For the last two decades, patients and transplant clinicians have found themselves being suddenly confronted with the hostile galaxy of BK polyomavirus (BKPyV) while surfing through the kidney transplant universe. Deep thought consultation then revealed the existence of underappreciated worlds full of challenging experiences and poor outcomes as well as daring suggestions on how to rescue the journey and to reduce short- and longer-term damage. This seemingly endless odyssey has been accompanied by an expanding and contracting information space, occasionally brightened by short-lived shooting stars, most of them with limited impact for down-to-earth practice. What is more, the mere existence of the BK galaxy, its focal impact and dire costs eventually needed to be communicated to the key passengers of this journey, patients and their relatives, most of whom had possibly never heard of this nebulous conglomeration before. Two years ago, however, a brave mission was concluded by 55 people who had accepted the challenging invitation by The Transplantation Society (TTS) to embark on six working groups with the task to better chart and tackle this not so remote galaxy centering around BK polyomavirus. The TTS International BK Polyomavirus Consensus Group safely now returned and published together one of the most updated and comprehensive reports, The Second International Consensus Guidelines on the Management of BK Polyomavirus in Kidney Transplantation [1].
Given its significant content and claim, what is a reasonable and lean approach to the BK galaxy, a hitchhiker’s guide facilitating clinical translation and implementation of the new TTS BKPyV guidelines? While the underlying mantra remains regular screening and prompt response to BK polyomavirus-DNAemia by reducing immunosuppression, there are three fixed stars with their own gravity fields, nevertheless clearly interconnected in this travel guide: the infographic, the timeline, and the flow-chart. Rather than being stunned or scared by the collection of tables and their detailed Swiss army knife-like content for every eventuality, we suggest the following approach:
First, consider the infographic, which is miraculously concise given the encyclopedic character of the updated TTS BK polyomavirus guidelines (Supplementary Figure S1). There, the main recommendations are summarized in their proactive character and directly prepare the quest for more professional and detailed information.
Second, review the conceptual timeline after kidney transplantation, which paradigmatically leads through the relevant sequence of virology, immunity and pathology, integrates diagnostic measures and management considerations, and allows for cross-comparison at a given time point (Supplementary Figure S2).
Third, walk through the flowchart and explore the suggested decision tree, which gives specific reference to the respective recommendations elaborated in tables of the TTS BK polyomavirus guidelines (Supplementary Figure S3).
These three steps allow to obtain overview, concepts and a first sense of detail - but being primed for now reviewing the current practice in your center is perhaps the most valuable item:
On the positive side, this helps to identify the “have” of tools, procedures and staff that are already existing in current center practice as well as those “must haves” that are not optimally used or clearly missing.
Given the heavy load and the multidisciplinary character, we presume that the task of harmonizing and successfully translating the updated TTS BK polyomavirus guidelines is best accomplished by a team approach (Figure 1). Perhaps a tabular comparison of “is” versus “suggested” or “have” versus “must have” provides a first overview and can be complemented by “priorities” and “timelines” to realization in order to create a helpful and traceable planning tool.
FIGURE 1
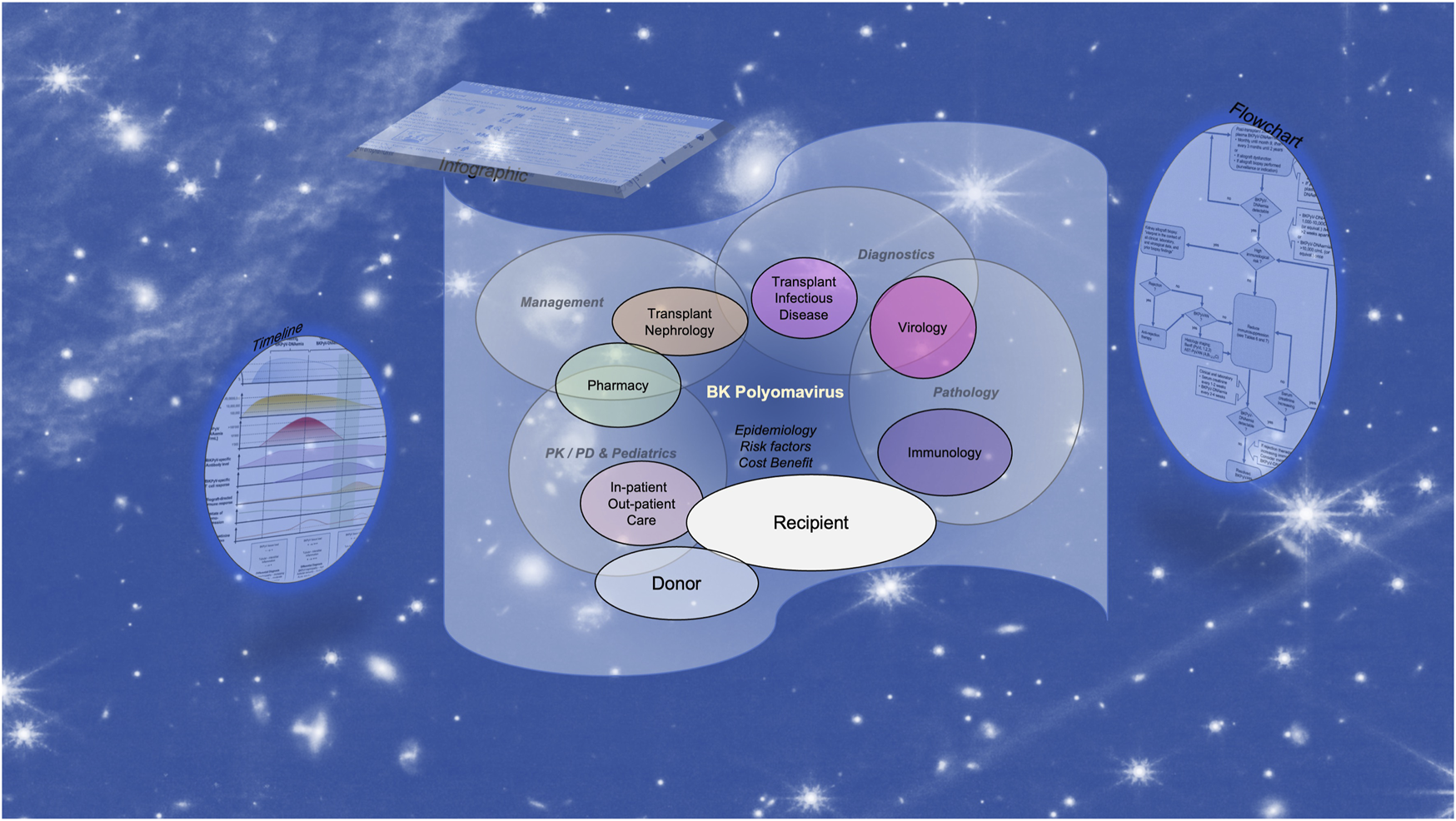
The multidisciplinary challenge of implementing the updated TTS BK polyomavirus guidelines for developing and updating standard operating procedures.
One of the key deliverables is the timely reduction of maintenance immunosuppression guided by a significant plasma BK polyomavirus DNA load. For standard immunological risk and patients with baseline renal function, there is no biopsy required. There is currently no data from randomized clinical trials supporting superiority of either management strategy 1 (first step reducing mycophenolate) or of strategy 2 (first reducing tacrolimus). Though frequently mentioned or considered for other reasons, we like to emphasize that there currently is, for no other opportunistic complication posttransplant, more consistent and better documented evidence of feasibility, for rates of success or harm than for the deliberate reduction of immunosuppression for BK polyomavirus. Clearly, trigger and timing remain key determinants [2].
To develop the local standard operating procedure, the active participation of all different experts and providers is expected to not only build and expand a broad foundation of knowledge, but also competence for critical (re-)evaluation. Deviation from the current recommendations always remains an option, but then they are the result of active informed decision instead of ignorance. Broad foundation of knowledge also prepares the transplantation team for participation in randomized clinical trials, which are particularly lacking for management decisions.
The new TTS BK polyomavirus guidelines also identify areas of uncertainty and unmet clinical need, where more excellent research is needed and expected to make a difference for patients on their hopefully timeless journey of kidney transplantation. This starts at transplantation with the investigations addressing the value of donor urine virus loads, donor and recipient BK polyomavirus-specific antibodies, virus-specific cell mediated immunity, biomarkers of allograft damage and differentials of T cell mediated rejection or antibody-mediated rejection, as well as therapeutic, preemptive or prophylactic transfer of humoral and cellular immune effectors. But even for the lower hanging fruits, more conclusive data from randomized clinical trials must be considered valuable. These include evaluation of other tantalizing forces in our management universe such as switching to mTOR inhibitors, perhaps combined with low-dose cyclosporine instead of tacrolimus [3], or for patients with persisting BK polyomavirus-DNAemia on tacrolimus monotherapy to switch to belatacept for maintenance.
Importantly, all of the TTS working group members and their leaders are committed to assist transplant clinicians with their expertise in the management of difficult cases as well as in establishing the best local standard operating procedures. Indeed, the challenges of BK polyomavirus and how it affects a significant part of kidney transplant recipients should be explained to the patients and relatives pre-transplant when preparing for one of the otherwise most successful journeys in modern medicine. As a disclaimer known from others, it remains to conclude that “The Guide is definitive. Reality is frequently inaccurate” [4].
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HH and CK identified the need for this communication and designed project, HH wrote the first draft of the manuscript and CK and HH finalized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the personal appointment grant MM2109 to HH by the University of Basel, Basel, Switzerland and an SNF grant 310030_212589 to HH. The TTS BK polyomavirus guidelines were supported by independent funding of Roche diagnostics provided to The Transplantation Society for the realizing the BK Polyomavirus guidelines hybrid meeting in Basel, April 7–8, 2022.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13873/full#supplementary-material
References
1.
Kotton CN Kamar N Wojciechowski D Eder M Hopfer H Randhawa P et al The Second International Consensus Guidelines on the Management of BK Polyomavirus in Kidney Transplantation. Transplantation (2024) 108:1834–66. 10.1097/TP.0000000000004976
2.
Hirsch HH Mengel M Kamar N . BK Polyomavirus Consensus. Clin Infect Dis (2022) 75(11):2046–7. 10.1093/cid/ciac594
3.
Caillard S Meyer N Solis M Bertrand D Jaureguy M Anglicheau D et al Everolimus versus Mycophenolate Mofetil for BK Polyomavirus infection in kidney transplant recipients: insights from the randomized controlled BKEVER trial. Kidney Int. (2024). in press. 10.1016/j.kint.2024.09.018
4.
Adams D . The Hitchhiker’s Guide to the Galaxy. Pan Books 0-330-25864-8; 1979.
Summary
Keywords
kidney transplantation, guidelines, BK virus, polyoma, nephropathy, BK polyomavirus
Citation
Hirsch HH and Kotton CN (2024) A Hitchhiker’s Guide to the BK Galaxy. Transpl Int 37:13873. doi: 10.3389/ti.2024.13873
Received
01 October 2024
Accepted
24 October 2024
Published
31 October 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Hirsch and Kotton.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans H. Hirsch, hans.hirsch@unibas.ch
ORCID: Hans H. Hirsch, orcid.org/0000-0003-0883-0423; Camille N. Kotton, orcid.org/0000-0001-7320-2234
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.