Dear Editors,
Solid organ transplant recipients continue to face a heightened risk of severe COVID-19, despite a decrease in virus virulence since the emergence of Omicron [1]. Managing preventive and therapeutic strategies in this population poses challenges due to their reduced vaccine response, potential drug-drug interactions with nirmatrelvir-ritonavir, and the ability of variants to escape neutralizing monoclonal antibodies (mAbs) [2, 3]. Neutralization is a surrogate marker of protection for both active (from previous infection or vaccination) and passive immunity (from monoclonal antibodies), and it is utilized for immunobridging of newly available therapeutic antibodies [3, 4]. However, its use in optimizing care for immunocompromised patients is rare, partly due to the absence of a well-defined protective threshold. The emergence of SARS-CoV-2 variants that evade neutralization necessitates ongoing evaluation of therapeutic mAbs and provides an opportunity to explore the relationship between neutralization activity and clinical outcomes. Here, we evaluated the in vitro neutralizing activity of sotrovimab and other therapeutic mAbs against XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants. We also retrospectively investigated the neutralization against these variants of sera from kidney transplant recipients (KTR) who received sotrovimab.
Our initial focus was to assess the in vitro neutralizing activity of mAbs that had been utilized since the end of 2021 (namely sotrovimab, cilgavimab-tixagevimab, and imdevimab-casirivimab). As controls, we analyzed the neutralizing activity against the ancestral D614G strain. Neutralization of authentic SARS-CoV-2 isolates were performed with the S-Fuse assay as described in Supplementary Material and previously [5]. Sotrovimab exhibited neutralizing activity against the XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants, albeit at low levels (with ED50 titers of 0.70 μg/mL, 1.18 μg/mL, and 1.41 μg/mL, respectively, as opposed to 0.04 μg/mL against the D614G variant, Supplementary Figure S1). The cilgavimab-tixagevimab combination and imdevimab-casirivimab displayed no discernible neutralizing activity.
Given this weak but consistent in vitro activity of sotrovimab against these variants, our subsequent investigation delved into its in vivo neutralization, using the same assay and sera retrieved from 18 KTR followed at Strasbourg University Hospital. These patients had received sotrovimab treatment for confirmed COVID-19, and had accessible post-sotrovimab serum samples during BA.1 and BA.2 breakthrough period spanning from January to March 2022. The administration of sotrovimab was conducted intravenously at a dose of 500 mg. The median age of this cohort was 60.5 years (interquartile range [IQR] 45.2–70.2 years). The median time from transplantation to COVID-19 diagnosis was 2.47 years (IQR 0.34–8.54 years). All but one patient had been vaccinated against SARS-CoV-2, but only two of them demonstrated an effective vaccine response with an anti-spike antibody titer above 264 BAU/mL (Supplementary Table S1). The measurement of in vivo neutralizing activity was conducted after a median of 34 days (IQR 18–51.5 days) following sotrovimab administration. All patients’ sera displayed significant serum neutralization against the D614G variant, with a median ED50 titer of 7,641 (IQR 934–11,859). In contrast, although a majority of sera exhibited neutralizing activity above the threshold against XBB.1.5. (n = 17/18), XBB.1.16.1 (n = 16/18), and XBB.1.9.1 (n = 17/18) variants, the titers were low and significantly reduced compared to the neutralization titers against D614G, with median ED50 titers of 31 (IQR 26–121, p = 0.04), 24 (IQR 14–85, p < 0.0001), and 24 (IQR 11–75, p < 0.0001) for XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants, respectively (Figure 1). The neutralizing titers for XBB.1.5, XBB.1.16.1, and XBB.1.9.1 were reduced by a median of 87-fold, 115-fold, and 154-fold, respectively, compared to D614G.
FIGURE 1
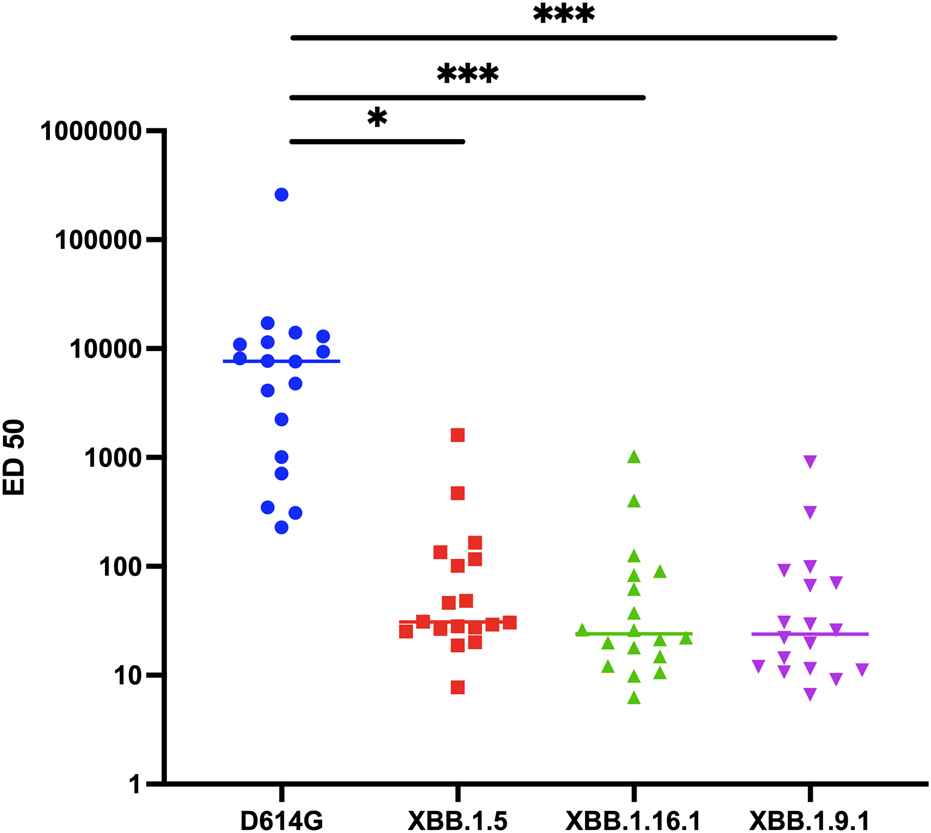
Neutralization activity against D614G, XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants in sera of COVID-19 kidney transplant recipients (n = 18) receiving Sotrovimab infusion. Results are effective dilution 50% (ED50; titers) as calculated with the S-Fuse assay. Each dot is an individual. Lines indicate medians. The dashed lines indicate the limits of detection. *p = 0.04 according to Friedman test with Dunn’s multiple comparison correction and Spearman non-parametric correlation test. ***p < 0.0001 according to Friedman test with Dunn’s multiple comparison correction and Spearman non-parametric correlation test.
In a subgroup of 10 patients, sera neutralization was assessed before and after sotrovimab administration. After administration of sotrovimab, neutralization activity increased slightly against XBB.1.5 (from a median of 15.61–38.72, p = 0.01), XBB.1.16.1 (from a median of 10–26.19, p = 0.004), and XBB.1.9.1 (from a median of 10–25.66, p = 0.004), Supplementary Figure S2.
Notably, non-hospitalized patients exhibited higher median titers compared to hospitalized patients for each variant: 74.7 (IQR 30.2–142) vs. 26 (IQR 20.4–27.2, p < 0.01) for XBB.1.5, 48.4 (IQR 24.2–93.2) vs. 11.3 (IQR 10.2–11.8, p < 0.01) for XBB.1.9.1, and 49.8 (IQR 21.6–117.1) vs. 11.4 (IQR 9.0–20.0, p < 0.01) for XBB.1.16.1 (Supplementary Figure S3). Advanced age also correlated with lower neutralizing titers against XBB.1.5 and XBB.1.9.1 (Spearman correlation coefficients: −0.49, p = 0.04 and −0.54, p = 0.02 respectively). None of the other clinical and demographic characteristics were found to be associated with neutralizing titers.
Collectively, the data presented here indicate a persistent in vitro and in vivo neutralization of sotrovimab against XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants. These variants are no longer circulating and the current dominant variants (JN.1 and derivatives) fully evades sotrovimab [6]. Nevertheless, our study raises interesting observations on the therapeutic use of mAbs, as it shows a residual antiviral activity of sotrovimab even after its discontinuation from the clinical setting. The minimum dose of mAb necessary to achieve adequate protection has not been established. Data on adintrevimab have shown that a low level of neutralization may be sufficient to provide clinical effectiveness against omicron BA.1 and BA.1.1 [7]. Conversely, during the BA.2 wave, increasing the dosage of tixagevimab-cilgavimab led to a rise in serum neutralizing activity and a decreased risk of COVID-19 breakthrough infections [8]. As higher doses of mAbs administration were found to be safe [9], it may be interesting to consider an increase in the dosage of therapeutic mAbs to boost efficacy against variants harboring partial escape. Indeed, the neutralizing activity against circulating variants is correlated with protection against COVID-19 infection, whether the immunity is passive or active [4]. Furthermore, sotrovimab exhibits an antibody-dependent cellular cytotoxicity (ADCC) activity against the XBB.1.5 variant [5]. Whether such non-neutralizing activities of antibodies contribute to the clinical efficacy of mAbs deserves further investigations. Altogether, our data and the literature suggest that a better mechanistical characterization of antibody activities against variants is needed to optimize patient care.
It is essential to acknowledge the limitations of our study. Being a retrospective, single-center study with a relatively limited sample size and lacking a control group, the findings should be interpreted with caution. We must also consider the potential impact of natural anti-COVID-19 immunity in this population infected with the BA.1 or BA.2 variant. However, it is important to note that the XBB.1.5 variant has the ability to evade antibodies generated after infection by these variant [10].
Providing data on the link between serum neutralization and mAbs efficacy (in our case sotrovimab and XBB.1.5, XBB.1.16.1, and XBB.1.9.1 variants) enables to create a framework to associate neutralization to clinical efficacy over the course of SARS-CoV-2 evolution and to help predict the efficacy of future therapies against future variants.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the local ethics committees (identifier: DC-2013-1990 and DC-2021-4460), and written informed consent was obtained from all participants.
Author contributions
Concept and design: IB and SC. Experimental strategy and design: TB and OS. Laboratory experiments: MJ-G and IS. Cohort management and clinical research: IB and SC. Viral strains and key reagents: AB, OD, and ES-L. Statistical analysis: IB and SC. Manuscript writing and editing: IB and SC. Critical revision of the manuscript for important intellectual content: All authors. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Work in TB and OS lab is funded by Institut Pasteur, Urgence COVID-19 Fundraising Campaign of Institut Pasteur, the ANR JCJC program (ANR-23-CE15-0039-01), Fondation pour la Recherche Médicale (FRM), ANRS-MIE, the Vaccine Research Institute (ANR-10-LABX-77), Labex IBEID (ANR-10-LABX-62-IBEID), ANR / FRM Flash Covid PROTEO-SARS-CoV-2, Programme Hubert Curien Maimonide, Coronamito, HERA european funding, Sanofi and IDISCOVR. ESL lab is funded by the INCEPTION program (Investissements d’Avenir grant ANR-16-CONV-0005), the NIH PICREID program (Award Number U01AI151758), the HERA European program DURABLE (101102733) and the Labex IBEID (ANR-10-LABX-62-IBEID). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank members of the Virus and Immunity Unit for discussions and help in the preparation of this manuscript. We thank Laurent Hocqueloux and Thierry Prazuck for providing the clinical antibodies for in vitro studies. We thank the Nephrology clinical research team (Danielle Roy, Annie Menguy, and Fanny Husselstein). We thank Dr. Djoubi from massif des Vosges Hospital and Dr. Vanessa Cocquerelle from Laboratory Deux Rives for providing the patient samples.
Conflict of interest
TB and OS have a pending patent application for an anti-RBD mAb not used in this study (PCT/FR2021/070522). IB received travel grant and payment or honoraria for lectures from Astra Zeneca. SC has received consultancy fees and has served on advisory boards for Astra Zeneca, Alexion, Chiesi, Pierre Fabre, and Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13272/full#supplementary-material
Abbreviations
BAU, binding arbitrary units; COVID-19, coronavirus disease 2019; KTRs, kidney transplant recipients; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mAbs, monoclonal antibodies.
References
1.
WeissAHendrickxRStensgaardEJellingsøMSommerMOA. Kidney Transplant and Dialysis Patients Remain at Increased Risk for Succumbing to COVID-19. Transplantation (2023) 107(5):1136–8. 10.1097/TP.0000000000004462
2.
AveryRK. Update on COVID-19 Therapeutics for Solid Organ Transplant Recipients, Including the Omicron Surge. Transplantation (2022) 106(8):1528–37. 10.1097/TP.0000000000004200
3.
CharmetantXEspiMBenotmaneIBarateauVHeibelFBuronFet alInfection or a Third Dose of mRNA Vaccine Elicits Neutralizing Antibody Responses Against SARS-CoV-2 in Kidney Transplant Recipients. Sci Transl Med (2022) 14(636):eabl6141. 10.1126/scitranslmed.abl6141
4.
FollmannDO’BrienMPFintziJFayMPMontefioriDMatejaAet alExamining Protective Effects of SARS-CoV-2 Neutralizing Antibodies After Vaccination or Monoclonal Antibody Administration. Nat Commun (2023) 14(1):3605. 10.1038/s41467-023-39292-w
5.
BruelTVrignaudLLPorrotFStaropoliIPlanasDGuivel-BenhassineFet alSotrovimab Therapy Elicits Antiviral Activities Against Omicron BQ.1.1 and XBB.1.5 in Sera of Immunocompromised Patients. Med N Y N (2023) 4(10):664–7. 10.1016/j.medj.2023.07.007
6.
UriuKItoJKosugiYTanakaYLMugitaYGuoZet alTransmissibility, Infectivity, and Immune Evasion of the SARS-CoV-2 BA.2.86 Variant. Lancet Infect Dis (2023) 23(11):e460–e461. 10.1016/S1473-3099(23)00575-3
7.
SchmidtPNarayanKLiYKakuCIBrownMEChampneyEet alAntibody-Mediated Protection Against Symptomatic COVID-19 Can Be Achieved at Low Serum Neutralizing Titers. Sci Transl Med (2023) 15(688):eadg2783. 10.1126/scitranslmed.adg2783
8.
Al JurdiAMorenaLCoteMBetheaEAzziJRiellaLV. Tixagevimab/Cilgavimab Pre-Exposure Prophylaxis Is Associated With Lower Breakthrough Infection Risk in Vaccinated Solid Organ Transplant Recipients During the Omicron Wave. Am J Transpl (2022) 22(12):3130–6. 10.1111/ajt.17128
9.
MoyaJTemechMParraSJuarezEHernandez-LoyRMoises GutierrezJCet alSafety, Virology, Pharmacokinetics, and Clinical Experience of High-Dose Intravenous Sotrovimab for the Treatment of Mild to Moderate COVID-19: An Open-Label Clinical Trial. Open Forum Infect Dis (2023) 10(7):ofad344. 10.1093/ofid/ofad344
10.
AbbadAYellinTSinghGFriedMRaskinATcheouJet alSARS-CoV-2 BA.1 and BA.2 Breakthrough Infections Boost Antibody Responses to Early Omicron Subvariants but Not BQ.1.1 or XBB.1.5. Cell Rep Med (2024) 5(3):101474. 10.1016/j.xcrm.2024.101474
Summary
Keywords
sotrovimab, COVID-19, immunocompromised, kidney transplant recipients, monoclonal antibodies, SARS-CoV-2
Citation
Benotmane I, Jungbauer-Groznica M, Staropoli I, Planas D, Dehan O, Brisebarre A, Simon-Loriere E, Fafi-Kremer S, Schwartz O, Bruel T and Caillard S (2024) In Vitro and In Vivo Neutralizing Efficacy of Monoclonal Antibodies Against Sars-Cov-2 Variants in Kidney Transplant Recipients. Transpl Int 37:13272. doi: 10.3389/ti.2024.13272
Received
18 May 2024
Accepted
01 July 2024
Published
16 July 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Benotmane, Jungbauer-Groznica, Staropoli, Planas, Dehan, Brisebarre, Simon-Loriere, Fafi-Kremer, Schwartz, Bruel and Caillard.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilies Benotmane, ilies.benotmane@chru-strasbourg.fr
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.