- 1Department of Immunology and Inflammation, Imperial College London, Hammersmith Campus, London, United Kingdom
- 2Imperial College Renal and Transplant Centre, Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, United Kingdom
- 3Department of Histopathology, Northwest London Pathology NHS Trust, Charing Cross Hospital, London, United Kingdom
Angiotensin II type-1 receptor antibody (AT1R-Ab) has been mooted as a potential effector of both acute and chronic antibody mediated rejection (AMR). A growing body of literature on the topic is now coming under scrutiny in the context of the evolving Banff AMR diagnostic classification system and refinement of recommendations for histocompatibility testing by the Sensitization in Transplantation Assessment of Risk (STAR) workgroup. This mini-review discusses the latest understanding of pathophysiological mechanisms, clinical evidence for the pathogenicity of AT1R-Ab, and methods of laboratory testing.
Introduction
Criteria for the diagnosis of antibody mediated rejection (AMR) in kidney allografts were first incorporated into the Banff Classification in 2001 and comprise both active and chronic components [1]. Classification criteria for AMR in other solid organ transplants have evolved at different rates [2–5]. Conventionally, donor human leucocyte antigen (HLA) proteins have been understood as the primary target of recipient alloimmune response, and are a major driver of late allograft rejection and loss [6]. However, as has become apparent in recent years, accounting for the HLA system alone is not the panacea for all immune-mediated transplant injury [7]. The latest Banff iteration has considered this gap in immunological understanding with the creation of a subcategory of C4d-negative microvascular inflammation/injury with absence of detectable circulating HLA donor-specific antibodies (HLA-DSA) [8].
In 2005, two significant papers on the concept of immune response in kidney transplantation were published. Opelz et al., in an international study of over 4,000 kidney transplant recipients from HLA-identical sibling donors, demonstrated that the presence of panel reactive antibodies >50% was associated with long-term allograft loss, suggesting that non-HLA antibodies may play a role in chronic rejection [9]. Separately, Dragun et al. reported the presence of agonistic IgG1 and IgG3 antibodies to angiotensin II type-1 receptor (AT1R) in the sera of kidney transplant recipients who had vascular rejection refractory to steroid treatment [10].
Since then, specific and sensitive tests for HLA-DSA have been developed and AT1R antibodies (AT1R-Ab) have become the most widely studied non-HLA antibody in transplantation, with conflicting reports on their association with allograft outcomes [11–14]. A comprehensive review of Dragun et al.’s contribution to our understanding of AT1R in transplantation was recently published [15]. This mini-review aims to highlight the latest research on pathophysiological mechanisms; to discuss methods of laboratory testing; and to outline current gaps in knowledge and potential for future research (Table 1).
Current Understanding of Pathophysiology of AT1R-Ab Mediated Rejection and Endothelial Injury
Angiotensin II, a potent vasoconstrictor that influences endothelial function, inflammation and fibrosis, primarily mediates its effect through AT1R, a G-protein coupled receptor (GPCR) [16–18]. Expression of AT1R is widespread but not ubiquitous, and concentrations on cell membranes fluctuate dependent on genetic and environmental factors [19]. AT1R-Ab function as receptor agonists [19]. They may be present at the time of organ transplantation, or develop de novo after transplantation.
Kidney transplant histological features in the context of AT1R-Ab positivity have been reported. Min et al. report that glomerulitis and peritubular capillaritis were the commonest biopsy findings amongst AT1R-Ab positive recipients [20]. In another cohort of 65 paediatric recipients, AT1R-Ab were associated with the presence of glomerulitis or arteritis [21]. In a prospective study, Lefaucheur et al. contemporaneously assessed AT1R-Ab and HLA-DSA serostatus at the time of indication and surveillance biopsies at or within 1 year of transplantation in 1,845 people. Recipients positive for HLA-DSA plus AT1R-Ab had the lowest allograft survival. Higher levels of circulating AT1R-Ab were associated with glomerultis, peritubular capillaritis, and intimal arteritis. Among recipients with histological rejection, AT1R-Ab positivity was associated with lower prevalence of complement deposition in peritubular capillaries (p < 0.001) [22]. This circumstantial evidence that AT1R-Ab can mediate vascular injury in a manner independent of complement corroborates the index cohort of Dragun et al., but is not a histological finding borne out uniformly in all studies.
Mechanistic studies have highlighted cellular signalling mechanisms influenced by AT1R-Ab. Catar et al. treated human microvascular cells with AT1R-Ab that had been isolated from seropositive patients with transplant vasculopathy. AT1R receptor signalling was sustained via beta2-arrestin recruitment to the cell membrane and mTOR complexes were activated with consequent impairment of endothelial repair capability [23]. These effects were terminated with pharmacological mTOR inhibition. Moll et al. determined that IgG derived from sera of kidney transplant recipients with vasculopathy stimulated secretion of tumour necrosis factor alpha from human microvascular endothelial cells with subsequent THP-1 monocyte activation [24]. The same effect was not demonstrated using IgG derived from the sera of a control cohort. Although the investigators do not explicitly state that AT1R-ab are implicated, this pro-inflammatory mechanism is proposed to act via GPCR-directed PAR1 signalling [24]. These in vitro models offer potential targets for therapeutic intervention.
Clinical Studies of AT1R Antibodies in Solid Organ Transplantation
Most studies of AT1R-Ab have been undertaken in kidney transplant recipients (Table 2). In 2022, a meta-analysis by Kang et al of 21 studies concluded that recipients with AT1R-Ab were at greater risk of AMR (RR 1.96, 95% CI 1.61–2.33) and allograft failure (RR 2.37, 95% CI 1.50–3.75) [25]. The studies varied in size, but three, one of which has already been discussed [22], were notably larger. In a longitudinal study of 351 recipients, using positivity threshold >15 u/mL, Taniguchi reported that de novo AT1R-Ab and dual AT1R-Ab plus HLA-DSA positivity were associated with allograft loss [12]. Giral et al., in a cohort of 599 at a threshold of >10 U/mL, reported AT1R-Ab positivity in 47.2% of participants at time of transplantation, who had a 2.6 fold greater risk of allograft failure beyond 3 years [11]. Not included in the meta-analysis, Deltombe et al., using a positivity threshold of >10 u/mL, found no association between AT1R-Ab status and transplant outcomes in a cohort of 387 patients [13]. In 62 paediatric recipients, AT1R-Ab was associated with AMR using a positivity threshold of 9.5 u/mL [26]. More recently, two observational studies using positivity threshold of >17 u/mL did not show clear association with AMR [27, 28].
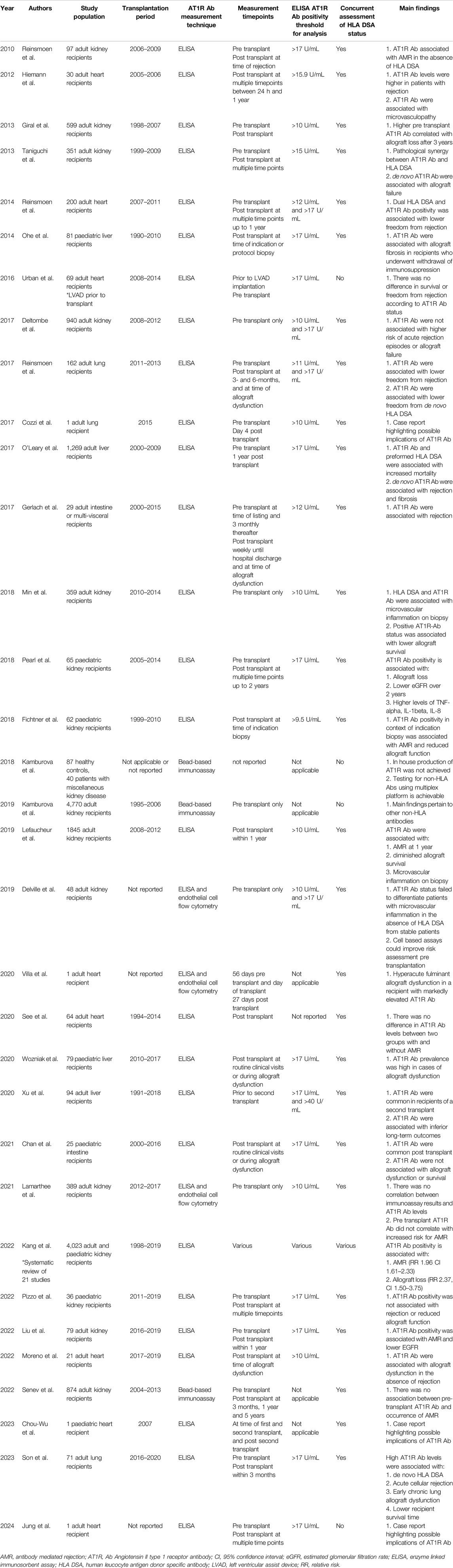
Table 2. Studies of angiotensin II type 1 receptor antibody (AT1R Ab) in solid organ transplantation.
In heart transplantation, a prospective study of 30 recipients demonstrated that persistently elevated AT1R-Ab levels were associated with AMR and microvasculopathy [29]. Elevated AT1R-Ab were reported in a cohort of 21 recipients with allograft dysfunction in the absence of AMR [30]. In a cohort of 200 recipients, concomitant de novo HLA-DSA and AT1R-Ab were associated with diminished freedom from AMR, and recipients with rising AT1R-Ab titres were more likely to be diagnosed with cardiac allograft vasculopathy (CAV) [31]. Characteristically, CAV does not often respond to conventional treatment of coronary artery disease and is a common cause of cardiac allograft failure [32]. In two studies of patients who underwent bridging to transplantation with a left ventricular assist device (LVAD), one reported that 63.8% of participants tested negative for AT1R-Ab initially and subsequently became positive post-LVAD, and another reported that higher titres of AT1R-Ab at time of transplant had worse outcomes [33, 34]. Vascular injury related to LVAD complements the auto-antigen exposure and auto-antibody recruitment theory previously described [35]. A number of illustrative case reports have been published in the literature: a fatal case of hyperacute AMR in a recipient with no HLA-DSA but high AT1R-Ab levels on the day of transplantation [36]; a paediatric case whereby a second allograft was lost in context of high AT1R-Ab [37]; and a case of AMR, CAV and persistently elevated AT1R-Ab levels despite repeated plasmapheresis, intravenous immunoglobulin and steroids [38]. Conversely, when testing for an array of 44 non-HLA antibodies in a cohort of 64 heart transplant recipients, 67% of whom had AMR, no association with AT1R-Ab was found [39]. In light of these findings, a recent Banff report specifically discussed the role of non-HLA antibodies such as AT1R-Ab in heart transplantation and advocated for development of standardized diagnostic tests, and prospective clinical trials to explore role in rejection and assess efficacy of treatments [3].
Regarding lung transplantation, in a multi-centre study of 162 recipients, 46% were positive for AT1R-Ab prior to transplantation, and frequency of de novo HLA-DSA and AMR was greater in those positive for AT1R-Ab [40]. In a cystic fibrosis patient, rapid onset AMR ensued in the absence of HLA-DSA but positive AT1R-Ab serostatus [41]. In 71 recipients, chronic lung allograft dysfunction at 3 years was more common in the AT1R-Ab positive group compared to the negative (58.3% vs. 11.8%, p < 0.001) [42].
Conventionally, liver allografts are perceived as lower immunological risk than other solid organ transplants. In 81 paediatric patients who received living donor transplants and subsequently had withdrawal of immunosuppression, AT1R-Ab >17 u/mL was evident in 65% of patients with advanced fibrosis, a greater proportion than those without fibrosis (p = 0.02) [43]. Two pertinent findings from a large study of 1,269 liver transplant recipients who had sera tested for AT1R-Ab and HLA-DSA, found that de novo AT1R-Ab was associated with increased risk of rejection and fibrosis progression, and histology from the de novo AT1R-Ab subgroup showed distinctive sinusoidal C4d staining spatially related to activated stellate cells [44]. In 79 paediatric recipients, those with active allograft dysfunction were more likely to be AT1R-Ab positive compared to those with stable function (89% vs. 29%, p = 0.001). Those with both AT1R-Ab and HLA-DSA were more likely to progress to allograft loss [45]. Regarding 94 patients receiving a second liver transplant, 51.1% had AT1R-Ab >17 u/mL at time of second transplant, and those with an AT1R-Ab level >40 u/mL were more likely to experience allograft loss [46].
As regards intestinal transplantation, in 29 recipients AMR was more common in those with positive AT1R-Ab versus those without (55% vs. 11%, p < 0.01) [47]. In 25 paediatric recipients, 68% had AT1R-Ab >17 u/mL pre-transplant; these levels did not vary significantly when sera were tested sequentially, and there was no association with allograft dysfunction. No explicit comment was made regarding AMR [48].
Current Techniques in Testing for AT1R-Ab and Related Problems
The most commonly used testing platform for AT1R-Ab is the enzyme linked immunosorbent assay (ELISA) available from CellTrend GmbH Luckenwalde Germany which can test 40 serum samples in 1 run. AT1R is pre-coated on the microtiter plate. During the first incubation, AT1R-Ab in samples are immobilized on the plate and detected with labelled anti-human IgG. The intensity of the colour in the subsequent enzymatic substrate reaction correlates with the concentration of AT1R-Ab. Reinsmoen et al. were the first to use this technique in a clinical study [49].
Senev et al. used the solid-phase Luminex assay to retrospectively test pre- and post-transplantation sera of 874 recipients for 82 different non-HLA antibodies including AT1R-Ab. There was an association between the burden of pretransplant non-HLA antibodies and development of AMR without HLA-DSA (HR 1.3 per 10 antibodies p = 0.02) and microvascular inflammation (HR 1.13 per 10 antibodies p = 0.04). Only four antigens were identified as independent risk factors for AMR histology, AT1R was not among them [50]. Kamburova et al created a solid-phase assay to test sera for 14 specific non-HLA antibodies. Production of AT1R proteins was hampered by protein cleavage before excretion in the culture supernatant, therefore AT1R-Ab was largely excluded from reported results [51]. Nevertheless, in a study by the same group, AT1R was included as an antigen in the same assay screening for non-HLA antibodies in pre-transplant sera of 4,770 recipients; no association between AT1R-Ab status and graft survival was observed [14].
Alternatives to solid-phase assays have been reported. Delville et al. used a cell-based crossmatch assay to identify pre-formed IgG antibodies to glomerular endothelial cells in a small cohort of highly selected transplant recipients. All participants had microvascular inflammation on early biopsies but no circulating HLA-DSA; 26% were positive for AT1R-Ab at threshold 10 U/mL [52]. Lamarthee et al. refined this strategy by using CRISPR/Cas9 to render glomerular endothelial cells devoid of HLA -A-B-C & -DR expression to develop a non-HLA antibody detection immunoassay (NHADIA). In an unselected cohort of 389 recipients, pre-transplant NHADIA values were associated with AMR histology (p = 0.0082) and microvascular inflammation (0.0024). However, using a positivity threshold of 10 U/mL and presumably measured using ELISA technique, there was no correlation between AT1R-Ab levels and NHADIA values [53]. In a novel approach, Lammerts et al. isolated endothelial cells from the perfusion fluid of 102 donor kidneys and propagated a biobank of machine perfusion-derived primary renal endothelial cells (MP-PRECs) to primarily study anti-HLA mediated cytotoxicity, but noted the technique could have utility for investigating non-HLA antibody mediated disease also [54]. Of course, in the context of studying AT1R-Ab, this will be contingent on MP-PRECs expressing AT1R.
Current Research Gaps and Potential Future Directions
The role of AT1R-Ab in transplantation continues to be investigated. In vitro mechanistic studies have outlined how AT1R-Ab influence signalling pathways to cause vascular injury. A steadily growing number of small to medium sized clinical studies highlight a link between AT1R-Ab status, both pre-transplant and de novo, and development of AMR, with possible pathological synergy in cases of concomitant HLA-DSA positivity. These studies are hampered by heterogeneous study design and inconsistent outcome reporting with variable thresholds of antibody positivity. A large, prospective two-centre study using solid-phase ELISA assay firmly established a link between AT1-Ab and a phenotype of AMR, but this link has not been borne out in similarly large retrospective studies using cell-based assays. There is no explanation for this discordance at present. The Bradford Hill criteria should be borne in mind when considering these and future studies investigating the role of non-HLA antibodies in causality of AMR [55].
Regarding ELISA specifically, a particular advantage is the capacity to bulk test; for instance, screening the stored sera of a transplant wait-list population. There are a number of disadvantages. For interpolation of results, a standard curve must be created for each ELISA kit. This introduces a degree of inter-assay variability making comparison of results between kits difficult. If antibody titres in a sample are high and the result is beyond the upper limit of the standard curve, the test needs to be performed again at greater dilution factor to obtain a discrete concentration value. Furthermore, as highlighted by Kamburova et al., details of the manufacturing processes of commercially available ELISA assays are obscure, preventing in-house replication of these assays and necessary reagents [51].
In heart transplantation, CAV is considered a manifestation of chronic rejection and is associated with both non-HLA antibodies and HLA-DSA [56]. As a medium-sized vasculopathy, it is possible that CAV is analogous with some types of the morphologically heterogenous radiologic abnormality of transplant renal artery stenosis (TRAS). Kidney recipients with a post-anastomotic TRAS lesion are more likely to have de novo class II HLA-DSA, suggesting a possible immune-mediated pathological process for some [57]. No studies assessing the relationship between AT1R-Ab and TRAS have been performed. In liver transplantation, reports of a distinctive histological pattern of sinusoidal C4d staining are in contrast to the reports of C4d negativity in many kidney studies. Mechanistic studies to date have elucidated injurious aberrations to cell-signalling pathways, but perhaps it is too soon to discount a potential complement-mediated mechanism also.
The creation in the Banff 2022 report of the descriptive phenotype microvascular injury, C4d-negative, anti-HLA DSA-negative has focussed the minds of researchers on finding a cause. The burgeoning number of non-HLA antibodies that have been studied and subsequently implicated in allograft injury represents both an opportunity and a challenge for transplantation medicine. Given the broad array of potential antigenic targets, there has been no recommendation to test for specific non-HLA antibodies as part of a transplant recipient’s immunological evaluation [58]. The Sensitization in Transplantation: Assessment of Risk (STAR) workgroup, initially established to aggregate cross-discipline knowledge on HLA histocompatabilty, has turned its attention to non-HLA antibodies in its latest report [59]. Acknowledging that studies to date have not firmly established temporal causality between AT1R-Ab status and development of AMR, the report nevertheless advocates for the development of standardized high-throughput testing for non-HLA antibodies [59]. Indeed, given that treatment options such as pharmacological receptor blockade and plasmapheresis have been reported as beneficial, it could be argued that access to validated testing for AT1R-Ab is an unmet clinical need for transplant recipients. For strategies to mitigate the potential pathologic effects of non-HLA antibodies in transplantation, readers are referred to the review by Kardol-Hoefnagel and Otten [60].
Large prospective studies are likely to be required to determine the clinically significant threshold of AT1R-Ab positivity. To take a pragmatic approach, expanding testing capabilities for patients on the transplant wait list first could be prioritised to allow us to better understand the epidemiological burden of AT1R-Ab positivity prior to transplantation, stratify patients at risk of developing chronic rejection related to AT1R-Ab, and identify recruits to prospective randomised controlled trials of therapeutic intervention.
Author Contributions
PM wrote the article. MW and CR reviewed and edited the article. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. MW and CR are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13280/full#supplementary-material
References
1. Racusen, LC, Colvin, RB, Solez, K, Mihatsch, MJ, Halloran, PF, Campbell, PM, et al. Antibody-Mediated Rejection Criteria - An Addition to the Banff 97 Classification of Renal Allograft Rejection. Am J Transpl (2003) 3(6):708–14. doi:10.1034/j.1600-6143.2003.00072.x
2. Berry, GJ, Burke, MM, Andersen, C, Bruneval, P, Fedrigo, M, Fishbein, MC, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the Standardization of Nomenclature in the Pathologic Diagnosis of Antibody-Mediated Rejection in Heart Transplantation. J Heart Lung Transpl (2013) 32(12):1147–62. doi:10.1016/j.healun.2013.08.011
3. Fedrigo, M, Berry, GJ, Coutance, G, Reed, EF, Lin, CY, Giarraputo, A, et al. Report of the 2022 Banff Heart Concurrent: Focus on Non-Human Leukocyte Antigen Antibodies in Rejection and the Pathology of “Mixed” Rejection. Am J Transpl (2023) 24:533–41. doi:10.1016/j.ajt.2023.10.004
4. Roux, A, Levine, DJ, Zeevi, A, Hachem, R, Halloran, K, Halloran, PF, et al. Banff Lung Report: Current Knowledge and Future Research Perspectives for Diagnosis and Treatment of Pulmonary Antibody-Mediated Rejection (AMR). Am J Transpl (2019) 19(1):21–31. doi:10.1111/ajt.14990
5. Bellamy, COC, O'Leary, JG, Adeyi, O, Baddour, N, Batal, I, Bucuvalas, J, et al. Banff 2022 Liver Group Meeting Report: Monitoring Long-Term Allograft Health. Am J Transpl (2024) 24:905–17. doi:10.1016/j.ajt.2024.03.008
6. Nankivell, BJ, and Alexander, SI. Rejection of the Kidney Allograft. N Engl J Med (2010) 363(15):1451–62. doi:10.1056/NEJMra0902927
7. Jackson, AM, Sigdel, TK, Delville, M, Hsieh, SC, Dai, H, Bagnasco, S, et al. Endothelial Cell Antibodies Associated With Novel Targets and Increased Rejection. J Am Soc Nephrol (2015) 26(5):1161–71. doi:10.1681/ASN.2013121277
8. Naesens, M, Roufosse, C, Haas, M, Lefaucheur, C, Mannon, RB, Adam, BA, et al. The Banff 2022 Kidney Meeting Report: Reappraisal of Microvascular Inflammation and the Role of Biopsy-Based Transcript Diagnostics. Am J Transpl (2024) 24(3):338–49. doi:10.1016/j.ajt.2023.10.016
9. Opelz, G, Collaborative Transplant Study. Non-HLA Transplantation Immunity Revealed by Lymphocytotoxic Antibodies. Lancet. (2005) 365(9470):1570–6. doi:10.1016/S0140-6736(05)66458-6
10. Dragun, D, Müller, DN, Bräsen, JH, Fritsche, L, Nieminen-Kelhä, M, Dechend, R, et al. Angiotensin II Type 1-Receptor Activating Antibodies in Renal-Allograft Rejection. N Engl J Med (2005) 352(6):558–69. doi:10.1056/NEJMoa035717
11. Giral, M, Foucher, Y, Dufay, A, Duong Van Huyen, JP, Renaudin, K, Moreau, A, et al. Pretransplant Sensitization Against Angiotensin II Type 1 Receptor Is a Risk Factor for Acute Rejection and Graft Loss. Am J Transpl (2013) 13(10):2567–76. doi:10.1111/ajt.12397
12. Taniguchi, M, Rebellato, LM, Cai, J, Hopfield, J, Briley, KP, Haisch, CE, et al. Higher Risk of Kidney Graft Failure in the Presence of Anti-Angiotensin II Type-1 Receptor Antibodies. Am J Transpl (2013) 13(10):2577–89. doi:10.1111/ajt.12395
13. Deltombe, C, Gillaizeau, F, Anglicheau, D, Morelon, E, Trébern-Launay, K, Le Borgne, F, et al. Is Pre-Transplant Sensitization Against Angiotensin II Type 1 Receptor Still a Risk Factor of Graft and Patient Outcome in Kidney Transplantation in the Anti-HLA Luminex Era? A Retrospective Study. Transpl Int (2017) 30(11):1150–60. doi:10.1111/tri.13009
14. Kamburova, EG, Gruijters, ML, Kardol-Hoefnagel, T, Wisse, BW, Joosten, I, Allebes, WA, et al. Antibodies Against ARHGDIB Are Associated With Long-Term Kidney Graft Loss. Am J Transpl (2019) 19(12):3335–44. doi:10.1111/ajt.15493
15. Lefaucheur, C, Louis, K, Philippe, A, Loupy, A, and Coates, PT. The Emerging Field of Non-Human Leukocyte Antigen Antibodies in Transplant Medicine and Beyond. Kidney Int (2021) 100(4):787–98. doi:10.1016/j.kint.2021.04.044
16. Zaman, MA, Oparil, S, and Calhoun, DA. Drugs Targeting the Renin-Angiotensin-Aldosterone System. Nat Rev Drug Discov (2002) 1(8):621–36. doi:10.1038/nrd873
17. Zhang, H, Unal, H, Gati, C, Han, GW, Liu, W, Zatsepin, NA, et al. Structure of the Angiotensin Receptor Revealed by Serial Femtosecond Crystallography. Cell (2015) 161(4):833–44. doi:10.1016/j.cell.2015.04.011
18. Balakumar, P, and Jagadeesh, G. Structural Determinants for Binding, Activation, and Functional Selectivity of the Angiotensin AT1 Receptor. J Mol Endocrinol (2014) 53(2):R71–92. doi:10.1530/JME-14-0125
19. Philogene, MC, Johnson, T, Vaught, AJ, Zakaria, S, and Fedarko, N. Antibodies Against Angiotensin II Type 1 and Endothelin A Receptors: Relevance and Pathogenicity. Hum Immunol (2019) 80(8):561–7. doi:10.1016/j.humimm.2019.04.012
20. Min, JW, Lee, H, Choi, BS, Park, CW, Yang, CW, Kim, YS, et al. Clinical Impact of Pre-Transplant Antibodies Against Angiotensin II Type I Receptor and Major Histocompatibility Complex Class I-Related Chain A in Kidney Transplant Patients. Ann Lab Med (2018) 38(5):450–7. doi:10.3343/alm.2018.38.5.450
21. Pearl, MH, Zhang, Q, Palma Diaz, MF, Grotts, J, Rossetti, M, Elashoff, D, et al. Angiotensin II Type 1 Receptor Antibodies Are Associated With Inflammatory Cytokines and Poor Clinical Outcomes in Pediatric Kidney Transplantation. Kidney Int (2018) 93(1):260–9. doi:10.1016/j.kint.2017.06.034
22. Lefaucheur, C, Viglietti, D, Bouatou, Y, Philippe, A, Pievani, D, Aubert, O, et al. Non-HLA Agonistic Anti-Angiotensin II Type 1 Receptor Antibodies Induce a Distinctive Phenotype of Antibody-Mediated Rejection in Kidney Transplant Recipients. Kidney Int (2019) 96(1):189–201. doi:10.1016/j.kint.2019.01.030
23. Catar, RA, Wischnewski, O, Chen, L, Heidecke, H, Rutz, C, Schülein, R, et al. Non-HLA Antibodies Targeting Angiotensin II Type 1 Receptor and Endothelin-1 Type A Receptors Induce Endothelial Injury via β2-Arrestin Link to mTOR Pathway. Kidney Int (2022) 101(3):498–509. doi:10.1016/j.kint.2021.09.029
24. Moll, G, Luecht, C, Gyamfi, MA, da Fonseca, DLM, Wang, P, Zhao, H, et al. Autoantibodies From Patients With Kidney Allograft Vasculopathy Stimulate a Proinflammatory Switch in Endothelial Cells and Monocytes Mediated via GPCR-Directed PAR1-TNF-α Signaling. Front Immunol (2023) 14:1289744. doi:10.3389/fimmu.2023.1289744
25. Kang, ZY, Liu, C, Liu, W, and Li, DH. Effect of Anti-Angiotensin II Type 1 Receptor Antibodies on the Outcomes of Kidney Transplantation: A Systematic Review and Meta-Analysis. Nephrol Dial Transpl (2022) 37(6):1171–80. doi:10.1093/ndt/gfab344
26. Fichtner, A, Süsal, C, Schröder, C, Höcker, B, Rieger, S, Waldherr, R, et al. Association of Angiotensin II Type 1 Receptor Antibodies With Graft Histology, Function and Survival in Paediatric Renal Transplant Recipients. Nephrol Dial Transpl (2018) 33(6):1065–72. doi:10.1093/ndt/gfy008
27. Pizzo, H, Mirocha, J, Choi, J, Garrison, J, Haas, M, Zhang, X, et al. Pre-Transplant Angiotensin II Receptor Type I Antibodies in Pediatric Renal Transplant Recipients: An Observational Cohort Study. Pediatr Transpl (2022) 26(8):e14400. doi:10.1111/petr.14400
28. Liu, C, Kang, ZY, Yin, Z, Xiao, Y, Liu, W, Zhao, Y, et al. Levels of Angiotensin II Type-1 Receptor Antibodies and Endothelin-1 Type-A Receptor Antibodies Correlate With Antibody-Mediated Rejection and Poor Graft Function in Kidney-Transplantation Patients. Transpl Immunol (2022) 74:101674. doi:10.1016/j.trim.2022.101674
29. Hiemann, NE, Meyer, R, Wellnhofer, E, Schoenemann, C, Heidecke, H, Lachmann, N, et al. Non-HLA Antibodies Targeting Vascular Receptors Enhance Alloimmune Response and Microvasculopathy After Heart Transplantation. Transplantation (2012) 94(9):919–24. doi:10.1097/TP.0b013e3182692ad2
30. Moreno, JD, Verma, AK, Kopecky, BJ, Dehner, C, Kostelecky, N, Vader, JM, et al. Angiotensin II Type 1 Receptor Antibody-Mediated Rejection Following Orthotopic Heart Transplant: A Single-Center Experience. Transplantation (2022) 106(2):373–80. doi:10.1097/TP.0000000000003712
31. Reinsmoen, NL, Lai, CH, Mirocha, J, Cao, K, Ong, G, Naim, M, et al. Increased Negative Impact of Donor HLA-Specific Together With Non-HLA-Specific Antibodies on Graft Outcome. Transplantation (2014) 97(5):595–601. doi:10.1097/01.TP.0000436927.08026.a8
32. Nikolova, AP, and Kobashigawa, JA. Cardiac Allograft Vasculopathy: The Enduring Enemy of Cardiac Transplantation. Transplantation (2019) 103(7):1338–48. doi:10.1097/TP.0000000000002704
33. Urban, M, Slavcev, A, Gazdic, T, Ivak, P, Besik, J, and Netuka, I. The Impact of Angiotensin II Type 1 Receptor Antibodies on Post-Heart Transplantation Outcome in Heart Mate II Bridged Recipients. Interact Cardiovasc Thorac Surg (2016) 22(3):292–7. doi:10.1093/icvts/ivv344
34. Chau, VQ, Flattery, M, Nicholson, KS, McDougan, F, Gupta, G, Uber, P, et al. Elevated AT1R Antibody and Morbidity in Patients Bridged to Heart Transplant Using Continuous Flow Left Ventricular Assist Devices. J Card Fail (2020) 26(11):959–67. doi:10.1016/j.cardfail.2020.06.010
35. Cardinal, H, Dieudé, M, and Hébert, MJ. The Emerging Importance of Non-HLA Autoantibodies in Kidney Transplant Complications. J Am Soc Nephrol (2017) 28(2):400–6. doi:10.1681/ASN.2016070756
36. Villa, C, Mesa, K, Cristy Smith, M, Mooney, DM, Coletti, A, and Klohe, E. Hyperacute Graft Dysfunction in an Orthotopic Heart Transplant in the Presence of Non-HLA Antibodies. Am J Transpl (2020) 20(2):593–9. doi:10.1111/ajt.15564
37. Chou-Wu, E, Kemna, M, Ross, S, Youngs, D, Law, Y, and Gimferrer, I. Association of MICA and AT1R Antibodies With Antibody-Mediated Rejection and Cardiac Allograft Vasculopathy in a Pediatric Heart Transplant Recipient. Transpl Immunol (2023) 78:101811. doi:10.1016/j.trim.2023.101811
38. Jung, R, Ly, K, Taniguchi, M, Arriola, AG, Gravante, C, Shinn, D, et al. Improved Graft Function Following Desensitization of Anti-AT(1)R and Autoantibodies in a Heart Transplant Recipient Negative for Donor-Specific Antibodies With Antibody-Mediated Rejection: A Case Report. Int J Mol Sci (2024) 25(4):2218. doi:10.3390/ijms25042218
39. See, SB, Mantell, BS, Clerkin, KJ, Ray, B, Vasilescu, ER, Marboe, CC, et al. Profiling Non-HLA Antibody Responses in Antibody-Mediated Rejection Following Heart Transplantation. Am J Transpl (2020) 20(9):2571–80. doi:10.1111/ajt.15871
40. Reinsmoen, NL, Mirocha, J, Ensor, CR, Marrari, M, Chaux, G, Levine, DJ, et al. A 3-Center Study Reveals New Insights Into the Impact of Non-HLA Antibodies on Lung Transplantation Outcome. Transplantation (2017) 101(6):1215–21. doi:10.1097/TP.0000000000001389
41. Cozzi, E, Calabrese, F, Schiavon, M, Feltracco, P, Seveso, M, Carollo, C, et al. Immediate and Catastrophic Antibody-Mediated Rejection in a Lung Transplant Recipient With Anti-Angiotensin II Receptor Type 1 and Anti-Endothelin-1 Receptor Type A Antibodies. Am J Transpl (2017) 17(2):557–64. doi:10.1111/ajt.14053
42. Son, BS, Lee, HJ, Cho, WH, So, MW, Park, JM, and Yeo, HJ. Association of Positive Pre-Transplant Angiotensin II Type 1 Receptor Antibodies With Clinical Outcomes in Lung Transplant Recipients. Transpl Immunol (2023) 80:101901. doi:10.1016/j.trim.2023.101901
43. Ohe, H, Uchida, Y, Yoshizawa, A, Hirao, H, Taniguchi, M, Maruya, E, et al. Association of Anti-Human Leukocyte Antigen and Anti-Angiotensin II Type 1 Receptor Antibodies With Liver Allograft Fibrosis After Immunosuppression Withdrawal. Transplantation (2014) 98(10):1105–11. doi:10.1097/TP.0000000000000185
44. OʼLeary, JG, Demetris, AJ, Philippe, A, Freeman, R, Cai, J, Heidecke, H, et al. Non-HLA Antibodies Impact on C4d Staining, Stellate Cell Activation and Fibrosis in Liver Allografts. Transplantation (2017) 101(10):2399–409. doi:10.1097/TP.0000000000001853
45. Wozniak, LJ, Hickey, MJ, Chan, AP, Venick, RS, Farmer, DG, Busuttil, RW, et al. Angiotensin II Type-1 Receptor Antibodies Are Associated With Active Allograft Dysfunction Following Pediatric Liver Transplantation. Transplantation (2020) 104(12):2547–56. doi:10.1097/TP.0000000000003206
46. Xu, Q, McAlister, VC, Leckie, S, House, AA, Skaro, A, and Marotta, P. Angiotensin II Type I Receptor Agonistic Autoantibodies Are Associated With Poor Allograft Survival in Liver Retransplantation. Am J Transpl (2020) 20(1):282–8. doi:10.1111/ajt.15571
47. Gerlach, UA, Lachmann, N, Ranucci, G, Sawitzki, B, Schoenemann, C, Pratschke, J, et al. Non-HLA Antibodies May Accelerate Immune Responses After Intestinal and Multivisceral Transplantation. Transplantation (2017) 101(1):141–9. doi:10.1097/TP.0000000000001439
48. Chan, AP, Guerra, MR, Rossetti, M, Hickey, MJ, Venick, RS, Marcus, EA, et al. Non-HLA AT1R Antibodies Are Highly Prevalent After Pediatric Intestinal Transplantation. Pediatr Transpl (2021) 25(3):e13987. doi:10.1111/petr.13987
49. Reinsmoen, NL, Lai, CH, Heidecke, H, Haas, M, Cao, K, Ong, G, et al. Anti-Angiotensin Type 1 Receptor Antibodies Associated With Antibody Mediated Rejection in Donor HLA Antibody Negative Patients. Transplantation (2010) 90(12):1473–7. doi:10.1097/TP.0b013e3181fd97f1
50. Senev, A, Ray, B, Lerut, E, Hariharan, J, Heylen, C, Kuypers, D, et al. The Pre-Transplant Non-HLA Antibody Burden Associates With the Development of Histology of Antibody-Mediated Rejection After Kidney Transplantation. Front Immunol (2022) 13:809059. doi:10.3389/fimmu.2022.809059
51. Kamburova, EG, Kardol-Hoefnagel, T, Wisse, BW, Joosten, I, Allebes, WA, van der Meer, A, et al. Development and Validation of a Multiplex Non-HLA Antibody Assay for the Screening of Kidney Transplant Recipients. Front Immunol (2018) 9:3002. doi:10.3389/fimmu.2018.03002
52. Delville, M, Lamarthée, B, Pagie, S, See, SB, Rabant, M, Burger, C, et al. Early Acute Microvascular Kidney Transplant Rejection in the Absence of Anti-HLA Antibodies Is Associated With Preformed IgG Antibodies Against Diverse Glomerular Endothelial Cell Antigens. J Am Soc Nephrol (2019) 30(4):692–709. doi:10.1681/ASN.2018080868
53. Lamarthée, B, Burger, C, Leclaire, C, Lebraud, E, Zablocki, A, Morin, L, et al. CRISPR/Cas9-Engineered HLA-Deleted Glomerular Endothelial Cells as a Tool to Predict Pathogenic Non-HLA Antibodies in Kidney Transplant Recipients. J Am Soc Nephrol (2021) 32(12):3231–51. doi:10.1681/ASN.2021050689
54. Lammerts, RGM, Lagendijk, LM, Tiller, G, Dam, WA, Lancaster, HL, Daha, MR, et al. Machine-Perfused Donor Kidneys as a Source of Human Renal Endothelial Cells. Am J Physiol Ren Physiol (2021) 320(5):F947–F962. doi:10.1152/ajprenal.00541.2020
55. Fedak, KM, Bernal, A, Capshaw, ZA, and Gross, S. Applying the Bradford Hill Criteria in the 21st Century: How Data Integration Has Changed Causal Inference in Molecular Epidemiology. Emerg Themes Epidemiol (2015) 12:14. doi:10.1186/s12982-015-0037-4
56. Gates, KV, Pereira, NL, and Griffiths, LG. Cardiac Non-Human Leukocyte Antigen Identification: Techniques and Troubles. Front Immunol (2017) 8:1332. doi:10.3389/fimmu.2017.01332
57. Willicombe, M, Sandhu, B, Brookes, P, Gedroyc, W, Hakim, N, Hamady, M, et al. Postanastomotic Transplant Renal Artery Stenosis: Association With De Novo Class II Donor-Specific Antibodies. Am J Transpl (2014) 14(1):133–43. doi:10.1111/ajt.12531
58. Bestard, O, Thaunat, O, Bellini, MI, Böhmig, GA, Budde, K, Claas, F, et al. Alloimmune Risk Stratification for Kidney Transplant Rejection. Transpl Int (2022) 35:10138. doi:10.3389/ti.2022.10138
59. Tambur, AR, Bestard, O, Campbell, P, Chong, AS, Barrio, MC, Ford, ML, et al. Sensitization in Transplantation: Assessment of Risk 2022 Working Group Meeting Report. Am J Transpl (2023) 23(1):133–49. doi:10.1016/j.ajt.2022.11.009
Keywords: AT1R antibody, AT1R, non-HLA antibody, rejection, antibody mediated rejection
Citation: Martin PJP, Willicombe M and Roufosse C (2024) Angiotensin II Type-1 Receptor Antibody in Solid Organ Transplantation – Is It Time to Test?. Transpl Int 37:13280. doi: 10.3389/ti.2024.13280
Received: 20 May 2024; Accepted: 15 October 2024;
Published: 13 November 2024.
Copyright © 2024 Martin, Willicombe and Roufosse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul James Patrick Martin, cGF1bC5tYXJ0aW45QG5ocy5uZXQ=
 Paul James Patrick Martin
Paul James Patrick Martin Michelle Willicombe1,2
Michelle Willicombe1,2 Candice Roufosse
Candice Roufosse