers were not very prevalent, generally with consistency across countries. One of those lesser reported barriers reflected cost as a barrier, ranked 23rd overall, with a prevalence of 3.6%, the highest being in Australia (9.8%), followed by Brazil (7.1%) and the US (6.5%). Although such a barrier reflects differences in countries’ healthcare systems, the intracluster correlation of this particular barrier only showed 1.2% of the variability to be situated at the country-level (1.2%), the rest being patient-level variability (98.8%).
Sensitivity Analysis
A sensitivity analysis compared the current ranking of barriers, obtained using frequencies of the dichotomized items (as presented in Table 1) with a ranking based on patients’ mean score, calculated from their responses on the scale from 1 to 5. The Spearman correlation between the two ranking systems was r = .99, indicating that their results were almost identical, thereby validating our dichotomization of item scores.
Discussion
This study assessed an extensive set of barriers to immunosuppressives intake among a worldwide sample of HTx recipients and found an average of three reported barriers per recipient, with some recipients reporting no barriers, while others up to twenty-two. The five most frequently mentioned barriers were sleeping during a prescribed intake time, being away from home, forgetfulness, interruptions to daily routine and being busy. Half of the sample reported at least one of these five, and one-fifth reported all five to be present simultaneously. These most frequent barriers can be grouped into three themes, which have been mentioned in the literature before:
- Sleeping through an intended intake moment, the most frequently mentioned barrier and among the top-3 of barriers in eight of the eleven countries, has been mentioned among renal transplant [33] and chronic heart failure [34] patients, in studies linking daytime sleepiness to poor medication adherence.
- The two barriers interruptions to daily routine and being away from home, and a third related barrier of inconvenient intake times, appeared in more than half of our participating countries and in over ten percent of the participants in six countries. These were previously reported in liver or kidney transplant recipients [14, 16, 17, 23], indicating that stringent intake times of IS can be a challenge at times when the normal schedule is disrupted.
- One of most frequently reported barriers within the transplant literature is forgetfulness. [12, 14, 16, 17, 20, 21] Although not ranked first within our sample, this barrier ties together with barriers referring to difficulties to remember intake of IS, or of lacking reminder support, making this theme one of the important barriers, probably not entirely independent from the previous theme of routine disruptions. As to factors that could explain forgetfulness, is linked with being busy among younger peopl, or to a decline in cognitive abilities among the aged [35]. A person’s personality type also seems to make a difference, since having a more compulsive or anxious personality type support adherence [36]. The meaning of forgetfulness seems to vary somewhat between high and low adherers: qualitative studies have shown that for the former group, forgetting refers to an occasional lapse, whereas for the latter, forgetfulness normalizes a consistent behavioral pattern [37–39].
Despite there being considerable differences between the top-ranked barriers among countries, most of the variability in number of reported barriers was still situated at the recipients level, as shown by the yet small intracluster correlations at the level of countries.
Even the barrier related to the healthcare system – concerning the cost of the IS, had most of its variability situated at the recipient level, despite it being largely determined by policies of healthcare coverage. In European countries, where the healthcare system covers largely the costs of organ transplants and its related expenses (e.g., IS) [40], the prevalence of the cost barrier was expectedly low; while the highest frequencies were recorded in Australia, where almost ten percent of the participants reported cost of IS as a barrier, followed by Brazil and the US. The relatively high frequency of this barrier in Australia and the USA is in line with the findings of a study that showed that their chronically ill patients reported high out-of-pocket costs for healthcare [28], a cause of financial stress [23, 28] and a source of cost-related non-adherence [41]. Unexpectedly, Brazilian recipients also reported a relatively high perception of perceived unaffordability, in spite of the fact that financial coverage for IS also applies to Brazil [40, 42], and that cost-related nonadherence was among the lowest in the Brazilian subsample [41].
Study Limitations
We investigated barriers to adherence only using the 28 IMAB items, which admittedly primarily focused on patient level barriers. Having the focus primarily on patient level is a limitation in our study. The IMAB could be expanded with additional barriers identified through quantitative and/or qualitative research. Especially barriers at the meso level pointing to barriers in the clinical work flow and organization in transplant centers such as limited time for patient education, not addressing adherence issues during an outpatient clinic visit or lack of trust in or access to healthcare providers might also be considered to be included in a barriers instrument [24]. Another limitation is that although large and with a diverse sample, the Bright study was only cross-sectional, hence, variability and changes in barrier experience over the course of a heart transplantation could not be well documented.
Implications for Practice and Research
HTx recipients face multiple barriers to adherence to IS. Barriers are proximal determinants of health behaviors and can guide the development of adherence enhancing or remediating interventions. With regard to adherence-enhancing, the advised approach is to first assess adherence and important determinants, such as barriers, in order to identify the patients at risk and deliver a multicomponent behavioral change intervention using shared decision making. Given the impactful nature of poor adherence to IS on clinical outcomes and economic costs [2], health professionals can assess actual and potential barriers a person with a transplant is faced with as this information provides direction in choosing tailored medication adherence interventions. Assessment of barriers in a research study is different from assessing barriers in daily clinical practice. Implementing regular barriers assessment in clinical practice, optimally combined with the assessment of medication adherence as a 5th vital sign (see COMMIT guidelines) [43], calls for careful consideration of context in view of clinical work flow to support the successful implementation in clinical practice (e.g., eHealth tools available for ePROM assessment). Moreover, the information collected needs to enrich clinical decision making. Decision tools integrated in the electronic medical record provide guidance how specific barriers can be linked to adherence interventions. Ribaut et al. have mapped components that can be used [44]. Well-designed interventions also prepare the transplant team and the organization for adherence management [45]. The implementation can be facilitated by dedicated education of transplant clinicians, not only providing the necessary knowledge but primarily with (communication) skills and organizing transplant care based on principles of chronic illness management, so that time and resources are specifically invested in patient’s self management support throughout the transplant journey [2]. An intervention program that successfully implemented all of these principles in a cost-effective way is published by Hooper et al. [46].
As mentioned earlier, barriers instruments can be continuously enriched with multi-level barriers generated from the literature and/or also from clinical observation. The IMAB is a good starting point, however, could be further extended.
Conclusion
We found limited international variability in primarily person-level barriers in our study. Understanding of barriers in variable contexts guides intervention development to support adherence to the immunosuppressive regimen in real-world settings. Implementation of barriers assessment in daily clinical practice needs specific considerations to guide successful implementation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by the University Hospitals of Leuven (Belgium) ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
KD, LB, FD, CR, and SD were involved in the study design. KD, GB, and SD participated in the manuscript writing. KD and GB analysed the data. All authors contributed to the article and approved the submitted version.
Group Members of The BRIGHT Study Team Consists of
Lut Berben, Institute of Nursing Science, Department Public Health, Faculty of Medicine, University of Basel and UKBB, Basel, Switzerland; Patricia Davidson Johns Hopkins School of Nursing, Baltimore, USA and University of Wollongong Australia, NSW, Australia; Maria G. Crespo-Leiro (Complexo Hospitalario Universitario A Coruña (CHUAC), CIBERCV, INIBIC, Universidade da Coruña (UDC), La Coruña, Spain); Sandra Cupples (U.S. Department of Veterans Affairs, Veterans Health Administration, Washington DC, United States); Paolo De Simone (Azienda Ospedaliero-Universitaria Pisana, Ospedale Cisanello, Pisa, Italy); Albert Groenewoud (Astellas Pharma Europe Ltd., United Kingdom); Christiane Kugler (Hannover Medical School, Hannover, Germany); Linda Ohler (George Washington University, Washington DC, United States); Johan Van Cleemput (University Hospitals Leuven, Leuven, Belgium); Alain Jean Poncelet (Cliniques Universitaires Saint-Luc, Brussels, Belgium); Laurent Sebbag (Hôpital Louis Pradel, Lyon, France); Magali Michel (Hôpital Nord Laennec, Nantes, France); Andrée Bernard (Hôpital Universitaire Pitié-Salpêtrière, Paris, France); Andreas Doesch (University Hospital Heidelberg, Heidelberg, Germany and Asklepios Hospital Bad Salzungen, Bad Salzungen, Germany); Ugolino Livi (University Hospital Udine, Udine, Italy); Luciano Potena (University of Bologna, Bologna, Italy); Vicens Brossa-Loidi (Hospital de Sant Pau, Barcelona, Spain); Javier Segovia-Cubero (Hospital Puerta de Hierro, Madrid, Spain); Luis Almenar-Bonet (Hospital Universitari i Politècnic La Fe de Valencia, Valencia and Hospital Universitari i Politècnic La Fe de Valencia, Valencia, Spain. CIBERCV Hospital Universitari i Politècnic La Fe de Valencia, Valencia, Spain. CIBERCV); Carmen Segura Saint-Gerons (Hospital Univeritario Reina Sofia, Córdoba, Spain); Paul Mohacsi (University Hospital of Bern, Bern, Switzerland); Eva Horvath and Stalder-Ochsner Irene (University Hospital Zurich, Zurich, Switzerland); Cheryl Riotto (Papworth Hospital, Cambridge, United Kingdom); Gareth Parry (Freeman Hospital, Newcastle, United Kingdom); Ashi Firouzi (Royal Brompton and Harefield NHS Foundation Trust, London, United Kingdom); Stella Kozuszko (Toronto General Hospital, Toronto, Canada); Haissam Haddad (University of Ottawa Heart Institute, Ottawa, ON, Canada); Annemarie Kaan (St Paul’s Hospital, Vancouver, BC, Canada); Grant Fisher (London Health Sciences Centre, London, ON, Canada); Tara Miller (Duke University Hospital, Durham, NC, United States); Maureen Flattery (Virginia Commonwealth University Health System, Richmond, VA, United States); Kristin Ludrosky/Nancy Albert (Cleveland Clinic, OH, United States); Bernice Coleman (Cedars-Sinai Medical Center, Los Angeles, CA, United States); Jacqueline Trammell & Flavio Epstein (Kaiser Permanente Santa Clara Medical Center, Santa Clara, CA, United States); Katherine St. Clair, Andrew Kao (St. Luke’s Hospital, Kansas City, MO, United States); Maria Molina (Hospital of the University of Pennsylvania, Philadelphia, PA, United States); Karyn Ryan Canales (Ochsner Medical Center, New Orleans, LA, United States); Samira Scalso de Almeida (Hospital Israelita Albert Einstein, São Paulo and Hospit.al Municipal Vila Santa Catarina - Ministerio da Saude PROAD/-SUS, Sao Paulo, BraziL); Bartira de Aguiar Roza, Paulista School of Nursing, Federal University of Sao Paulo, Sao Paolo, Brazil; ; Andrea Cotait Ayoub (Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil); Fernanda Barone (Instituto do Coração da Universidade de São Paulo, São Paulo Brazil); Michelle Harkess (St. Vincent’s Hospital, Sydney, Australia); Joanne Maddicks-Law (The Prince Charles Hospital, Brisbane, Australia).
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The BRIGHT study is funded by research grants of the International Transplant Nurses Society (ITNS) in 2008, the International Society for Heart and Lung Transplantation (ISHLT) in 2012, the Swiss Academy of Medical Sciences (SAMW) in 2013 as well as an unrestricted research grant from Astellas Pharma. None of the grants has a grant number. None of the organizations that provided funding has access to the data nor were involved in the preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank the patients and clinicians of the 36 HTx centers for their participation in the BRIGHT study. Special thanks to Sandra Schönfeld; Eva Baumgartner, Christine Vetter, and Remon Helmy for their support in this study. We also thank the junior and senior peers of the Master Seminar at the Institute of Nursing Science, University of Basel, Switzerland for their support during the writing process of this article and Chris Shultis for editing this paper.
References
1. Berben, L, Denhaerynck, K, Dobbels, F, Engberg, S, Vanhaecke, J, Crespo-Leiro, MG, et al. Building Research Initiative Group: Chronic Illness Management and Adherence in Transplantation (BRIGHT) Study: Study Protocol. J Adv Nurs (2015) 71:642–54. doi:10.1111/jan.12519
2. De Geest, S, Ribaut, J, Denhaerynck, K, and Dobbels, F. Adherence Management in Transplantation: Creating Impact in REAL World Settings Based on State of the Art Evidence. In: D Cukor, S Cohen, and PL Kimmel, editors. Psychosocial Aspects of Chronic Kidney Disease: Exploring the Impact of CKD, Dialysis, and Transplantation on Patients. London: Academic Press (2021). p. 409–48.
3. Dew, MA, Kormos, RL, Roth, LH, Murali, S, DiMartini, A, and Griffith, BP. Early Post-Transplant Medical Compliance and Mental Health Predict Physical Morbidity and Mortality One to Three Years After Heart Transplantation. J Heart Lung Transplant (1999) 18:549–62. doi:10.1016/S1053-2498(98)00044-8
4. Dobbels, F, De Geest, S, van Cleemput, J, Droogne, W, and Vanhaecke, J. Effect of LATE Medication Non-compliance on Outcome After Heart Transplantation: A 5-YEAR Follow-Up. J Heart Lung Transplant (2004) 23:1245–51. doi:10.1016/j.healun.2003.09.016
5. De Geest, S, Abraham, I, Moons, P, Vandeputte, M, Van Cleemput, J, Evers, G, et al. LATE Acute Rejection and Subclinical Noncompliance WITH Cyclosporine Therapy in Heart Transplant Recipients. J Heart Lung Transplant (1998) 17:854–63.
6. De Geest, S, Denhaerynck, K, and Dobbels, F. Clinical and Economic Consequences of Non-Adherence to Immunosuppressive Drugs in Adult Solid Organ Transplantation. In: International Transplantation Updates. Barcelona, Spain: Permanyer Publications (2011).
7. Pinsky, BW, Takemoto, SK, Lentine, KL, Burroughs, TE, Schnitzler, MA, and Salvalaggio, PR. Transplant Outcomes and Economic Costs Associated WITH Patient Noncompliance to Immunosuppression. Am J Transplant : official J Am Soc Transplant Am Soc Transpl Surgeons (2009) 9:2597–606. doi:10.1111/j.1600-6143.2009.02798.x
8. Vrijens, B, De Geest, S, Hughes, DA, Przemyslaw, K, Demonceau, J, Ruppar, T, et al. A New Taxonomy for Describing and Defining Adherence to Medications. Br J Clin Pharmacol (2012) 73:691–705. doi:10.1111/j.1365-2125.2012.04167.x
9. Fishbein, M. The ROLE of Theory in HIV Prevention. AIDS Care Psychol Socio-medical Aspects AIDS/HIV (2000) 12:273–8. doi:10.1080/09540120050042918
10. Glasgow, RE. Perceived Barriers to Self-Management and Preventive Behaviors (2016). Available from: http://cancercontrol.cancer.gov/brp/research/constructs/barriers.html (Accessed June 17, 2016).
11. Cedillo-Galindo, H, and Gracida, C. Barriers and Strategies for Taking Medicines in Adult Patients WITH Renal Transplantation. Transplant Proc (2011) 43:3364–6. doi:10.1016/j.transproceed.2011.09.084
12. Chisholm-Burns, M, Pinsky, B, Parker, G, Johnson, P, Arcona, S, Buzinec, P, et al. Factors Related to Immunosuppressant Medication Adherence in Renal Transplant Recipients. Clin Transplant (2012) 26:706–13. doi:10.1111/j.1399-0012.2011.01589.x
13. Gordon, EJ, Prohaska, TR, Gallant, MP, and Siminoff, LA. Adherence to Immunosuppression: A Prospective Diary Study. Transplant Proc (2007) 39:3081–5. doi:10.1016/j.transproceed.2007.02.100
14. Lamba, S, Nagurka, R, Desai, KK, Chun, SJ, Holland, B, and Koneru, B. Self-Reported Non-Adherence to Immune-Suppressant Therapy in Liver Transplant Recipients: Demographic, Interpersonal, and Intrapersonal Factors. Clin Transplant (2012) 26:328–35. doi:10.1111/j.1399-0012.2011.01489.x
15. Muduma, G, Shupo, FC, Dam, S, Hawken, NA, Aballéa, S, Odeyemi, I, et al. Patient Survey to Identify Reasons for Non-adherence and Elicitation of Quality of LIFE Concepts Associated with Immunosuppressant Therapy in Kidney Transplant Recipients. Patient preference and adherence (2016) 10:27–36. doi:10.2147/ppa.s96086
16. Schmid-Mohler, G, Pechula, TM, Wüthrich, RP, Denhaerynck, K, and De Geest, S. Non-Adherence to Immunosuppressive Medication in Renal Transplant Recipients Within the Scope of the Integrative Model of Behavioral Prediction: A Cross-Sectional Study. Clin Transplant (2010) 24:213–22. doi:10.1111/j.1399-0012.2009.01056.x
17. Weng, FL, Chandwani, S, Kurtyka, KM, Zacker, C, Chisholm-Burns, MA, and Demissie, K. Prevalence and Correlates of Medication Non-adherence Among Kidney Transplant Recipients MORE THAN 6 Months Post-Transplant: A Cross-Sectional Study. BMC Nephrol (2013) 14:261. doi:10.1186/1471-2369-14-261
18. Cossart, AR, Staatz, CE, Campbell, SB, Isbel, NM, and Cottrell, WN. Investigating Barriers to Immunosuppressant Medication Adherence in Renal Transplant Patients. Nephrology (Carlton) (2019) 24:102–10. doi:10.1111/nep.13214
19. Wadhwani, SI, Nichols, M, Klosterkemper, J, Cirincione, R, Whitesell, K, Owen, D, et al. Implementing a Process to Systematically Identify and Address POOR Medication Adherence in Pediatric Liver Transplant Recipients. Pediatr Qual Saf (2020) 5:e296. doi:10.1097/pq9.0000000000000296
20. Ganjali, R, Ghorban Sabbagh, M, Nazemiyan, F, Mamdouhi, F, Badiee Aval, S, Taherzadeh, Z, et al. Factors Associated WITH Adherence to Immunosuppressive Therapy and Barriers in Asian Kidney Transplant Recipients. Immunotargets Ther (2019) 8:53–62. doi:10.2147/ITT.S212760
21. Taj, SM, Baghaffar, H, Alnajjar, DK, Almashabi, NK, and Ismail, S. Prevalence of Non-Adherence to Immunosuppressive Medications in Kidney Transplant Recipients: Barriers and Predictors. Ann Transpl (2021) 26:e928356. doi:10.12659/AOT.928356
22. Morales, JM, Varo, E, and Lazaro, P. Immunosuppressant Treatment Adherence, Barriers to Adherence and Quality of LIFE in Renal and Liver Transplant Recipients in Spain. Clin Transplant (2012) 26:369–76. doi:10.1111/j.1399-0012.2011.01544.x
23. Moayed, MS, Khatiban, M, Nassiri Toosi, M, Khodaveisi, M, Soltanian, AR, and Ebadi, A. Barriers to Adherence to Medical CARE Programs in Liver Transplant Recipients: A Qualitative Study. Int J Organ Transpl Med (2019) 10:115–26.
24. Memory, KE, Wilkinson, TJ, Smith, AC, and Lightfoot, CJ. A Qualitative Exploration of the Facilitators and Barriers to Self-Management in Kidney Transplant Recipients. J Nephrol (2022) 35:1863–72. doi:10.1007/s40620-022-01325-w
25. Eaton, CK, Lee, JL, Simons, LE, Devine, KA, Mee, LL, and Blount, RL. Clinical Cutoffs for Adherence Barriers in Solid Organ Transplant Recipients: How MANY Is Too MANY? J Pediatr Psychol (2015) 40:431–41. doi:10.1093/jpepsy/jsu102
26. Denhaerynck, K, Berben, L, Dobbels, F, Russell, CL, Crespo-Leiro, MG, Poncelet, AJ, et al. Multilevel Factors Are Associated WITH Immunosuppressant Nonadherence in Heart Transplant Recipients: The International BRIGHT Study. Am J Transplant : official J Am Soc Transplant Am Soc Transpl Surgeons (2018) 18:1447–60. doi:10.1111/ajt.14611
27. Varnell, CD, Rich, KL, Zhang, B, Carle, AC, Pai, ALH, Modi, AC, et al. Predicting Acute Rejection in Children, Adolescents, and Young Adults WITH a Kidney Transplant by Assessing Barriers to Taking Medication. Pediatr Nephrol (2021) 36:2453–61. doi:10.1007/s00467-021-04946-8
28. Schoen, C, Osborn, R, Squires, D, and Doty, MM. Access, Affordability, and Insurance Complexity Are Often Worse in the United States Compared to Ten Other Countries. Health Aff (2013) 32:2205–15. doi:10.1377/hlthaff.2013.0879
29. Schoen, C, Osborn, R, Squires, D, Doty, MM, Pierson, R, and Applebaum, S. How Health Insurance Design Affects Access to CARE and Costs, By Income, in Eleven Countries. Health Aff (2010) 29:2323–34. doi:10.1377/hlthaff.2010.0862
30. Berben, L, Dobbels, F, Engberg, S, Hill, MN, and De Geest, S. An Ecological Perspective on Medication Adherence. West J Nurs Res (2012) 34:635–53. doi:10.1177/0193945911434518
31. ISHLT. International Society of Heart and Lung Transplantation (2011). Available from: http://www.ishlt.org/(Accessed June 17, 2016).
32. Transplant360. Identifying Medication Adherence Barriers (IMAB) Questionnaire (2014). Available from: http://www.transplant360.com/(Accessed June 17, 2016).
33. Burkhalter, H, Wirz-Justice, A, Cajochen, C, Weaver, TE, Steiger, J, Fehr, T, et al. Daytime Sleepiness in Renal Transplant Recipients Is Associated WITH Immunosuppressive Non-Adherence: A Cross-Sectional, Multi-Center Study. Clin Transplant (2014) 28:58–66. doi:10.1111/ctr.12279
34. Riegel, B, Moelter, ST, Ratcliffe, SJ, Pressler, SJ, De Geest, S, Potashnik, S, et al. Excessive Daytime Sleepiness Is Associated WITH POOR Medication Adherence in Adults WITH Heart Failure. J Card Fail (2011) 17:340–8. doi:10.1016/j.cardfail.2010.11.002
35. Neupert, SD, Patterson, TR, Davis, AA, and Allaire, JC. Age Differences in Daily Predictors of Forgetting to TAKE Medication: The Importance of Context and Cognition. Exp Aging Res (2011) 37:435–48. doi:10.1080/0361073X.2011.590757
36. DiMatteo, MR, Lepper, HS, and Croghan, TW. Depression Is a RISK Factor for Noncompliance WITH Medical Treatment: Meta-Analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch Intern Med (2000) 160:2101–7. doi:10.1001/archinte.160.14.2101
37. Drabble, SJ, O'Cathain, A, Arden, MA, Hutchings, M, Beever, D, and Wildman, M. When Is Forgetting Not Forgetting? A Discursive Analysis of Differences in Forgetting TALK Between Adults WITH Cystic Fibrosis WITH Different Levels of Adherence to Nebulizer Treatments. Qual Health Res (2019) 29:2119–31. doi:10.1177/1049732319856580
38. Kalichman, SC, Kalichman, MO, and Cherry, C. Forget About Forgetting: Structural Barriers and Severe Non-adherence to Antiretroviral Therapy. AIDS Care Psychol Socio-medical Aspects AIDS/HIV (2017) 29:418–22. doi:10.1080/09540121.2016.1220478
39. Freeman, R, Gwadz, M, Francis, K, and Hoffeld, E. Forgetting to TAKE HIV Antiretroviral Therapy: A Qualitative Exploration of Medication Adherence in the Third Decade of the HIV Epidemic in the United States. SAHARA J (2021) 18:113–30. doi:10.1080/17290376.2021.1989021
40. Renkes, AC. Financial Barriers to Organ Transplantation: A Comparative Analysis. SPNHA Rev (2012) 8:33–42.
41. Schonfeld, S, Denhaerynck, K, Berben, L, Dobbels, F, Russell, CL, Crespo-Leiro, MG, et al. Prevalence and Correlates of Cost-Related Medication Nonadherence to Immunosuppressive Drugs After Heart Transplantation: The International Multicenter Cross-Sectional Bright Study. J Cardiovasc Nurs (2020) 35:519–29. doi:10.1097/JCN.0000000000000683
42. Medina-Pestana, JO, Galante, NZ, Tedesco-Silva, H, Harada, KM, Garcia, VD, Abbud-Filho, M, et al. Kidney Transplantation in Brazil and Its Geographic Disparity. Jornal brasileiro de nefrologia : 'orgao oficial de Sociedades Brasileira e Latino-Americana de Nefrologia (2011) 33:472–84.
43. Neuberger, JM, Bechstein, WO, Kuypers, DR, Burra, P, Citterio, F, De Geest, S, et al. Practical Recommendations for Long-Term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist By the Consensus on Managing Modifiable RISK in Transplantation (COMMIT) Group. Transplantation (2017) 101:S1-S56–S56. doi:10.1097/TP.0000000000001651
44. Ribaut, J, Leppla, L, Teynor, A, Valenta, S, Dobbels, F, Zullig, LL, et al. Theory-Driven Development of a Medication Adherence Intervention Delivered by eHealth and Transplant TEAM in Allogeneic STEM CELL Transplantation: The SMILe Implementation Science Project. BMC Health Serv Res (2020) 20:827. doi:10.1186/s12913-020-05636-1
45. Kostalova, B, Ribaut, J, Dobbels, F, Gerull, S, Mala-Ladova, K, Zullig, LL, et al. Medication Adherence Interventions in Transplantation LACK Information on How to Implement Findings from Randomized Controlled Trials in Real-World Settings: A Systematic Review. Transpl Rev (Orlando) (2022) 36:100671. doi:10.1016/j.trre.2021.100671
Keywords: heart transplantation, medication adherence, immunosuppressant nonadherence, immunosuppressant medication, barrier
Citation: Denhaerynck K, Berger Wermuth G, Dobbels F, Berben L, Russell CL and De Geest S (2024) International Variability of Barriers to Adherence to Immunosuppressive Medication in Adult Heart Transplant Recipients. A Secondary Data Analysis of the BRIGHT Study. Transpl Int 37:12874. doi: 10.3389/ti.2024.12874
Received: 19 February 2024; Accepted: 05 August 2024;
Published: 29 August 2024.
Copyright © 2024 Denhaerynck, Berger Wermuth, Dobbels, Berben, Russell and De Geest. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina De Geest, c2FiaW5hLmRlZ2Vlc3RAdW5pYmFzLmNo
 Kris Denhaerynck
Kris Denhaerynck Gabriele Berger Wermuth1
Gabriele Berger Wermuth1 Fabienne Dobbels
Fabienne Dobbels Cynthia L. Russell
Cynthia L. Russell Sabina De Geest
Sabina De Geest