Abstract
As transplant programmes have evolved to allow a wider donor pool, organ acceptance decisions have become increasingly complex and lack transparency and equality. Clinical scoring tools exist but there is limited consensus on their use. From a prospective observation of consecutive deceased-donor kidney offers in a large urban transplant centre, a simple score was developed based on donor age and other risk characteristics, excluding ischemia time and graft histology. The score was validated in subsequent cohorts of consecutive offers in the United Kingdom and Germany. In the development cohort of 389 kidney offers, 110 (28%) were transplanted and 175 (45%) declined. Nine risk factors were incorporated into a score based on age, but adjusted for the number of risk factors present, making an “adjusted donor age,” with offers separated into equal quintiles by decade. The score was validated in a UK cohort of 380 subsequent offers, and a German cohort of 431 offers. In both cohorts adjusted donor age discriminated between favourable and poor post-transplant outcomes (C-statistic 0.77 in the United Kingdom, 95% CI 0.65–0.88, and 0.71 in Germany, 95% CI 0.64–0.77). Adjusted donor age is a simple score quantifying deceased donor kidney quality, which is consistent with current practice and predicts post-transplant outcome.
Introduction
As transplant programmes evolve to tackle the rising burden of end stage kidney disease, the age and comorbidity of those considered as deceased donors has increased [1, 2]. Organ acceptance decisions are therefore increasingly complex, with practices varying significantly between clinicians and institutions, with limited patient involvement in the decision-making process, leading to a system that lacks transparency and equality [3]. It is a major challenge therefore for clinicians to ensure that the appropriate organ acceptance decisions are made, and that this process is accountable and communicated effectively with patients.
A number of studies have identified donor factors predictive of post-transplant outcome, including donor age and aspects of medical history such as hypertension, stroke, diabetes and kidney function [4]. To support acceptance decisions, clinical tools have been devised which combine several factors into a single numerical score, to indicate quality of the donor kidney, including the Deceased Donor Score [5], Donor Risk Grade [6], Kidney Donor Risk Index [7] and UK Kidney Donor Risk Index [8].
However, scoring systems so far developed have no meaning which is intuitive to patients, and are not calibrated to the donor pool, with over half of all offers placed in the highest category of risk. They provide limited assistance therefore in comparing the current offer to possible future offers, and do little to enhance patient understanding or facilitate a shared decision. There is limited consensus therefore on the use of such tools in the organ acceptance decision process [9]. The hypothesis of this study is that it is possible to develop a valid clinical tool based on age, which can easily be understood by patients, to assist with shared decision making in organ acceptance from deceased kidney donors.
Patients and Methods
In two large urban transplant centres, three sequential studies were carried out. The first was a prospective observation of deceased-donor kidney offers in a single UK centre during 2018, collecting donor and recipient characteristics, decisions and clinical outcomes. A simple score was developed based on donor age and other offer characteristics, termed “adjusted donor age,” and calibrated to separate all offers into quintiles according to quality. The second was a validation of this score in a subsequent UK cohort of consecutive offers. The third was a further validation in a cohort of consecutive offers in Germany. As a retrospective study of routinely available data, the protocols were approved by the National Research Ethics Service (IRAS Ref 308076) in the United Kingdom, and the local Ethics Committee in Germany (S-187/2022) without requirements for individual consent.
Variables were selected from published donor-scoring literature (age, gender, comorbidities, donor cardiac death, cause of death, length of admission, HLA mismatch) supplemented with additional variables that are commonly considered (smoking, alcohol excess, proteinuria, cardiac arrest duration). Efforts were made to distinguish between acute kidney injury (using urine output and creatinine rise from baseline) and baseline creatinine clearance, which was estimated by the Cockcroft-Gault equation, using a simplified formula for adjusted body weight: (weight+70)/2 (male), (weight+55)/2 (female), and using the average of pre-admission, initial-admission and lowest-during-admission for baseline creatinine. Variables were subsequently excluded from consideration if there was limited or conflicting evidence for outcome prediction in prior literature and the development cohort. Ischaemia times were not considered since they are usually unknown at the time of the initial decision, and similarly graft histology was not included since it is so rarely available in either institution.
The unit of analysis was the offer of a donor kidney for a particular (named) recipient. Where both kidneys were offered from the same donor (for different recipients), they were considered as separate offers. In a minority of cases no recipient was specified by the allocation system and clinical teams were able to select any suitable recipient. Kidneys declined for one recipient but also thought unsuitable for any recipient were analysed as a single offer for that recipient. Initial acceptance decisions were made by clinicians only, via joint agreement between a nephrologist and a transplant surgeon. Decline decision types were defined as “exclusion” if due to a single qualitative factor occasionally with recipient involvement in the decision (e.g., recent donor cancer) or as “quality” if due to the combination of quantitative variables such as age, creatinine clearance and comorbidities, usually without involvement of the recipient. Decisions were defined as “other” if initially accepted but then not transplanted due to factors outside the control of the clinical team. Most commonly this would be due to a prolonged agonal phase in donation after circulatory death (DCD) donors, leading to withdrawal of the offer, but sometimes recipient factors were involved, for example, if the recipient was unwell or unavailable.
The influence of variables on acceptance decisions was assessed with Fisher’s exact test comparing transplanted with declined offers. Outcome prediction was assessed by logistic regression with poor outcome defined as organ failure or GFR below 30 mL/min/1.72 m2 at 3 months after transplantation, using an average of three consecutive outpatient creatinine measurements. Analyses were performed using Microsoft Excel, JASP (Jeffreys’ Amazing Statistics Program, JASP Team, 2020) and RStudio (R Team, 2021).
Results
The development cohort consisted of 389 consecutive kidney offers, from 302 deceased donors (aged 6–84). The majority of offers (93%) were for specified recipients (aged 24–78), with donor and recipient characteristics for all offers provided in Table 1. Out of all offers, 110 (28%) were transplanted, 175 (45%) were declined and 104 (27%) initially accepted but then not transplanted due to factors outside the control of the clinical team. Of the 175 offers declined by the clinical team, 43 (11% of all offers) were declined due to an exclusion factor, with the remaining 132 (34% of all offers) declined due to quality concerns.
TABLE 1
| Acceptance decisions (N = 389) | Post-transplant outcome univariate (N = 110) | Selectione | ||||||
|---|---|---|---|---|---|---|---|---|
| All offers | Transplant (110) | Declined (175) | p-valuea | ORb | 95% CI | p-value | ||
| Donor | ||||||||
| Age (years) | 60 (51–71) | 56 (47–67) | 68 (59–76) | 0.001 | 1.07 | 1.03–1.12 | 0.002 | |
| Male gender | 205 (53) | 59 (54) | 84 (48) | 0.330 | 0.40 | 0.18–0.91 | 0.066 | Excluded |
| CrC (mL/min) continuousc | 86 (70–112) | 89 (74–113) | 75 (60–92) | 0.002 | 0.96 | 0.94–0.98 | 0.000 | |
| <70d | 102 (26) | 24 (22) | 67 (38) | 0.006 | 6.61 | 2.75–15.9 | 0.000 | |
| Creatinine ≥100% rise | 42 (11) | 8 (7) | 29 (17) | 0.029 | 0.54 | 0.09–3.31 | 0.579 | |
| Urine <75 mL/h | 143 (37) | 36 (33) | 77 (44) | 0.082 | 4.02 | 1.77–9.11 | 0.005 | |
| Proteinuria (>1+) | 189 (49) | 41 (37) | 113 (65) | 0.000 | 1.11 | 0.43–2.84 | 0.836 | Excluded |
| Hypertension | 168 (43) | 34 (31) | 94 (54) | 0.000 | 2.78 | 1.24–6.24 | 0.037 | |
| Diabetes | 53 (14) | 8 (7) | 31 (18) | 0.014 | 1.35 | 0.33–5.50 | 0.725 | |
| Vascular event | 42 (11) | 2 (2) | 31 (18) | 0.000 | 2.02 | 0.26–15.8 | 0.572 | |
| Alcohol excess | 69 (18) | 18 (16) | 31 (18) | 0.873 | 0.40 | 0.12–1.31 | 0.204 | Excluded |
| Smoking | 158 (41) | 40 (36) | 68 (39) | 0.802 | 0.77 | 0.32–1.88 | 0.631 | Excluded |
| Donor cardiac death | 151 (39) | 35 (32) | 68 (39) | 0.310 | 3.34 | 1.49–7.51 | 0.014 | |
| Stroke (cause of death) | 167 (43) | 46 (42) | 78 (45) | 0.806 | 0.74 | 0.33–1.66 | 0.536 | Excluded |
| Arrest duration >30 min | 67 (17) | 21 (19) | 32 (18) | 0.876 | 0.99 | 0.97–1.01 | 0.444 | Excluded |
| Admission ≥10 days | 41 (11) | 6 (6) | 21 (12) | 0.095 | 4.42 | 1.08–18.0 | 0.082 | |
| HLA mismatch ≥4 | 82 (21) | 24 (22) | 37 (21) | 0.882 | 1.55 | 1.01–2.37 | 0.093 | |
| Recipient (N = 363) | ||||||||
| Age (years) | 56 (48–63) | 55 (47–65) | 56 (47–62) | 0.535 | 1.03 | 0.99–1.08 | 0.114 | |
| Weight (kg) | 75 (66–85) | 74 (64–87) | 75 (67–86) | 0.639 | 1.05 | 1.02–1.09 | 0.003 | |
| CRF > 50% | 80 (22) | 23 (20.9) | 33 (22) | 0.647 | 0.75 | 0.23–2.48 | 0.636 | |
| Wait time (years) | 3.4 (2.1–5.0) | 2.8 (1.7–4.2) | 3.5 (2.2–4.9) | 0.009 | 1.16 | 0.93–1.45 | 0.193 | |
Offer characteristics, decision and outcome post-transplant in the development cohort (N = 389).
Data provided as number (%) or median (IQR), results shaded if p < 0.10. HLA, human leukocyte antigen; CRF, calculated reaction frequency.
p value comparing transplanted with declined.
OR, odds ratio for poor outcome (GFR <30) at 3 months.
CrC, creatinine clearance, as continuous variable.
CrC: creatinine clearance, as threshold dependent on recipient weight: 60 (<65 kg), 70 (65–85 kg), 80 (>85 kg).
Variables excluded as contributors to adjusted donor age are shown (see text).
Several donor characteristics were associated with acceptance decisions including age, creatinine clearance, creatinine rise, urine output, proteinuria, hypertension, diabetes, vascular events and length of the donor’s hospital admission (Table 1). Decisions had to be made within a short time after receiving the offer, with 39% of decisions made between 21:00 and 06:00.
After 3 months, of the 110 recipients transplanted, 87 (79%) had a favourable clinical outcome, whereas 17 had poor transplant function (GFR below 30 mL/min/1.72 m2) and 6 kidneys had permanently failed. Donor characteristics predictive of poor transplant outcome (GFR <30 or failure) included age, gender, creatinine clearance, urine output, hypertension, cardiac death, length of admission and HLA mismatch (Table 1). Greater recipient weight also predicted poor outcome. Some of the counterintuitive relationships between donor factors and transplant outcome may be explained by collinearity between factors (Supplementary Table S1). In a sensitivity analysis, similar prediction characteristics were found using outcome at 12 months post-transplantation (Supplementary Table S2).
Donor variables without predictive ability, which were therefore excluded from further analysis, included proteinuria, alcohol excess, smoking, death from stroke and cardiac arrest duration. Gender was also excluded since its effect disappeared after adjustment for creatinine clearance (calculation of which includes gender). The number of kidneys transplanted from donors with diabetes or prior vascular events was small – these factors, which are increasingly prevalent amongst deceased donors, were retained since they exerted a marked influence on acceptance decisions, and would therefore be under-estimated as predictors of post-transplant outcome. Nine risk factors were therefore incorporated into a score based on age, but adjusted for the number of risk factors present, making an “adjusted donor age” (Figure 1). Thresholds and coefficients were selected to optimise prediction, with risk factors scaled by 4 years, so that the score remains largely age-dependent (like other scores such as KDRI) with 6 years subtracted to centre the distribution. Since recipient weight was a strong predictor of outcome, weight-dependent thresholds were used for donor creatinine clearance.
FIGURE 1
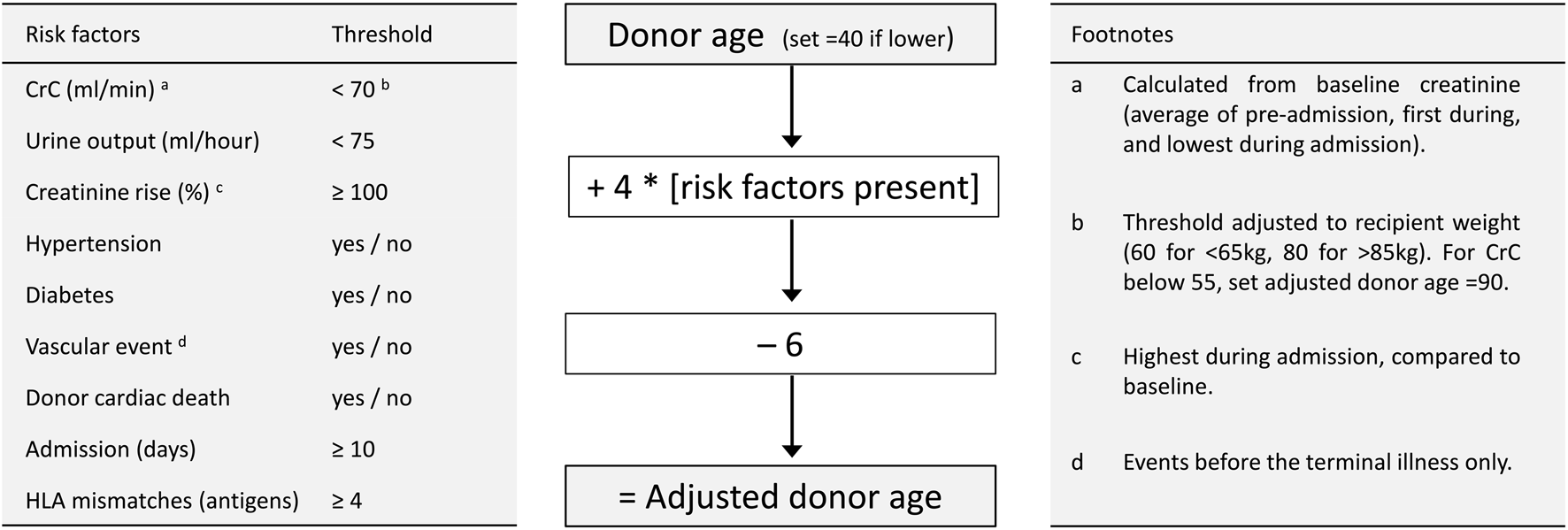
Calculation of adjusted donor age. Left panel describes 9 risk factors with thresholds for their presence. Central panel describes calculation of adjusted donor age. Right panel contains clarifying footnotes. CrC, creatinine clearance (by Cockroft-Gault formula); HLA, human leucocyte antigen.
As expected, the adjusted donor age was still strongly correlated with donor age with coefficient of determination (R2) 0.75, suggesting that 75% of the variation in adjusted donor age was explained by donor age. Age was therefore the dominant determinant of the score, as it is with published scoring systems: calculating the Kidney Donor Risk Index [7] and UK Kidney Donor Risk Index [8] in this cohort, gave coefficients of determination of 0.76 and 0.72, respectively for the relationship with donor age. Using published risk thresholds for either score however, over half of all offers from this cohort fell into the highest risk category, whereas offers were separated approximately into quintiles using decade of adjusted donor age (Table 2).
TABLE 2
| Risk category | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Adjusted donor age | <49 | 50–59 | 60–69 | 70–79 | >80 |
| Offers (number) | 89 (23) | 81 (21) | 62 (16) | 79 (20) | 78 (20) |
| Age (years) | 42 (32–49) | 54 (51–57) | 61 (59–65) | 71 (68–74) | 75 (69–78) |
| Risk factors presenta | 1 (0–2) | 2 (1–2.5) | 2 (2–3) | 3 (2–3) | 4 (3–5) |
| US KDRIb | <0.75 | 0.75–0.91 | 0.91–1.11 | 1.11–1.39 | >1.39 |
| Offers (number) | 28 (7) | 25 (6) | 51 (13) | 67 (17) | 218 (56) |
| Age (years) | 28 (20.5–32.5) | 39 (35–46) | 50 (47–53.5) | 54 (51.5–58) | 70 (64–75) |
| Risk factors presenta | 1.5 (0–3) | 1 (0–2) | 1 (0–3) | 2 (1–2.5) | 3 (2–5) |
| UK KDRIc | <0.87 | 0.87–1.02 | 1.02–1.34 | >1.34 | |
| Offers (number) | 41 (11) | 56 (14) | 85 (22) | 207 (53) | |
| Age (years) | 28 (23–35) | 53.5 (50–56) | 52 (47–55) | 70 (65–76) | |
| Risk factors presenta | 1 (0–2) | 2 (0–2) | 2 (1–3) | 3 (2–5) | |
Offer characteristics by adjusted donor age and established risk scores in the development cohort (N = 389).
Data provided as N (%) or median (IQR). KDRI, kidney donor risk index.
Risk factors are those given in Figure 1.
Rao, Transplantation, 2009, scaled to median offer and using updated quintile boundaries from 2018 USA cohort.
Watson, Transplantation, 2012, using original quartile boundaries from 2000 to 2007 UK cohort.
The adjusted donor age score was validated in separate cohorts from the UK and Germany. The UK validation cohort consisted of 377 consecutive offers, of which 96 (25%) were transplanted, 176 (47%) declined and 105 (28%) initially accepted but then not transplanted. Three months after transplantation, outcomes in this cohort were similar, with a favourable 3-month clinical outcome (GFR above 30 mL/min/1.72 m2) was seen in 78 recipients (81%). All risk factors were validated in this cohort by association with acceptance decisions or outcome or both, and greater recipient weight remained marginally predictive of poor outcome (Table 3).
TABLE 3
| Acceptance decisions (N = 377) | Post-transplant outcome univariate (N = 96) | Post-transplant outcome multivariatea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All offers | Transplant (96) | Declined (176) | p-valueb | ORc | 95% CI | p-value | ORc | 95% CI | p-value | |
| Donor | ||||||||||
| Age (years) | 61 (51–71) | 55 (48–66) | 66 (54–72) | 0.000 | 1.06 | 1.01–1.11 | 0.016 | 1.09 | 1.03–1.16 | 0.006 |
| CrC (mL/min) < 70d | 92 (24) | 21 (22) | 58 (33) | 0.069 | 4.00 | 1.33–12.1 | 0.014 | |||
| Creatinine ≥100% rise | 46 (12) | 8 (8) | 34 (19) | 0.022 | 0.60 | 0.07–5.18 | 0.640 | |||
| Urine <75 mL/h | 183 (49) | 36 (38) | 99 (56) | 0.004 | 1.08 | 0.38–3.08 | 0.893 | |||
| Hypertension | 153 (41) | 30 (31) | 92 (52) | 0.001 | 1.10 | 0.37–3.29 | 0.859 | |||
| Diabetes | 42 (11) | 5 (5) | 34 (19) | 0.001 | 1.00 | 0.00–>100 | 0.993 | |||
| Vascular event | 42 (11) | 3 (3) | 30 (17) | 0.000 | 1.00 | 0.00–>100 | 0.991 | |||
| Donor cardiac death | 189 (50) | 34 (35) | 94 (53) | 0.005 | 2.81 | 0.99–8.01 | 0.053 | |||
| Admission ≥10 days | 31 (8) | 3 (3) | 16 (9) | 0.082 | 9.50 | 0.81–>100 | 0.073 | |||
| HLA mismatch ≥4 | 156 (41) | 49 (51) | 61 (35) | 0.010 | 6.32 | 1.33–30.1 | 0.021 | |||
| Risk factors presente | 1.67 | 1.08–2.58 | 0.020 | 1.72 | 1.02–2.89 | 0.042 | ||||
| Recipient (N = 333) | ||||||||||
| Weight (kg) | 75 (65–88) | 78 (65–89) | 75 (66–90) | 0.729 | 1.02 | 1.00–1.04 | 0.095 | 1.04 | 1.01–1.07 | 0.006 |
Offer characteristics, decision and outcome post-transplant in the UK validation cohort.
Data provided as number (%) or median [IQR], results shaded if p < 0.10. HLA, human leukocyte antigen.
multivariable model adjusted for variables shown.
p value comparing transplanted with declined.
OR, odds ratio for poor outcome (GFR<30) at 3 months.
CrC, creatinine clearance, as threshold dependent on recipient weight: 60 (<65 kg), 70 (65–85 kg), 80 (>85 kg).
Total number of donor risk factors (from the above list) present.
The German validation cohort included 431 consecutive offers, of which 173 (40%) were transplanted, 224 (52%) declined and 34 (8%) initially accepted but then not transplanted (a smaller category due to the absence of DCD donors). A favourable 3-month clinical outcome (GFR above 30 mL/min/1.72 m2) was seen in 146 (84%). Apart from vascular events and length of admission, risk factors were also validated in this cohort by association with acceptance decisions or outcome or both (Table 4).
TABLE 4
| Acceptance decisions (N = 431) | Post-transplant outcome univariate (N = 173) | Post-transplant outcome multivariatea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All offers | Transplant (173) | Declined (224) | p-valueb | ORc | 95% CI | p-value | ORc | 95% CI | p-value | |
| Donor | ||||||||||
| Donor age (years) | 63 [52–78] | 60 [50–72] | 68 [56–80] | 0.000 | 1.08 | 1.04–1.13 | 0.000 | 1.07 | 1.03–1.11 | 0.001 |
| CrC (mL/min) < 70d | 123 (29) | 66 (32) | 57 (25) | 0.170 | 2.12 | 0.91–4.92 | 0.080 | |||
| Creatinine ≥100% rise | 75 (17) | 23 (11) | 52 (23) | 0.001 | 0.34 | 0.04–2.65 | 0.301 | |||
| Urine <75 mL/h | 109 (25) | 39 (19) | 70 (31) | 0.004 | 1.87 | 0.71–4.93 | 0.205 | |||
| Hypertension | 225 (52) | 104 (50) | 121 (54) | 0.492 | 6.24 | 2.06–18.9 | 0.001 | |||
| Diabetes | 62 (14) | 21 (10) | 41 (18) | 0.023 | 4.29 | 1.49–12.4 | 0.007 | |||
| Vascular event | 53 (12) | 23 (11) | 30 (13) | 0.566 | 1.09 | 0.29–4.06 | 0.896 | |||
| Admission ≥10 days | 37 (9) | 15 (7) | 22 (10) | 0.435 | 0.43 | 0.05–3.45 | 0.426 | |||
| HLA mismatch ≥4 | 92 (21) | 53 (26) | 39 (17) | 0.050 | 4.61 | 1.96–10.9 | 0.000 | |||
| Risk factors presente | 1.97 | 1.40–2.78 | 0.000 | 1.50 | 1.01–2.22 | 0.045 | ||||
| Recipient (N = 305) | ||||||||||
| Weight (kg) | 75 [64–88] | 76 [65–88] | 74 [63–87] | 0.728 | 1.00 | 0.97–1.02 | 0.906 | |||
Offer characteristics, decision and outcome post-transplant in the German validation cohort.
Data provided as number (%) or median [IQR], results shaded if p < 0.10. HLA, human leukocyte antigen.
multivariable model adjusted for variables shown.
p value comparing transplanted with declined.
OR, odds ratio for poor outcome (GFR<30) at 3 months.
CrC, creatinine clearance, as threshold dependent on recipient weight: 60 mL/min (<65 kg), 70 mL/min (65–85 kg), 80 mL/min (>85 kg).
Total number of donor risk factors (from the above list) present.
The ability of adjusted donor age to predict both acceptance decision and outcome after transplantation was analysed by calibration and discrimination in both validation cohorts. In the UK validation cohort adjusted donor age was well calibrated to decisions with each quintile increasing the rate of decline (28%, 24%, 37%, 58% and 79%) with OR 1.91 per quintile (95% CI 1.62–2.27, Figure 2). In Germany the rate of decline similarly increased with each quintile of adjusted donor age (37%, 44%, 44%, 52% and 68%) with OR 1.36 per quintile (95% CI 1.20–1.54, Figure 2). In both cohorts adjusted donor age discriminated between decisions (C-statistic 0.74 in the UK, 95% CI 0.69–0.79, and 0.72 in Germany, 95% CI 0.68–0.76).
FIGURE 2
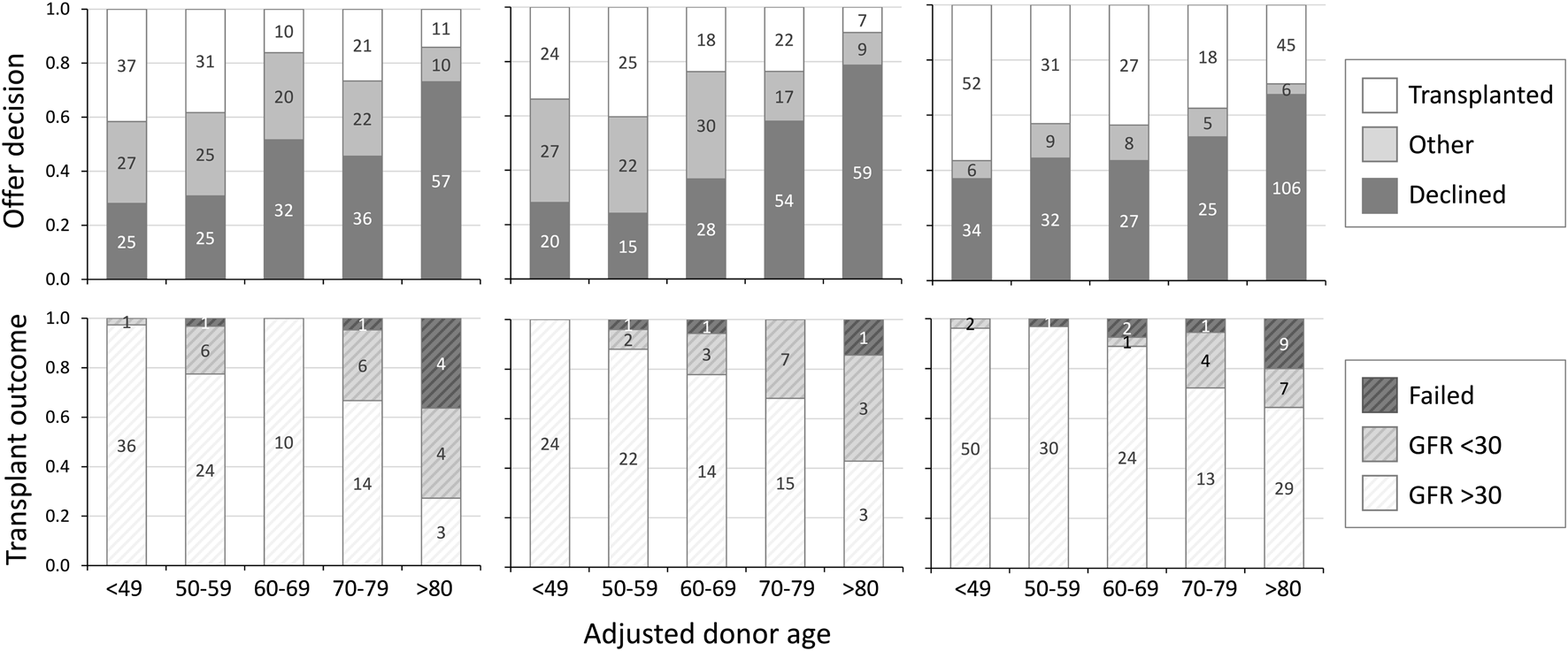
Offer decision and transplant outcome by adjusted donor age. Offer decision (upper panels) and transplant outcome at 3 months (lower panels) by adjusted donor age, in UK development cohort (left), UK validation cohort (centre) and German validation cohort (right). Decision “other” = offers accepted but not transplanted due to non-donor factors. GFR: glomerular filtration rate (ml/min/1.72 m2).
In both cohorts adjusted donor age was calibrated to 3-month post-transplant outcome with each quintile increasing the likelihood of a poor outcome post-transplantation: 0%, 12%, 22%, 32% and 57% in the UK with OR 2.29 per quintile (95% CI 1.39–3.77, Figure 2) and 4%, 3%, 11%, 28% and 36% in Germany with OR 2.09 (95% CI 1.49–2.92, Figure 2). In both cohorts adjusted donor age discriminated between post-transplant outcomes (C-statistic 0.77 in the UK, 95% CI 0.65–0.88, and 0.71 in Germany, 95% CI 0.64–0.77). Receiver operating characteristic curves illustrating the ability of adjusted donor age to discriminate between favourable and poor outcome offers in the combined cohort is shown in Figure 3.
FIGURE 3
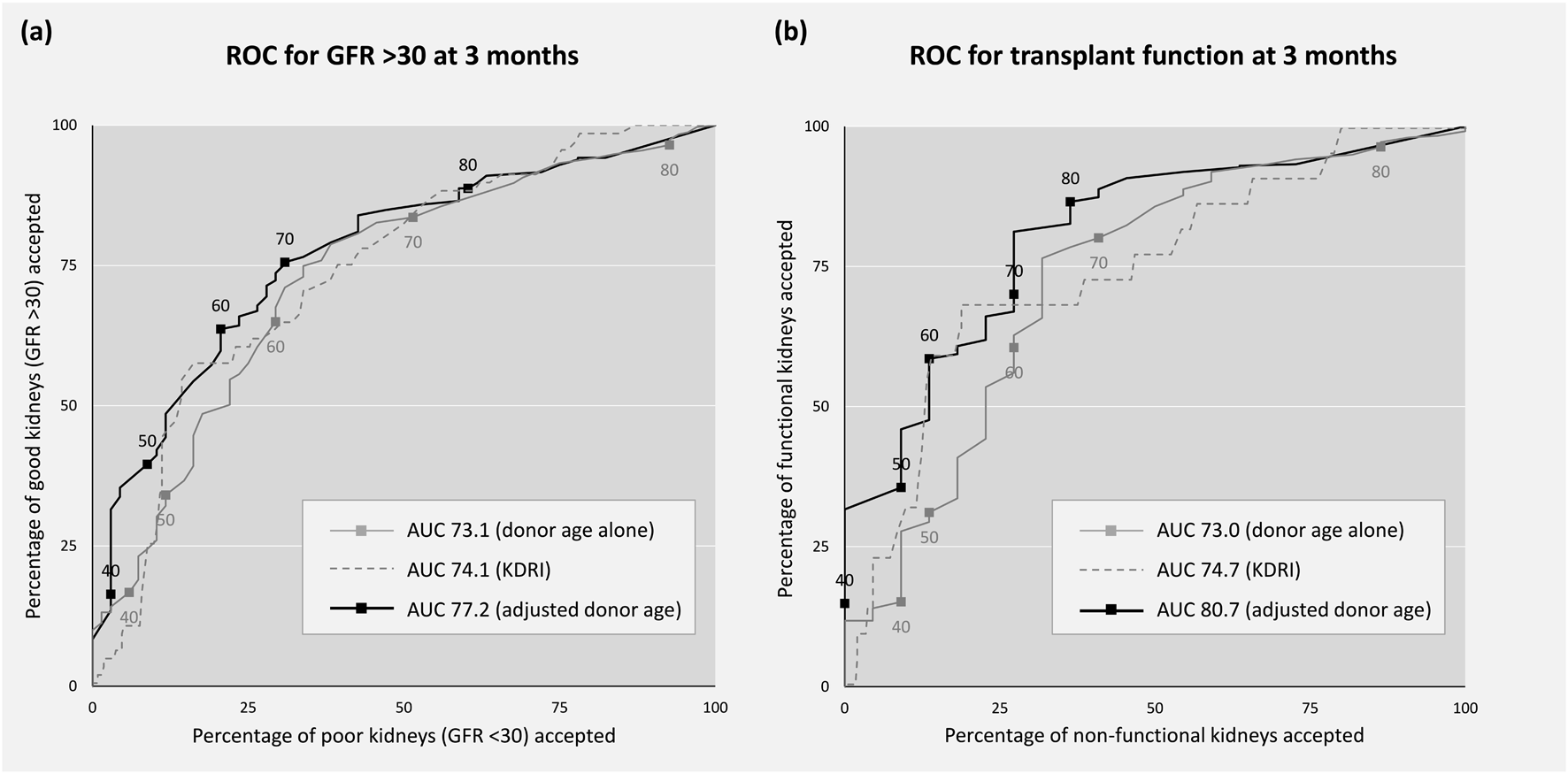
Receiver operating characteristic (ROC) curves. Sensitivity (good kidneys accepted) and 1-specificity (poor kidneys accepted) by threshold of donor age (grey), KDRI (grey dash) and adjusted donor age (black) in the combined cohort. Good outcome is defined as: (A) transplant GFR >30 at 3 months vs <30, and (B) transplant functioning at 3 months vs non-function. GFR, glomerular filtration rate (ml/min/1.72 m2), KDRI, kidney donor risk index.
Discussion
This paper describes a novel clinical tool for scoring the quality of a donor kidney offer, which is simple to calculate, calibrated to current acceptance practice, and predictive of outcome after transplantation. Such tools have appeared increasingly necessary as transplant procurement practices have evolved, from their conservative beginnings to an era of expanded criteria, allowing a much wider pool of potential donors. Studies have confirmed the survival benefit of transplantation from higher risk donors in selected recipients [10–12] but the greater variation in donor quality has made acceptance decisions increasingly complex and recipient-specific [13].
In making decisions about transplant offers, clinicians face a discrete choice within a skewed outcome distribution: kidney transplantation is usually successful, quickly and dramatically improving both quantity and quality of life. But an unsuccessful transplant, though much less common, may be fatal or disabling, or at best provide only a short reprieve from the burden of dialysis, often leaving the patient sensitised with limited prospects for re-transplantation. When to grasp opportunity, and when to play safe, is difficult to determine, not helped by the large number of donor and recipient factors which must be considered, the limited evidence base which lacks clear consensus, and the short timeframe within which decisions must be made, frequently at night.
Unsurprisingly marked variation in practice is seen between centres, with many kidneys being sequentially declined before finally being accepted and successfully transplanted – a process which leads to inequality, with marked regional differences in wait-time to transplantation [14]. That clinicians struggle with these decisions is also highlighted by the increased decline rate observed with night-time or weekend decisions [15, 16]. When clinical decisions are difficult due to complexity and time-pressure, yet stereotyped since the same concepts apply to every decision, a numerical tool offers a way to support decision making by framing the information, leading to consistency with less unwarranted variation.
Several clinical tools have previously been published which provide a numerical measure of the quality of a deceased donor kidney offer [5–8]. These have been developed from multivariate analysis of large registries, assessing the ability of donor characteristics to predict post-transplant outcome (either GFR at 6 months or time to transplant failure), leading to a score based heavily on donor age, but also including a small number of other characteristics, which partially overlap between studies. The “adjusted donor age” described in this study is similar to these tools in having age as the dominant contributor, and in predicting post-transplant outcome, but there are a number of important differences which are advantageous.
Firstly, currently available tools have boundaries which classify offers with respect to specific outcomes, rather than against the offer pool. Indeed, by the two most recently published tools [7, 8], over half of the offers in the development cohort would fall into the highest risk category. This is another significant limitation, since the acceptance decision is largely a comparative one, involving the likelihood of receiving a higher quality kidney offer within a short time. Decade boundaries of the adjusted donor age separate offers into quintiles, and although this might require recalibration over time or in different transplant programmes, the concept allows the current offer to be considered against future ones. The need for greater comparative thought in the decision process is obvious when one considers the significant number of offers declined despite belonging to the most favourable quintile, without an exclusion factor (i.e., on grounds of quality). Most often this arises from a failure to appreciate that the presence of several risk factors may be entirely offset by favourable donor age.
Secondly, although kidney function is an accepted predictor of post-transplant outcome, current tools base their estimate of function on terminal creatinine, thus failing to distinguish between chronic and acute kidney dysfunction, with recent studies suggesting the latter has only a much smaller impact on outcome. In contrast, the adjusted donor age is based on creatinine clearance estimated from baseline function, with creatinine rise and urine output as measures of acute dysfunction. Interestingly, whilst baseline creatinine clearance was a strong predictor of both decision and outcome in both cohorts, creatinine rise predicted decisions but not post-transplant outcome, suggesting it may be over-valued by decision makers. As the pool of potential donors expands, the frequency of offers with acute kidney injury will increase, so it will be increasingly important to distinguish between the large effect of chronic kidney disease and the lesser effect of acute kidney injury. Using different creatinine clearance thresholds according to recipient weight is also helpful in accounting for the negative impact of recipient size on outcome.
Finally, and most importantly, the adjusted donor age has an intuitive meaning: the age of the typical donor (with no unusual risk factors) equivalent in quality to the current offer. Since it can be readily understood, this would facilitate a discussion with the potential recipient and their involvement in the process. Though the value of shared decisions is widely appreciated, in current practice decisions are often made by clinicians only, or with limited patient involvement, in part due to the difficulty in expressing the balance of risk. Such communication is regarded as an essential part of the informed consent process [17] and linking new information to a familiar principal is believed to aid understanding [18]. This intuitive meaning, which also frames the offer within the whole distribution, may therefore facilitate patient understanding and involvement in a shared acceptance decision. Further study would be needed to assess patient feedback on the use of the score, as well as the influence on practice within centres.
There are several important limitations to this study, in particular the dual-centre and relatively small size for this type of study may reduce the ability to assess risk factors reliably. This is partially offset by the advantages of greater data granularity, the ability to understand the decision-making process, and consistency of other aspects of care which may influence outcome. There are clinical practice differences between the two centres, most notably in DCD transplantation which is not performed in Germany, leading, for example, to a much smaller group of accepted offers which did not proceed. Although the tool still validated reasonably well in the German cohort, it is possible that specific optimisation may enhance its utility in this setting.
The adjusted donor age score model does not incorporate graft histology or ischemia time. This is due to graft histology rarely playing a role in organ acceptance decisions in both the UK and Germany. Obtaining optimal pre-implantation graft histology results in the deceased donor setting is also challenging. Likewise, both warm and cold ischemia time are typically not known at the point of the initial offer acceptance decision, when the score is designed to be used. In the UK, a long warm ischaemia time is rare: typically, if there is an excessive delay between withdrawing life-support treatment and circulatory death, the retrieval is cancelled and the offer withdrawn.
As with all such tools, the outcome is only known for those kidneys which are accepted for transplantation, and a characteristic which is highly predictive of a declined offer, will be largely absent from the dataset of transplanted kidneys, and therefore have limited ability to also predict post-transplant outcome. The use of 3-month GFR as the outcome measure overlooks overall transplant survival, which is more meaningful to patients, though it may more easily capture the effect of donor-specific factors, the influence of which becomes diluted over time.
The study is specific to the UK and German deceased-donor transplant programs, and applicability beyond Europe is unknown. Whilst recalibration is likely to be necessary, the concept of an age-adjusted score to assist acceptance decisions and patient involvement should still be widely applicable. One drawback of all clinical tools including this study is outcome evidence restricted to those offers which proceeded to transplantation: the comparator needed is outcomes after not accepting the offer, such as time to the next offer, quality of that offer, and mortality or removal from the waiting list before transplantation is achieved. This outcome has received little research attention, but future studies will hopefully address this important knowledge gap.
The adjusted donor age score provides a transparent method of quantifying deceased donor kidney quality, which is consistent with current practice and predicts post-transplant outcome. Its intuitive meaning, which frames the offer against the donor distribution, may support organ acceptance decision making and facilitate meaningful patient involvement in the process.
Statements
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that were included in the study. The data will be shared on reasonable request to the corresponding author.
Ethics statement
As a retrospective study of routinely available data, the protocols were approved by the National Research Ethics Service (IRAS Ref 308076) in the UK, and the local Ethics Committee in Germany (S-187/2022) without requirements for individual consent.
Author contributions
DA, RB, CFM, FD, CM, and FK conceived the study. AA, DD, EM, CFM, FF, CN, CSp, LB, DG, MS, CSo, MM, AM, MZ, CM, and RB collected the data. DA and CFM performed the analysis. DA, RB, CFM, CM, and FK wrote the initial manuscript, which was revised and agreed by all authors. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The open access fee was paid from the Imperial College London Open Access Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13477/full#supplementary-material
Abbreviations
CRF, calculated rejection frequency; DCD, donation after circulatory death; GFR, glomerular filtration rate; HLA, anti-human leukocyte antigen; UK, United Kingdom.
References
1.
Peters-Sengers H Berger SP Heemskerk MBA Al Arashi D Homan van der Heide JJ Hemke AC et al Stretching the Limits of Renal Transplantation in Elderly Recipients of Grafts from Elderly Deceased Donors. J Am Soc Nephrol (2017) 28(2):621–31. 10.1681/ASN.2015080879
2.
Gridelli B Remuzzi G . Strategies for Making More Organs Available for Transplantation. N Eng J Med (2000) 343(6):404–10. 10.1056/NEJM200008103430606
3.
Wolfe RA LaPorte FB Rodgers AM Roys EC Fant G Leichtman AB . Developing Organ Offer and Acceptance Measures: When “Good” Organs Are Turned Down. Am J Transpl (2007) 7(5 Pt 2):1404–11. 10.1111/j.1600-6143.2007.01784.x
4.
Port FK Bragg-Gresham JL Metzger RA Dykstra DM Gillespie BW Young EW et al Donor Characteristics Associated With Reduced Graft Survival: An Approach to Expanding the Pool of Kidney Donors. Transplantation (2002) 74(9):1281–6. 10.1097/00007890-200211150-00014
5.
Nyberg SL Matas AJ Kremers WK Thostenson JD Larson TS Prieto M et al Improved Scoring System to Assess Adult Donors for Cadaver Renal Transplantation. Am J Transpl (2003) 3(6):715–21. 10.1034/j.1600-6143.2003.00111.x
6.
Schold JD Kaplan B Baliga RS Meier-Kriesche HU . The Broad Spectrum of Quality in Deceased Donor Kidneys. Am J Transpl (2005) 5(4 Pt 1):757–65. 10.1111/j.1600-6143.2005.00770.x
7.
Rao PS Schaubel DE Guidinger MK Andreoni KA Wolfe RA Merion RM et al A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation (2009) 88(2):231–6. 10.1097/TP.0b013e3181ac620b
8.
Watson CJ Johnson RJ Birch R Collett D Bradley JA . A Simplified Donor Risk Index for Predicting Outcome After Deceased Donor Kidney Transplantation. Transplantation (2012) 93(3):314–8. 10.1097/TP.0b013e31823f14d4
9.
Mohan S Chiles MC . Achieving Equity Through Reducing Variability in Accepting Deceased Donor Kidney Offers. Clin J Am Soc Nephrol (2017) 12(8):1212–4. 10.2215/CJN.06220617
10.
Lloveras J Arcos E Comas J Crespo M Pascual J . A Paired Survival Analysis Comparing Hemodialysis and Kidney Transplantation From Deceased Elderly Donors Older Than 65 Years. Transplantation (2015) 99(5):991–6. 10.1097/TP.0000000000000474
11.
Bae S Massie AB Thomas AG Bahn G Luo X Jackson KR et al Who Can Tolerate a Marginal Kidney? Predicting Survival After Deceased Donor Kidney Transplant by Donor-Recipient Combination. Am J Transpl (2019) 19(2):425–33. 10.1111/ajt.14978
12.
Ojo AO Hanson JA Meier-Kriesche H Okechukwu CN Wolfe RA Leichtman AB et al Survival in Recipients of Marginal Cadaveric Donor Kidneys Compared With Other Recipients and Wait-Listed Transplant Candidates. J Am Soc Nephrol (2001) 12(3):589–97. 10.1681/ASN.V123589
13.
Waiser J Schreiber M Budde K Fritsche L Böhler T Hauser I et al Age-Matching in Renal Transplantation. Nephrol Dial Transpl (2000) 15(5):696–700. 10.1093/ndt/15.5.696
14.
Huml AM Albert JM Thornton JD Sehgal AR . Outcomes of Deceased Donor Kidney Offers to Patients at the Top of the Waiting List. Clin J Am Soc Nephrol (2017) 12(8):1311–20. 10.2215/CJN.10130916
15.
King KL Husain SA Cohen DJ Mohan S . Deceased Donor Kidneys Are Harder to Place on the Weekend. Clin J Am Soc Nephrol (2019) 14(6):904–6. 10.2215/CJN.00620119
16.
Mohan S Foley K Chiles MC Dube GK Patzer RE Pastan SO et al The Weekend Effect Alters the Procurement and Discard Rates of Deceased Donor Kidneys in the United States. Kidney Int (2016) 90(1):157–63. 10.1016/j.kint.2016.03.007
17.
Paling J . Strategies to Help Patients Understand Risks. BMJ (2003) 327(7417):745–8. 10.1136/bmj.327.7417.745
18.
Edwards A Elwyn G Mulley A . Explaining Risks: Turning Numerical Data Into Meaningful Pictures. BMJ (2002) 324(7341):827–30. 10.1136/bmj.324.7341.827
Summary
Keywords
kidney transplantation, deceased donor, patient empowerment, acceptance, decision making
Citation
Bright R, Mahler CF., Adwaney A, Dosani D, Morganti E, Friedl F, Nusshag C, Speer C, Benning L, Göth D, Schaier M, Sommerer C, Mieth M, Mehrabi A, Zeier M, Morath C, Dor FJ.M.F., Kälble F and Ashby D (2024) Adjusted Donor Age: A Clinical Score to Support Organ Acceptance Decisions in Deceased-Donor Kidney Transplantation. Transpl Int 37:13477. doi: 10.3389/ti.2024.13477
Received
02 July 2024
Accepted
25 October 2024
Published
07 November 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Bright, Mahler, Adwaney, Dosani, Morganti, Friedl, Nusshag, Speer, Benning, Göth, Schaier, Sommerer, Mieth, Mehrabi, Zeier, Morath, Dor, Kälble and Ashby.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Damien Ashby, damien.ashby@nhs.net
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.