- 1Professor E. Pijanowski Catering School Complex in Warsaw, Warsaw, Poland
- 2Institute of Food Science, Department of Food Technology and Assessment, Warsaw University of Life Sciences - SGGW (WULS-SGGW), Warsaw, Poland
Fatty acid profiles are crucial for the functionality and viability of lactobacilli used in food applications. Tween 80™, a common culture media additive, is known to influence bacterial growth and composition. This study investigated how Tween 80™ supplementation impacts the fatty acid profiles of six mesophilic lactobacilli strains (Lacticaseibacillus spp., Limosilactobacillus spp., Lactiplantibacillus plantarum). Analysis of eleven strains revealed 29 distinct fatty acids. Tween 80™ supplementation significantly altered their fatty acid composition. Notably, there was a shift towards saturated fatty acids and changes within the unsaturated fatty acid profile. While some unsaturated fatty acids decreased, there was a concurrent rise in cyclic derivatives like lactobacillic acid (derived from vaccenic acid) and dihydrosterculic acid (derived from oleic acid). This suggests that despite the presence of Tween 80™ as an oleic acid source, lactobacilli prioritize the synthesis of these cyclic derivatives from precursor unsaturated fatty acids. Myristic acid and dihydrosterculic acid levels varied across strains. Interestingly, palmitic acid content increased, potentially reflecting enhanced incorporation of oleic acid from Tween 80™ into membranes. Conversely, cis-vaccenic acid levels consistently decreased across all strains. The observed fatty acid profiles differed from previous studies, likely due to a combination of factors including strain-specific variations and growth condition differences (media type, temperature, harvesting point). However, this study highlights the consistent impact of Tween 80™ on the fatty acid composition of lactobacilli, regardless of these variations. In conclusion, Tween 80™ significantly alters fatty acid profiles, influencing saturation levels and specific fatty acid proportions. This work reveals key factors, including stimulated synthesis of lactobacillic acid, competition for oleic acid incorporation, and strain-specific responses to myristic and dihydrosterculic acids. The consistent reduction in cis-vaccenic acid and the presence of cyclic derivatives warrant further investigation to elucidate their roles in response to Tween 80™ supplementation.
Introduction
The fatty acid composition of bacterial cells of the lactobacilli determines their survival and depends on many environmental factors (Johnsson et al., 1995; Partanen et al., 2001; Corcoran et al., 2007; Montanari et al., 2010; Li et al., 2011; Tan et al., 2012; Terraf et al., 2012; Al-Naseri et al., 2013; Hansen et al., 2015; Reitermayer et al., 2018). Corcoran et al. (2007) proved this by showing that oleic acid (C18:1,cis-9) and cis-vaccenic acid (C18:1,cis-11) have a protective effect on Lacticaseibacillus rhamnosus GG cells suspended in artificial gastric juice. These researchers have shown that only in the case of these two fatty acids, the number of bacterial cells was greater than the number of cells in the control sample. Other tested fatty acids, including stearic (C18:0), elaidic (C18:1,trans-9), linoleic (C18:2,cis-9,cis-12), cis-9,trans-11-octadecadienoic, and trans-10,cis-12-octadecadienoic, caused a decrease in the number of viable cells (to a level of 3.89 log CFU/mL in case of trans-10,cis-12-octadecadienoic acid and to 4.75 log CFU/mL for stearic and elaidic acids). This phenomenon can be explained by the fact that oleic (C18:1,cis-9) and cis-vaccenic (C18:1,cis-11) acids are the substrates for the synthesis of fatty acid in various ways required for cell survival and modification of cell membrane fluidity. Lactic acid bacteria (LAB), lactobacilli including, are equipped with the mechanisms for transforming these fatty acids in their cyclic, polyunsaturated, or conjugated forms (Henderson and McNeill, 1966; Weeks and Wakil, 1970; Partanen et al., 2001; Corcoran et al., 2007; Tan et al., 2012). Transformation of linoleic (C18:2,cis-9,cis-12) or conjugated acids to oleic acid (C18:1,cis-9) requires the action of specific enzymes if LAB cells lack access to external sources of oleic acid. In the absence of external oleic acid, some LAB strains may resort to this conversion pathway to maintain a certain level of oleic acid, potentially leading to a decrease in the overall protective action of these polyunsaturated fatty acids.
One of the external sources of fatty acids for bacterial cells is their living environment, including culture media. A key example is oleic acid, a fatty acid found in many natural sources. However, bacteria can also utilize oleic acid provided in the form of Tween 80™. Tween 80™ is a nonionic surfactant, commonly used in the food industry as an emulsifier, dispersant, and stabilizer. It finds application in microbiology as a common additive to media for cultivating lactobacilli (Johnsson et al., 1995; Partanen et al., 2001; Montanari et al., 2010; Li et al., 2018). It could be also employed in food fermentation processes for its beneficial effects on microbial growth and product quality (Li et al., 2018). Chemically, Tween 80™ is a synthetic molecule derived from sorbitan (a cyclic polyol) and esterified with oleic acid (C18:1,cis-9). This combination results in a molecule with a hydrophilic head (sorbitan) and a hydrophobic tail (oleic acid), allowing Tween 80™ to act as a surface-active agent. Tween 80™ improves the solubility and bioavailability of nutrients, promoting microbial growth and fermentation efficiency. By facilitating nutrient uptake and metabolism, Tween 80™ can lead to higher yields of fermented products (Li et al., 2018). The combination of oleic acid (C18:1,cis-9) with sorbitol permits the solubility of fatty acid in aqueous solution and a uniform dispersion of particles in growth medium, thereby making it more accessible to acid bacterial cells. Corcoran et al. (2007) compared the effect of adding Tween 80™ to the survival of four strains of lactobacilli suspended in artificial gastric juice: Lacticaseibacillus rhamnosus GG, Lacticaseibacillus rhamnosus E800, Lacticaseibacillus paracasei subsp. paracasei NFBC 338, and Ligilactobacillus salivarius UCC 500. In each case they confirmed the protective effect of Tween 80™ on cell viability. No Tween 80™ resulted in a decrease in the number of LAB viable cells in at least two logarithmic cycles after incubation in artificial gastric juice for 90 min. The cited studies are confirmed by other references (Johnsson et al., 1995; Broadbent et al., 2014).
The most widely used methods in study cellular fatty acid (as methyl esters) of Firmicutes (including lactobacilli) is matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) based on the principle of ionization and mass analysis of molecules, and it is particularly well-suited for identifying bacteria because it can analyze intact bacterial cells rather than extracting and purifying specific molecules (Anderson et al., 2014; Foschi et al., 2017; Nacef et al., 2017). As a result of these advantages, MALDI-TOF is becoming the preferred method for the identification of lactobacilli in many laboratories. MALDI-TOF (matrix-assisted laser desorption ionization time-of-flight mass spectrometry) is another method that is increasingly being used for the study of bacterial cellular fatty acid. It is a rapid, sensitive, and easy-to-use technique that can provide accurate species identification. However, the Sherlock Microbial Identification System (by MIDI Inc.) is a reliable method for species identification, particularly for lactobacilli, and is often used in conjunction with other methods, such as sequencing, to provide a complete taxonomic profile (Brondz, 2002; Kunitsky et al., 2006; Alexander, 2012). The alternative method referred to in the statement is likely to be gas chromatography-mass spectrometry (GC-MS), which is a more sophisticated technique that can provide more detailed information about the composition of fatty acids. However, GC-MS is a more expensive and time-consuming method, so it is not always the most practical choice. MIDI is a faster and more cost-effective method, and it is often sufficient for routine identification of lactobacilli. In general, MIDI is a suitable choice for routine identification of lactobacilli when speed and cost are important, while MALDI-TOF is the more advanced method and is the best choice for routine identification and specialized applications. GC-MS is most appropriate for more detailed studies or when species identification is challenging.
This study investigates the influence of Tween 80™ (oleic acid source) supplementation in growth media on the FA profile of selected mesophilic lactobacilli strains. We aim to compare their abilities to modulate FA synthesis and transformation upon incorporation of this external oleic acid (C18:1,cis-9) source.
Materials and methods
Materials
Eleven lactobacilli strains of the lactobacilli (Table 1) deposited in the laboratory collection in the form of monocultures freeze-dried or frozen were used in our research. Cultures of each bacterial strain were conducted in three independent experiments. First, the cultures were revived in the MRS (de Mann, Rogosa, and Sharpe) broth supplemented with Tween 80™ (Sigma-Aldrich) in portions of 50 mL (incubation at 37°C for approx. 12 h). Tween 80™ is known to influence membrane fluidity and potentially impact fatty acid synthesis in lactobacilli (Al-Naseri et al., 2013; Zotta et al., 2017b; Reitermayer et al., 2018). Subsequently, the cultures were inoculated onto the surface of three parallel MRS agar plates with Tween 80™ and three parallel MRS agar plates without Tween 80™ (in two independent replicates for each growth medium). Cultures were incubated at 37°C for 24 h under anaerobic conditions. Anaerocult™ A (Merck) was used to produce an anaerobic milieu in the anaerobic jar™ (Merck). Anaerocult™ A is recommended for the cultivation of obligatory and facultative anaerobes. To minimize growth phase variation, we carefully harvested cells for biomass collection from the defined center sector of each plate using the secondary inoculum. This ensured a representative sample of the cultured population for fatty acid analysis (Asadi et al., 2015).
The extraction of fatty acids: separating and detecting conditions of fatty acid methyl esters using GC-MS
The fatty acids were extracted from the entire bacterial biomass according to recommendations (Haack et al., 1994; Buyer, 2002; Kankaanpaa et al., 2004). Each bacterial biomass sample was prepared for analysis and analyzed in two replicates. The chromatographic separation of fatty acid methyl esters was carried out by gas chromatography coupled with mass spectrometer (GC-MS QP 2010 Shimadzu, Shimadzu Corporation, Japan) using polar column 007-23-30-0.2F (30 m × 0.25 mm × 0.20 μm; Quadrex, Quadrex Corporation, United States). The sample was injected under a split ratio of 1:25 at the dispenser temperature equal to 230°C. The chromatographic separation was conducted in the following conditions: Initial temperature of column of 60°C, 2 min isotherm, the temperature increase of 4°C/min to 220°C, 10 min isotherm. The carrier gas was helium with a flow rate of 0.37 mL/min. The following detector conditions were used: Temperature of the ion source of 200°C, temperature of the line connecting GC with MS of 220°C, detector voltage of 1.45 kV, and quadrupole filter sweep in a range 50-400 m/z. The following standards were used for identifying fatty acids: oleic acid (Sigma Aldrich, United States), anteiso12-methyltetradecanoic acid (Sigma Aldrich, United States), 2-hydroxytetradecanoic acid (Sigma Aldrich, United States), nonadecanoic acid (Sigma Aldrich, United States), the BAME (bacterial acid methyl esters; Sigma Aldrich, United States), GLC-674 and GLC-617 (Nu-Chek-Prep., United States), and isomers of methyl esters of linoleic acid 18:2 (cis-9,trans-11 and trans-10,cis-12; Nu-Chek-Prep., United States). The composition of bacterial acid methyl esters (BAMEs) is presented in Supplementary Table 1S. In other cases of fatty acids (including lactobacillic acid), the comparisons were made with the literature data (Brondz, 2002; Christie et al., 2007; Montanari et al., 2010). The data obtained from GC-MS was analyzed using GCMS Solution v.2.50 (Shimadzu Corporation).
Statistical analysis
The statistical analysis was carried out using the statistical program Statistica v.10. The analysis of variance (ANOVA) was employed. In this study, ANOVA was complemented by Tukey’s test, conducted at a significance level of 0.05, to scrutinize the mutual influence of the factors and identify any statistically significant differences between them. Principal components analysis (PCA) was conducted to assess the relationship between fatty acid profiles and the presence or absence of Tween 80™ in the growth medium. In this case, PCA was used to identify the main components that influenced the variation in fatty acid profiles.
Results and discussion
Lacticaseibacillus rhamnosus
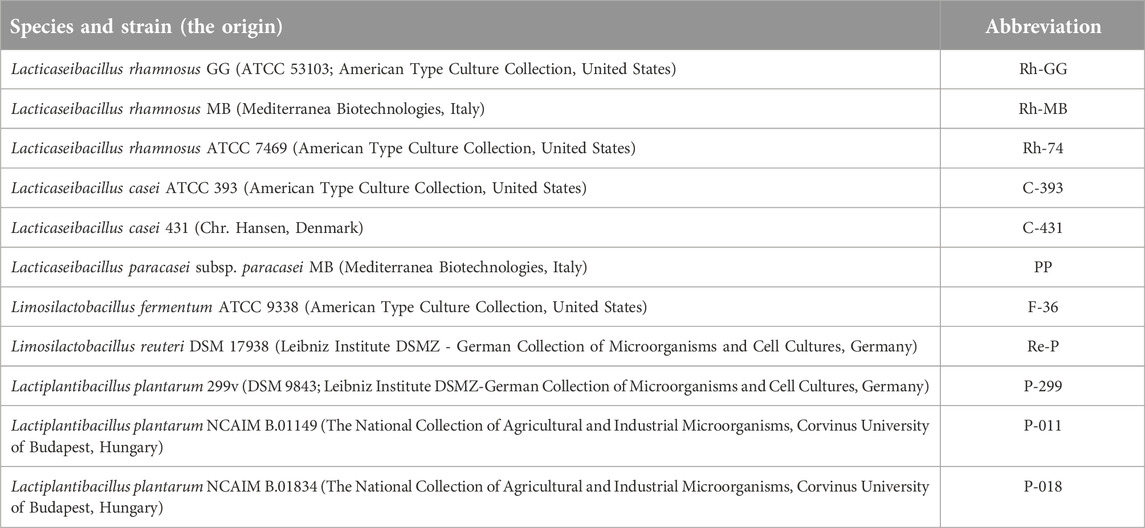
The determined fatty acid profile of L. rhamnosus GG, L. rhamnosus MB, and L. rhamnosus ATCC 4769 cells demonstrated the presence of 29 fatty acids after the incubation on the growth medium with Tween 80™ (Table 2). The strains of L. rhamnosus used in our study were characterized by an intense growth on the MRS agar without Tween 80™. The character of the growth of this species’ colonies was different from other lactobacilli species used for this study. The colonies on the line of loop movement were snow-white with characteristic papules and damming, and so obtaining the biomass required for the analysis was not difficult. Nineteen fatty acids were identified in the fatty acid profile of L. rhamnosus GG cells, while the presence of 21 fatty acids was noted in the case of L. rhamnosus MB and L. rhamnosus ATCC 7469 cells (Table 2).
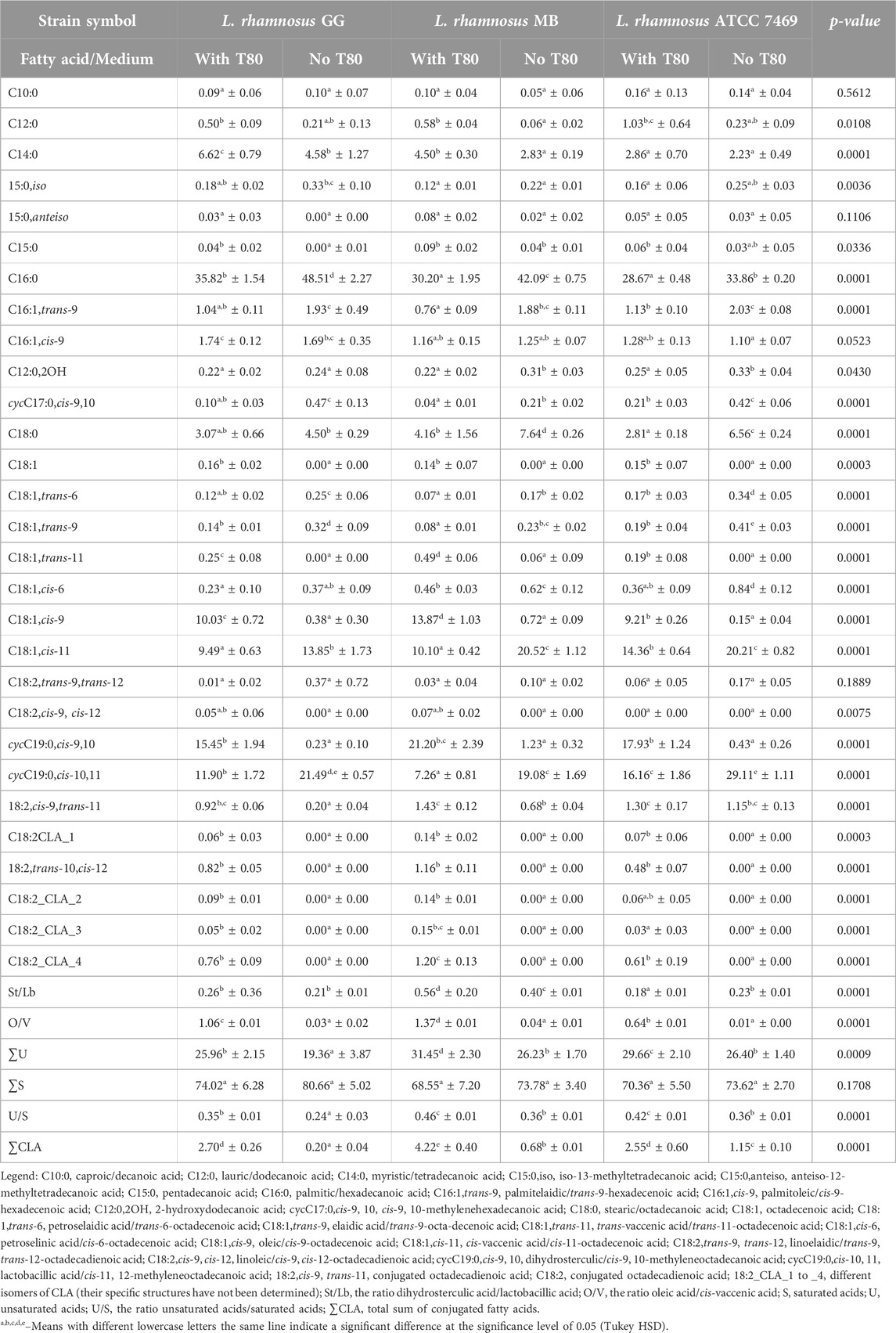
Table 2. The percentage fatty acids composition (mean ± SD, n = 6) in mesophilic Lacticaseibacillus rhamnosus strains profiles obtained from the cell cultured in a growth medium with Tween 80TM (with T80) and without Tween 80TM (no T80).
By comparing the test strains of L. rhamnosus species acid, similarities were observed in the sequence fatty acid dominant in the fatty acid profile of the cells. The comparative analysis (conducted using Tukey’s test) of the share of all dominant fatty acids in the fatty acid profiles obtained from the biomass of all strains showed significant differences. The fatty acid profile of the L. rhamnosus GG strain was dominated (the share >10%) by palmitic (C16:0), dihydrosterculic (cycC19:0,cis-9,10), lactobacillic (cycC19:0,cis-10,11), and oleic (C18:1,cis-9,) fatty acids. Meanwhile, the fatty acid profile of the L. rhamnosus MB strain was dominated (the share >10%) by palmitic acid, dihydrosterculic acid (cycC19:0,cis-9,10), oleic acid, and cis-vaccenic fatty (C18:1,cis-11) acids. As for the fatty acid profile of L. rhamnosus ATCC 7469 strain, it was dominated by palmitic (C16:0), dihydrosterculic (cycC19:0,cis-9,10), lactobacillic (cycC19:0,cis-10,11), and cis-vaccenic acid (C18:1,cis-11) acids (the share >10%). Their contribution to the fatty acid profile changed significantly when Tween 80™ was eliminated from the growth medium. The only fatty acids whose proportion in the fatty acid profile did not change significantly were the following fatty acids: caproic (C10:0), anteiso-12-methyltetradecanoic (C15:0,anteiso), palmitoleic (C16:1,cis-9), and linoelaidic (C18:2,trans-9,trans-12) fatty acids. In general, removal of Tween 80™ from the growth medium resulted in a decrease in the proportion of 14 fatty acids in the fatty acid profile, both saturated and unsaturated fatty acids, and an increase in the proportion of 11 fatty acids in the profile of fatty acids, mainly unsaturated fatty acids. Of course, these changes were not observed in every L. rhamnosus strains tested. Consequently, this resulted in a significant reduction in the calculated the ratio dihydrosterculic acid (cycC19:0,cis-9,10)/lactobacillic acid (cycC19:0,cis-10,11) (St/Lb ratio) with the exception for the L. rhamnosus MB strain, the ratio oleic acid (C18:1,cis-9)/cis-vaccenic acid (C18:1,cis-11) (O/V ratio), and the ratio unsaturated acids/saturated fatty acids (U/S ratio). It is worth noting that removal of Tween 80™ from the growth medium resulted in a significant reduction in the proportion of total unsaturated fatty acids, including conjugated fatty acids (CLA), in the fatty acid profile, but also did not result in a complete change in the proportion of saturated fatty acids in the fatty acid profile.
Kankaanpaa et al. (2004) found that L. rhamnosus GG cells grown with Tween 80™ contained oleic (21.5%), cis-vaccenic (21.8%), dihydrosterculic (23.5%), and lactobacillic (2.1%) acids as major fatty acids, along with conjugated acids at about 3%. Bacteria produce unsaturated fatty acids with various functionalities de novo (Zhu et al., 2019). These modifications, along with other fatty acids, increase the fluidity of the cytoplasmic membrane through their specific structures (Kaneda, 1991). The ability of the strains to synthesize cis-vaccenic acid and convert it to lactobacillic acid is noteworthy, with minimal de novo synthesis of oleic acid, especially in the absence of Tween 80™. The presence of Tween 80™ appears to inhibit the FAS system, as suggested by (Zhang et al., 2020). Our study indicates that all strains produced vaccenic acid even under anaerobic conditions, which is significant since anaerobic conditions favor the anaerobic vaccenic acid pathway. This contrasts with aerobic conditions in L. casei N87, which enhance growth, metabolism, and stress tolerance, influencing vaccenic acid synthesis (Ianniello et al., 2016). Refrigerated storage before biomass extraction can increase the proportion of unsaturated fatty acids (Zhu et al., 2019), which helps maintain membrane fluidity and protein activity (Santivarangkna et al., 2008; Parsons and Rock, 2013). The absence of oleic acid stimulates lactobacillic acid synthesis, while its presence increases dihydrosterculic acid levels (Kankaanpaa et al., 2004). This adaptive modification in fatty acid composition helps control membrane fluidity, aiding bacterial stress defense (Beal et al., 2001; Murga et al., 2001; Montanari et al., 2010; Pawar and Aranha, 2022). Environmental factors significantly influence unsaturated fatty acid proportions in lactobacilli membranes (Zotta et al., 2017a; Fonseca et al., 2019), with oxygen availability playing a crucial role (López de Felipe, 2023). Under aerobic conditions, lactobacilli can convert palmitoleic acid to cis-vaccenic acid (Cronan, 2002; Schujman and Mendoza, 2008; Jimenez et al., 2023). Corcoran et al. (2007) noted a rise in certain acids in L. rhamnosus GG cells when exposed to oleic acid (C18:1,cis-9). Our research found that adding oleic acid via Tween 80™ increased dihydrosterculic acid (cycC19:0,cis-9,10), oleic acid (C18:1,cis-9), and conjugated fatty acids, while reducing palmitic (C16:0) and stearic (C18:0) acids. Furthermore, L. rhamnosus GG, L. rhamnosus MB, and L. rhamnosus ATCC 7469 cells cultured without Tween 80™ showed lactobacillic acid (cycC19:0,cis-10,11) dominance in their fatty acid profiles. This difference is likely due to the lack of separation of cyclic acids in the Corcoran et al. (2007) study.
Lacticaseibacillus casei and Lacticaseibacillus paracasei
The identified fatty acid profile of L. casei 393, L. casei 431, and L. paracasei subsp. paracasei MB grown on MRS agar with Tween 80™ contained 29 and 27 fatty acids for L. casei and L. paracasei, respectively (Table 3). The predominant fatty acids in the fatty acid profile of the first of these strains were: palmitic acid (C16:0), dihydrosterculic acid (cycC19:0,cis-9,10), lactobacillic acid (cycC19:0,cis-10,11), and cis-vaccenic acid (C18:1,cis-11). Meanwhile, fatty acids such as palmitic acid, oleic acid (C18:1,cis-9), cis-vaccenic acid (C18:1,cis-11), and dihydrosterculic acid (cycC19:0,cis-9,10) were found to be dominant for the fatty acid profile of L. casei 431. On the other hand, dominant fatty acids in the L. paracasei subsp. paracasei MB fatty acid profile included oleic acid (C18:1,cis-9), dihydrosterculic acid (cycC19:0,cis-9,10), and palmitic acid (C16:0). While dihydrosterculic acid (cycC19:0,cis-9,10) could potentially be used to differentiate between L. casei and L. paracasei subsp. paracasei MB, the high proportion observed in this study might be due to stress experienced by the bacteria during growth (Rouch et al., 2002; Wu et al., 2020; Klein et al., 2022). Cyclic fatty acids, including dihydrosterculic acid (cycC19:0,cis-9,10), are known markers of stress in LAB. Further studies are needed to investigate the influence of growth conditions on the fatty acid profile of L. paracasei subsp. paracasei MB and confirm its potential as a differentiating factor. L. casei and L. paracasei grown without Tween 80™ displayed altered fatty acid profiles compared to cultures with Tween 80™. Notably, the absence of oleic acid (C18:1,cis-9) triggered cis-vaccenic (C18:1,cis-11) and lactobacillic acid (cycC19:0,cis-10,11) synthesis. Total saturated fatty acids, especially iso-13-methyltetradecanoic (C15:0,iso) and stearic (C18:0) acids, increased in bacteria lacking Tween 80™, although this effect was not universal. Conversely, unsaturated fatty acid content and the U/S ratio decreased without Tween 80™. Interestingly, total CLA and St/Lb and O/V ratios also significantly declined. These changes in L. casei and paracasei are distinct from those observed previously inL. rhamnosus.
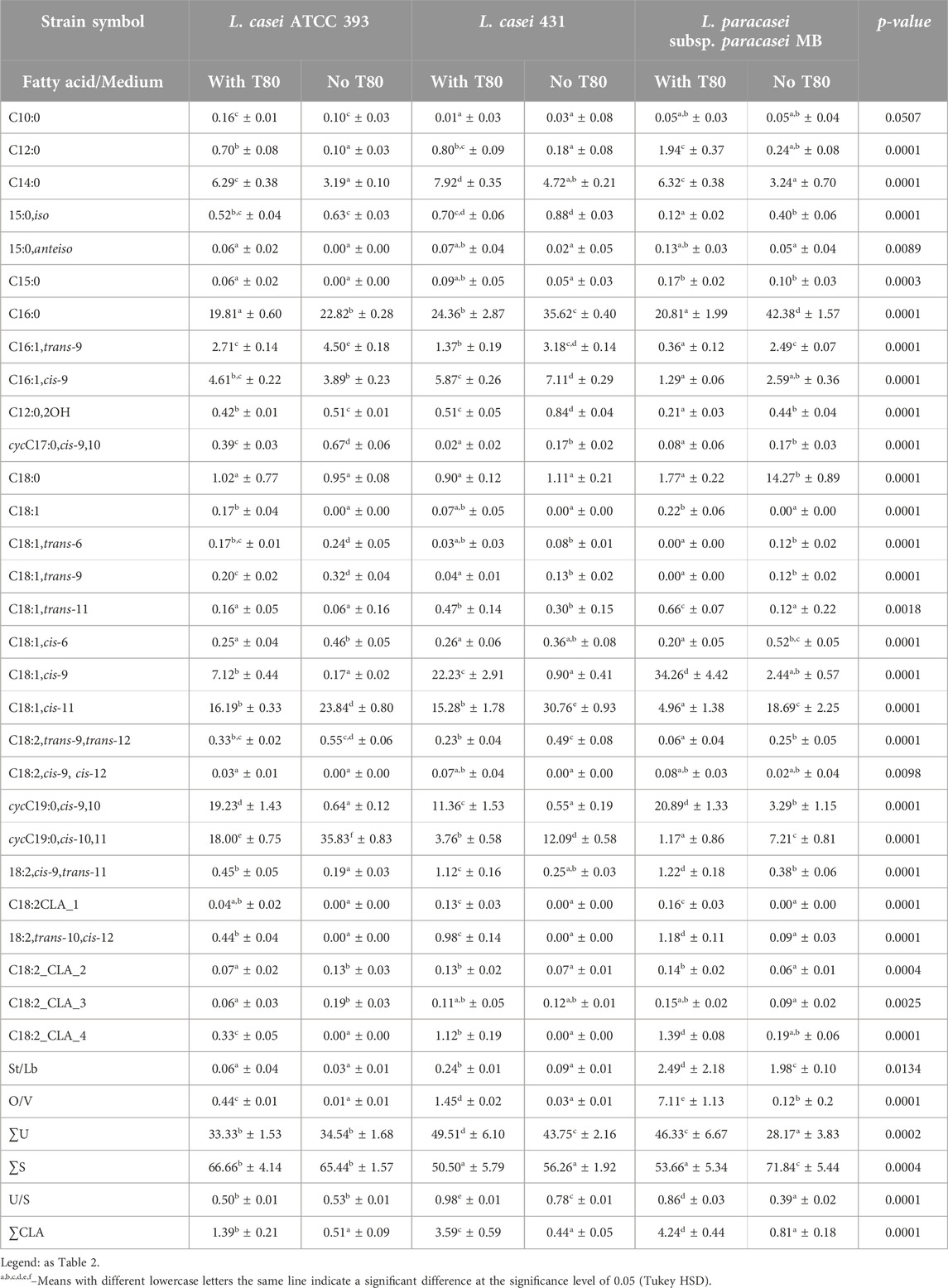
Table 3. The percentage fatty acids composition (mean ± SD, n = 6) in mesophilic Lacticaseibacillus casei and Lacticaseibacillus paracasei strains profiles obtained from the cell cultured in a growth medium with Tween 80TM (with T80) and without Tween 80TM (no T80).
We observed significant differences in the proportions of saturated, unsaturated, and cyclic fatty acids compared to previous studies (Rizzo et al., 1987; Kankaanpaa et al., 2004; Machado et al., 2004; Liong and Shah, 2005; Corsetti et al., 2010; Rodrigues et al., 2012). Notably, our findings revealed substantial levels of dihydrosterculic and lactobacillic acids, cyclic derivatives of oleic and vaccenic acids, respectively. These results contrast with some prior studies that may have missed these cyclic acids due to separation and detection challenges (Machado et al., 2004; Liong and Shah, 2005). For L. casei, our dominant fatty acids included dihydrosterculic and lactobacillic acids, alongside palmitic acid. This aligns with the findings of Rizzo et al. (1987) who reported palmitic, octadecenoic, and cyclopropane acids as the major components in L. casei ATCC 393. However, some discrepancies exist, such as the presence of palmitoleic acid in Machado et al. (2004) which we did not observe. These differences could be attributed to various factors, including strain variations and growth conditions. While comparisons for L. paracasei subsp. paracasei MB were limited due to a scarcity of data specifically for this subspecies, it’s important to acknowledge that the L. casei group taxonomy has undergone revisions in recent years. Some strains previously classified as L. casei may now be identified as L. paracasei. Future studies that incorporate a wider range of Lactobacillus species, including recently reclassified members of the casei group, along with standardized protocols, would offer valuable insights into the influence of growth media components on bacterial fatty acid composition.
Limosilactobacillus fermentum and Limosilactobacillus reuteri
The fatty acid profile of bacterial biomass of L. fermentum ATCC 9338 and L. reuteri DSM 17938 cultured on growth medium with Tween 80™ was characterized by the presence of 27 and 28 fatty acids, respectively (Table 4). We demonstrated that L. fermentum ATCC 9338 was characterized by the share of palmitic (C16:0), oleic (C18:1,cis-9), lactobacillic (cycC19:0,cis-10,11), cis-vaccenic (C18:1,cis-11), and dihydrosterculic fatty (cycC19:0,cis-9,10) acids, while L. reuteri DSM 17938 by palmitic, cis-vaccenic (C18:1,cis-11), dihydrosterculic (cycC19:0,cis-9,10), and lactobacillic (cycC19:0,cis-10,11) acids. Each of these strains demonstrated the ability to synthesize vaccenic and lactobacillic (cycC19:0,cis-10,11) acids, which were in a pool of dominant fatty acids. We also demonstrated the presence of oleic acid (C18:1,cis-9) and its cyclic derivative, dihydrosterculic acid (cycC19:0,cis-9,10) (Table 4). L. fermentum ATCC 9338 and L. reuteri DSM 17938 showed excellent growth on MRS agar without Tween 80™. The fatty acid profile of the first of these strains contained 20 fatty acids, while the second strain was characterized by 22 fatty acids. Removal of Tween 80™ from the growth medium resulted in statistically significant changes in the fatty acid profile of bacterial strains: an increase in the share of 8 fatty acids (5 unsaturated fatty acids and 3 saturated fatty acids) and a decrease in the share of 11 fatty acids (4 saturated fatty acids and 7 unsaturated fatty acids). This significantly reduced the calculated ratios of O/V and U/S, but increased the calculated St/Lb ratio. It is also worth noting that the changes observed were not the same across all bacterial strains tested, which, as with the lactobacilli strains discussed previously, allows for some differentiation.
Table 4. The percentage fatty acids composition (mean ± SD, n = 6) in mesophilic Limosilactobacillus fermentum and Limosilactobacillus reuteri strains profiles obtained from the cell cultured in a growth medium with Tween 80TM (with T80) and without Tween 80TM (no T80).
Our study confirmed previous findings (Johnsson et al., 1995) that Tween 80™ significantly alters the fatty acid profile of L. fermentum. Oleic (C18:1,cis-9) and dihydrosterculic (cycC19:0,cis-9,10) acids, undetectable without the surfactant, became enriched upon its addition. This effect occurred without affecting growth rate, suggesting independent processes. Furthermore, our results aligned with Rizzo et al. (1987) regarding the overall fatty acid composition of L. fermentum, highlighting the consistency of this profile across strains.
Taranto et al. (2006) determined the dominant fatty acid in L. reuteri CRL 1098 to be palmitic acid (57.7%). Our study found a similar proportion of palmitic acid (40.07%) in L. reuteri cells, suggesting a relatively consistent fatty acid profile for this species. Similar results were observed for stearic acid (3.86% and 3.28%, respectively) and the ratio of oleic acid (C18:1,cis-9) to cis-vaccenic acid (C18:1,cis-11) ratio (0.70% and 0.62%). Comparison of cyclic acids was difficult due to limitations in the cited study (Taranto et al., 2006). The ability of L. reuteri strains to synthesize CLA from linoleic acid (C18:2,cis-9,cis-12) has been demonstrated previously (Jenkins and Courtney, 2003; Hernandez-Mendoza et al., 2009; Macouzet et al., 2010). Our study confirmed CLA synthesis by L. reuteri DSM 17938 at a level comparable to other strains. The dominant CLA profile was cis-9,trans-11-octadecadienoic acid, and the strain exhibited the ability to synthesize CLA even without added oleic acid. Lactic acid bacteria possess the ability to transform unsaturated fatty acids in their conjugated form (i.e., CLA) (Kishino et al., 2002; Lin et al., 2002; Lin et al., 2003; Ogawa et al., 2005; Lin, 2006).
Lactiplantibacillus plantarum
Our study examined three strains of L. plantarum, all of which exhibited efficient growth on MRS agar with or without Tween 80™. Interestingly, the fatty acid profiles of L. plantarum 299v and L. plantarum B.01149cultured on growth medium with Tween 80™ exhibiting profiles with 24 fatty acids, and B.01834 exhibiting a 24-fatty acid profile. Cultivation on growth medium with the addition of Tween 80™ resulted in the fatty acid profile of all three strains examined being dominated by palmitic acid (C16:0), dihydrosterculic acid, cis-vaccinic acid and lactobacillic acid (cycC19:0,cis-10,11), with the involvement of the last two of these fatty acids differentiating the strains examined (Table 5). Removal of Tween 80™ from the growth medium resulted in a decrease in the share of 11 fatty acids (4 saturated and 7 unsaturated) in the fatty acid profile and an increase in the share of 9 fatty acids (6 saturated and 3 unsaturated) in the fatty acid profile. It is also worth mentioning that the overall contribution of CLA to the fatty acid profile of the tested strains was also statistically reduced due to the removal of Tween 80™ from the growth medium. As a result, the proportion of total saturated and unsaturated fatty acids in the fatty acid profile increased significantly in some bacterial strains. Instead, a significant increase in the St/Lb ratio and a significant decrease in the O/V ratio were observed.
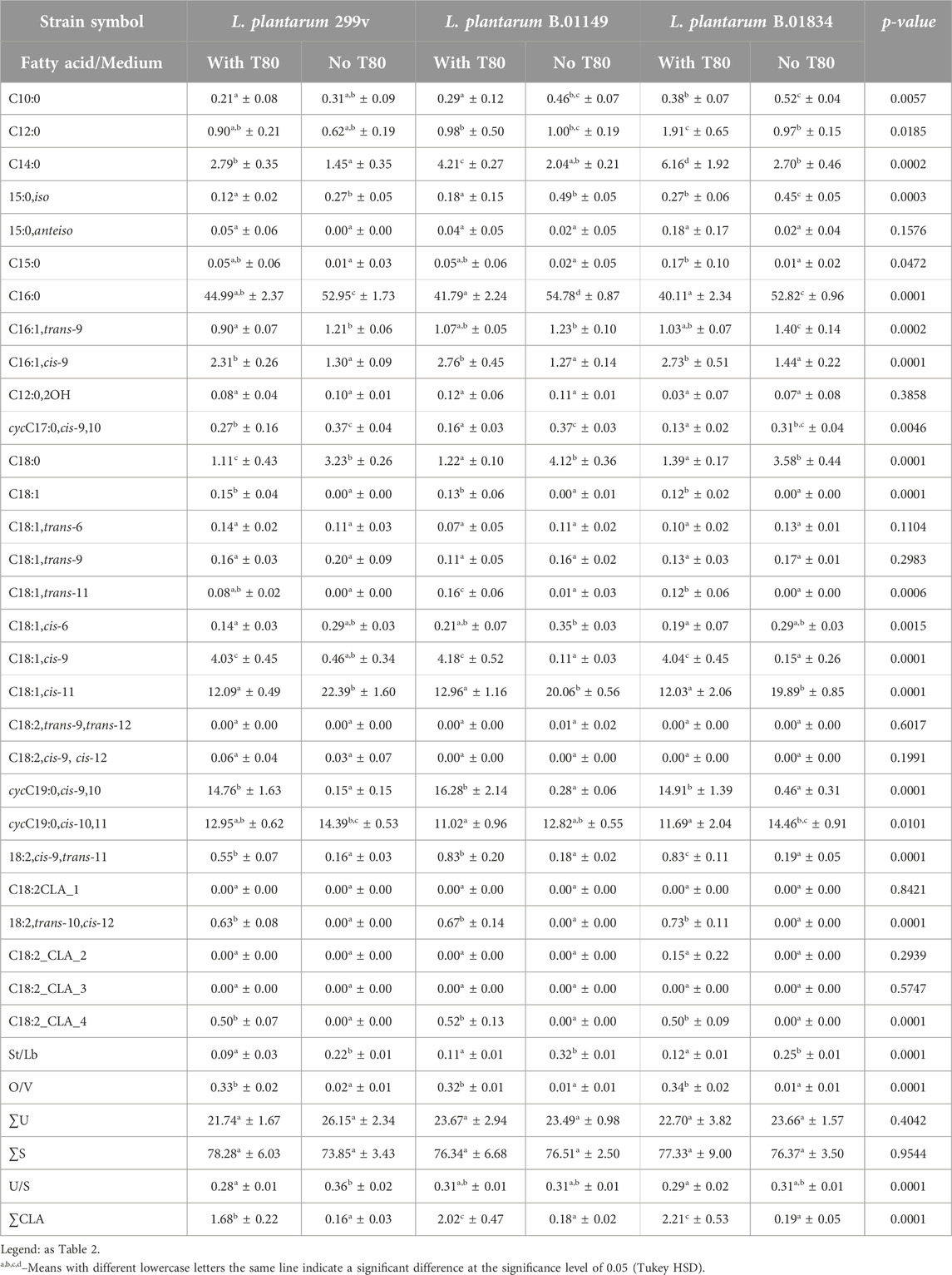
Table 5. The percentage fatty acids composition (mean ± SD, n = 6) in mesophilic Lactiplantibacillus plantarum strains profiles obtained from the cell cultured in a growth medium with Tween 80TM (with T80) and without Tween 80TM (no T80).
We identified discrepancies with Johnsson et al. (1995) regarding oleic acid (C18:1,cis-9) and lactobacillic acid (cycC19:0,cis-10,11) production by L. plantarum 2004. Our strains displayed a dominance of lactobacillic acid (11.02%–14.46%) and lower levels of oleic acid (up to 4.18%) compared to their findings. We attribute these differences to potential limitations in their methodology, such as sample size and incubation temperature. Our results for strains cultured without Tween 80™ showed a high proportion of palmitic acid (52.82%–54.78%), aligning with Rozès and Peres (1998). Both studies highlighted the prominence of C18:1 and cyclic C19:0 acids, although the specific compositions were not identical. Similarly, Russell et al. (1995) reported palmitic acid dominance, but stearic acid (C18:0) was not a major component in our analysis. These variations likely stem from differing incubation temperatures and analytical methods. Rizzo et al. (1987) identified palmitic (C16:0), cis-vaccenic (C18:1,cis-11), and cyclic C19:0 acids in L. plantarum ATCC 14917. However, their classification of the latter solely as lactobacillic acid (cycC19:0,cis-10,11) might not account for all possible isomers. L. plantarum’s ability to produce dihydrosterculic acid (cycC19:0,cis-9,10), as demonstrated by Johnsson et al. (1995), supports this notion. Our findings support this notion, revealing the presence of both lactobacillic (cycC19:0,cis-10,11) and dihydrosterculic (cycC19:0,cis-9,10) acids. Our research confirms the ability of L. plantarum strains to synthesize CLA (conjugated linoleic acid) from various substrates, as reported in previous studies (Ogawa et al., 2005; Kishino et al., 2009; Corsetti et al., 2010; Rodríguez-Alcalá et al., 2011). Studies by Kishino et al. (2009) CLA levels were significantly higher in cultures supplemented with Tween 80™ (1.67%–2.21%) compared to those without (0.16%–0.19%). The two predominant CLA isomers identified (cis-9,trans-11 and trans-10,cis-12) are consistent with Rodriguez-Alcalá et al. (2011). This study highlights the variability in fatty acid profiles of L. plantarum strains and emphasizes the need for standardized methodologies for accurate comparisons. We confirm the prevalence of palmitic (C16:0) and C18:1/cyclic C19:0 fatty acids, with lactobacillic acid (cycC19:0,cis-10,11) as the dominant cyclic form. Additionally, our findings support L. plantarum’s ability to produce CLA, with levels influenced by the presence of Tween 80™.
Principal components analysis
Fatty acids play a crucial role in the physiology and metabolism of lactobacilli. Their composition can influence various aspects of lactobacilli growth, function, and interaction with the environment. Understanding the fatty acid profiles of lactobacilli can provide valuable insights into their metabolic capabilities and potential applications. Of course, the fatty acid composition of lactobacilli can vary significantly depending on the strain and growth conditions. Principal components analysis revealed that growth medium supplementation with Tween 80™ significantly affected the fatty acid profiles of mesophilic lactobacilli strains, particularly the share of oleic acid (C18:1,cis-9), cis-vaccenic acid (C18:1,cis-11), lactobacillic acid (cycC19:0,cis-10,11), and dihydrosterculic acid (cycC19:0,cis-9,10). In general, the values of the first two principal components of the PCA statistical analysis of 57.53% are not too high and indicate that they do not fully explain the variability of the original data set. Nevertheless, the first two principal components may be statistically significant for the variables we are interested in. The PCA gives a quick overview of the connection between the different factors and the samples within. It should be noted that the first main component, which is 57.53% of the model variation, divided the cases into two main groups: the fatty acid profiles obtained from the growth medium with Tween 80™ and without Tween 80™ (Figure 1A). The first principal component separated the cases into two main groups based on the growth medium with or without Tween 80™ (Figure 1A). Notably, some L. casei strains did not cluster together, suggesting a potential taxonomic misclassification or high variability within this group. The high share of oleic acid (C18:1,cis-9) and CLA was important for the separation of the bacteria cultured on the growth medium with Tween 80™, while linoelaidic (C18:2,trans-9,trans-12), palmitelaidic (C16:1,trans-9), and cis-vaccenic (C18:1,cis-11) acids were important in case of growth medium without Tween 80™ (Figure 1B). This allowed for the separation of strains cultured on growth medium with Tween 80™ into two main groups: those cultured with Tween 80™ and cultured without Tween 80™. In contrast, no significant differentiation was observed for strains grown on the same growth medium without Tween 80™.
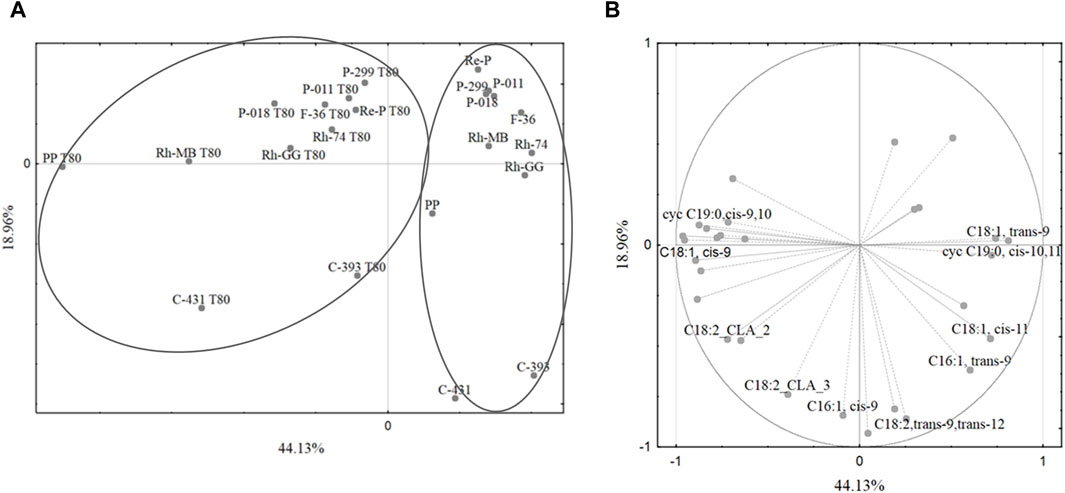
Figure 1. (A) The grouping of cases based on the shares of fatty acids in the mesophilic lactobacilli biomass. The projection of cases onto SG-1 (44.13%) and SG-2 (18.96%) plane. (B) The projection of variables (composition and shares of acid groups) onto SG-1 (44.13%) and SG-2 (18.96%) plane. The differentiation of mesophilic lactobacilli based on the fatty acids composition. Legend: as Table 2.
Recent taxonomic revisions suggest that some L. casei strains might be reclassified as L. paracasei (Ghosh et al., 2019). Unfortunately, we could not perform phylogenetic analysis on all our strains due to the lack of a genome sequence for C-431. Despite this uncertainty, we observed distinct fatty acid profiles among the three L. casei strains (potentially L. paracasei). However, further studies with a larger and well-characterized strain collection are necessary to confirm the discriminatory power of fatty acid profiles for differentiation between these closely related species.
The exact effects of the presence of Tween 80™ in the growth medium vary depending on the type of lactobacilli, but in general, Tween 80™ can increase saturated fatty acids and reduce unsaturated fatty acids. The presence of Tween 80™ in the growth medium generally increased the proportion of lactobacillic acid (cycC19:0,cis-10,11) in the fatty acid profile of all lactobacilli strains. This was due to the stimulation of the synthesis of lactobacillic acid (cycC19:0,cis-10,11) by lactobacilli cells in response to the absence of oleic acid (C18:1,cis-9) from Tween 80™. The presence of Tween 80™ in the growth medium had a mixed effect on the proportion of myristic acid (C14:0) and dihydrosterculic acid (cycC19:0,cis-9,10) in the fatty acid profile. In some lactobacilli strains, the proportion of myristic acid and dihydrosterculic acid (cycC19:0,cis-9,10) increased, while in other species, the proportion of myristic acid and dihydrosterculic acid (cycC19:0,cis-9,10) decreased. The presence of Tween 80™ in the growth medium significantly impacts the fatty acid profile of lactobacilli, generally increased the proportion of palmitic acid in the fatty acid profile of all lactobacilli strains. This was due to the incorporation of oleic acid (C18:1,cis-9) from Tween 80™ into the cell membranes of lactobacilli cells, which increased the demand for saturated fatty acids to maintain the fluidity of the cell membranes. While our data suggests a decrease in cis-vaccenic acid (C18:1,cis-11) in the fatty acid profile of all lactobacilli strains, further investigation is needed to elucidate the underlying mechanisms. Existing literature suggests that Tween 80™ might suppress early fatty acids synthesis, potentially affecting cis-vaccenic acid (C18:1,cis-11) production later in the growth cycle (Jacques et al., 1980; Zotta et al., 2017b; Reitermayer et al., 2018). Future studies employing time-course experiments could provide valuable insights into the dynamics of fatty acid biosynthesis and incorporation in the presence of Tween 80™, allowing for a more definitive understanding of potential competition or alternative pathways involved.
Conclusion
Our study investigated the influence of Tween 80™, a widely used surfactant in culturing media, on the fatty acid profile of selected mesophilic lactobacilli strains. The fatty acid composition of mesophilic lactobacilli cells plays a crucial role in determining their membrane properties, which are essential for various cellular functions, including cell growth, motility, and stress resistance. Our study investigated the impact of Tween 80™, a common surfactant used in bacterial culturing media, on the fatty acid profile of selected mesophilic lactobacilli strains. Tween 80™ has a significant impact on the fatty acid profile of lactobacilli. The presence of Tween 80™ in the growth medium can alter the proportion of various fatty acids, including saturated and unsaturated. The specific changes in fatty acid composition vary depending on the lactobacilli strains and species. One important aspect to consider is the role of the culturing environment in shaping the fatty acid profile of lactobacilli. Culture media can introduce external fatty acids, such as the oleic acid (C18:1,cis-9) present in Tween 80™, a common additive. Notably, oleic acid originating in Tween 80™ can be incorporated into lactobacilli membranes, contributing to the complex interplay between environmental factors and bacterial fatty acid biosynthesis in shaping membrane properties.
Our results reveal that the presence of Tween 80™ in the growth medium decreases the abundance of cis-vaccenic acid (C18:1,cis-11), a key contributor to membrane fluidity. This suggests that Tween 80™ may not only act as an external fatty acid source but also potentially compete with the bacteria’s own synthesis of cis-vaccenic acid (C18:1,cis-11). While competition with oleic acid (C18:1,cis-9) from Tween 80™ for membrane incorporation is a possibility, further investigation is needed. Existing literature suggests that Tween 80™ may initially suppress fatty acid synthesis, potentially impacting the availability of precursors for later cis-vaccenic acid (C18:1,cis-11) production. This decrease in cis-vaccenic acid (C18:1,cis-11) may lead to an increase in membrane rigidity, which could negatively impact cell growth, motility, and stress resistance.
Conversely, the addition of Tween 80™ to the growth medium had the opposite effect, increasing the proportion of cis-vaccenic acid (C18:1,cis-11), likely through precursor availability. This increase may help to maintain membrane fluidity, which is important for these cellular functions. The presence of Tween 80™ can increase the proportion of palmitic acid (C16:0) and lactobacillic acid (cycC19:0,cis-10,11). Palmitic acid is a saturated fatty acid that is involved in maintaining cell membrane structure and function. Lactobacillic acid (cycC19:0,cis-10,11) is a cyclopropane fatty acid that is unique to lactobacilli and is thought to play a role in their interactions with other organisms.
In conclusion, our study demonstrates that Tween 80™ significantly impact the fatty acid composition of lactobacilli. The presence of Tween 80™ can reduce the proportion of oleic acid and cis-vaccenic acid (C18:1,cis-11). Oleic acid is a major component of the cell membranes of lactobacilli, and Tween 80™ can compete with oleic acid for incorporation into the membranes. The results of our study are particularly relevant to the industrial application of mesophilic lactobacilli. These changes in fatty acid composition can in turn lead to changes in membrane properties, which could have implications for cell function.
Future studies could explore the specific mechanisms by which Tween 80™ influences fatty acid synthesis and the potential for strain-specific adaptations. Additionally, investigating the functional consequences of altered membrane fluidity on cell function would provide valuable insights for optimizing probiotic performance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MZ and DZ: data curation, conceptualization, formal analysis, visualization, writing–review and editing, and supervision. MZ: project administration, funding acquisition, and supervision. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Science Centre (NCN) in Poland, grant number N N312 121739.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Authors gratefully acknowledge the Institute of Food Sciences of Warsaw University of Life Sciences WULS–SGGW for supporting and providing necessary infrastructure and research stuff.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/abp.2024.13014/full#supplementary-material
References
Alexander, M. B. (2012). Rapid fatty acid and microbial identification using the MIDI sherlock® microbial identification system. Pharm. Regul. Aff. 2, 1–4. doi:10.4172/2167-7689.S1.001
Al-Naseri, A., Bowman, J. P., Wilson, R., Nilsson, R. E., and Britz, M. L. (2013). Impact of lactose starvation on the physiology of lactobacillus casei GCRL163 in the presence or absence of tween 80. J. Proteome Res. 12 (11), 5313–5322. doi:10.1021/pr400661g
Anderson, A. C., Sanunu, M., Schneider, C., Clad, A., Karygianni, L., Hellwig, E., et al. (2014). Rapid species-level identification of vaginal and oral lactobacilli using MALDI-TOF MS analysis and 16S rDNA sequencing. BMC Microbiol. 14 (1), 312. doi:10.1186/s12866-014-0312-5
Asadi, S. Z., Khosravi-Darani, K., Nikoopour, H., and Bakhoda, H. (2015). Evaluation of the effect of process variables on the fatty acid profile of single cell oil produced by mortierella using solid-state fermentation. Crit. Rev. Biotechnol. 35, 94–102. doi:10.3109/07388551.2013.804805
Beal, C., Fonseca, F., and Corrieu, G. (2001). Resistance to freezing and frozen storage of Streptococcus thermophilus is related to membrane fatty acid composition. J. Dairy Sci. 84 (11), 2347–2356. doi:10.3168/jds.S0022-0302(01)74683-8
Broadbent, J. R., Oberg, T. S., Hughes, J. E., Ward, R. E., Brighton, C., Welker, D. L., et al. (2014). Influence of polysorbate 80 and cyclopropane fatty acid synthase activity on lactic acid production by Lactobacillus casei ATCC 334 at low pH. J. Industrial Microbiol. Biotechnol. 41 (3), 545–553. doi:10.1007/s10295-013-1391-2
Brondz, I. (2002). Development of fatty acid analysis by high-performance liquid chromatography, gas chromatography, and related techniques. Anal. Chim. Acta, 465 (1), 1–37. doi:10.1016/S0003-2670(01)01467-2
Buyer, J. S. (2002). Rapid sample processing and fast gas chromatography for identification of bacteria by fatty acid analysis. J. Microbiol. Methods 51 (2), 209–215. doi:10.1016/S0167-7012(02)00081-7
Christie, W. W., Dobson, G., and Adlof, R. O. (2007). A practical guide to the isolation, analysis and identification of conjugated linoleic acid. Lipids 42 (12), 1073–1084. doi:10.1007/s11745-007-3107-8
Corcoran, B. M., Stanton, C., Fitzgerald, G. F., and Ross, R. P. (2007). Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiol. Read. Engl. 153 (Pt 1), 291–299. doi:10.1099/mic.0.28966-0
Corsetti, A., Tofalo, R., Schirone, M., Perpetuini, G., Quinto, M., and Suzzi, G. (2010). Bioconversion of linoleic acid to bioactive CLA isomers cis-9, trans-11 C18:2 and trans-10, cis-12 C18:2 by Lactobacillus casei- and Lactobacillus plantarum-group strains. J. Biotechnol. 150 (listopad), 338–339. doi:10.1016/j.jbiotec.2010.09.358
Cronan, J. E. (2002). Phospholipid modifications in bacteria. Curr. Opin. Microbiol. 5 (2), 202–205. doi:10.1016/s1369-5274(02)00297-7
Fonseca, F., Pénicaud, C., Tymczyszyn, E. E., Gómez-Zavaglia, A., and Passot, S. (2019). Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 103 (17), 6867–6883. doi:10.1007/s00253-019-10002-1
Foschi, C., Laghi, L., Parolin, C., Giordani, B., Compri, M., Cevenini, R., et al. (2017). Novel approaches for the taxonomic and metabolic characterization of lactobacilli: integration of 16S rRNA gene sequencing with MALDI-TOF MS and 1H-nmr. PLOS ONE 12 (2), e0172483. doi:10.1371/journal.pone.0172483
Ghosh, S., Sarangi, A. N., Mukherjee, M., Bhowmick, S., and Tripathy, S. (2019). Reanalysis of lactobacillus paracasei Lbs2 strain and large-scale comparative genomics places many strains into their correct taxonomic position. Microorganisms 7 (11), 487. doi:10.3390/microorganisms7110487
Haack, S. K., Garchow, H., Odelson, D. A., Forney, L. J., and Klug, M. J. (1994). Accuracy, reproducibility, and interpretation of fatty acid methyl ester profiles of model bacterial communities. Appl. Environ. Microbiol. 60 (7), 2483–2493. doi:10.1128/aem.60.7.2483-2493.1994
Hansen, M.-L. R. W., Petersen, M. A., Risbo, J., Hümmer, M., and Clausen, A. (2015). Implications of modifying membrane fatty acid composition on membrane oxidation, integrity, and storage viability of freeze-dried probiotic, lactobacillus acidophilus La-5. Biotechnol. Prog. 31 (3), 799–807. doi:10.1002/btpr.2074
Henderson, T. O., and McNeill, J. (1966). The control of fatty acid synthesis in Lactobacillus plantarum. Biochem. Biophysical Res. Commun. 25 (6), 662–669. doi:10.1016/0006-291X(66)90506-7
Hernandez-Mendoza, A., Lopez-Hernandez, A., Hill, C. G., and Garcia, H. S. (2009). Bioconversion of linoleic acid to conjugated linoleic acid by Lactobacillus reuteri under different growth conditions. J. Chem. Technol. Biotechnol. 84 (2), 180–185. doi:10.1002/jctb.2021
Ianniello, R. G., Zotta, T., Matera, A., Genovese, F., Parente, E., and Ricciardi, A. (2016). Investigation of factors affecting aerobic and respiratory growth in the oxygen-tolerant strain lactobacillus casei N87. PLOS ONE 11 (11), e0164065. doi:10.1371/journal.pone.0164065
Jacques, N. A., Hardy, L., Knox, K. W., and Wicken, A. J. (1980). Effect of tween 80 on the morphology and physiology of lactobacillus salivarius strain IV CL-37 grown in a chemostat under glucose limitation. Microbiology 119 (1), 195–201. doi:10.1099/00221287-119-1-195
Jenkins, J. K., and Courtney, P. D. (2003). Lactobacillus growth and membrane composition in the presence of linoleic or conjugated linoleic acid. Can. J. Microbiol. 49 (1), 51–57. doi:10.1139/w03-003
Jimenez, N. R., Maarsingh, J. D., Łaniewski, P., and Herbst-Kralovetz, M. M. (2023). Commensal lactobacilli metabolically contribute to cervical epithelial homeostasis in a species-specific manner. mSphere 8 (1), e0045222–22. doi:10.1128/msphere.00452-22
Johnsson, T., Nikkila, P., Toivonen, L., Rosenqvist, H., and Laakso, S. (1995). Cellular Fatty Acid profiles of lactobacillus and lactococcus strains in relation to the oleic Acid content of the cultivation medium. Appl. Environ. Microbiol. 61 (12), 4497–4499. doi:10.1128/aem.61.12.4497-4499.1995
Kaneda, T. (1991). Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55 (2), 288–302. doi:10.1128/mr.55.2.288-302.1991
Kankaanpaa, P., Yang, B., Kallio, H., Isolauri, E., and Salminen, S. (2004). Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl. Environ. Microbiol. 70 (1), 129–136. doi:10.1128/AEM.70.1.129-136.2004
Kishino, S., Ogawa, J., Ando, A., Omura, Y., and Shimizu, S. (2002). Ricinoleic acid and Castor oil as substrates for conjugated linoleic acid production by washed cells of lactobacillus plantarum. Biosci. Biotechnol. Biochem. 66 (10), 2283–2286. doi:10.1271/bbb.66.2283
Kishino, S., Ogawa, J., Yokozeki, K., and Shimizu, S. (2009). Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Appl. Microbiol. Biotechnol. 84 (1), 87–97. doi:10.1007/s00253-009-1949-0
Klein, D., Breuch, R., Reinmüller, J., Engelhard, C., and Kaul, P. (2022). Discrimination of stressed and non-stressed food-related bacteria using Raman-microspectroscopy. Foods 11 (10), 1506. doi:10.3390/foods11101506
Kunitsky, C. J., Osterhout, G., and Sasser, M. (2006). Identification of microorganisms using fatty acid methyl ester (FAME) analysis and the MIDI Sherlock ® microbial identification system. Available at: https://www.semanticscholar.org/paper/1-1-IDENTIFICATION-OF-MICROORGANISMS-USING-FATTY-(-Kunitsky-Osterhout/a1aa6423f0e19df3a204a3f7d07cf0bc07e17918.
Li, J. Y., Zhang, L. W., Du, M., Han, X., Yi, H. X., Guo, C. F., et al. (2011). Effect of tween series on growth and cis-9, trans-11 conjugated linoleic acid production of lactobacillus acidophilus F0221 in the presence of bile salts. Int. J. Mol. Sci. 12 (12), 9138–9154. doi:10.3390/ijms12129138
Li, Q., Lei, Y., Hu, G., Lei, Y., and Dan, D. (2018). Effects of Tween 80 on the liquid fermentation of Lentinus edodes. Food Sci. Biotechnol. 27 (4), 1103–1109. doi:10.1007/s10068-018-0339-8
Lin, T. Y. (2006). Conjugated linoleic acid production by cells and enzyme extract of Lactobacillus delbrueckii ssp. bulgaricus with additions of different fatty acids. Food Chem. 94 (3), 437–441. doi:10.1016/j.foodchem.2004.11.032
Lin, T. y., Lin, C. W., and Wang, Y. J. (2002). Linoleic acid isomerase activity in enzyme extracts from lactobacillus acidophilus and propionibacterium freudenreichii Ssp. shermanii. J. Food Sci. 67 (4), 1502–1505. doi:10.1111/j.1365-2621.2002.tb10312.x
Lin, T. Y., Lin, C.-W., and Wang, Y. J. (2003). Production of conjugated linoleic acid by enzyme extract of Lactobacillus acidophilus CCRC 14079. Food Chem. 83 (1), 27–31. doi:10.1016/S0308-8146(03)00032-3
Liong, M. T., and Shah, N. P. (2005). Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 88 (1), 55–66. doi:10.3168/jds.S0022-0302(05)72662-X
López de Felipe, F. (2023). Revised aspects into the molecular bases of hydroxycinnamic acid metabolism in lactobacilli. Antioxidants 12 (6), 1294. doi:10.3390/antiox12061294
Machado, M. C., López, C. S., Heras, H., and Rivas, E. A. (2004). Osmotic response in lactobacillus casei ATCC 393: Biochemical and biophysical characteristics of membrane. Archives Biochem. Biophysics 422 (1), 61–70. doi:10.1016/j.abb.2003.11.001
Macouzet, M., Lee, B. h., and Robert, N. (2010). Genetic and structural comparison of linoleate isomerases from selected food-grade bacteria. J. Appl. Microbiol. 109 (6), 2128–2134. doi:10.1111/j.1365-2672.2010.04844.x
Montanari, C., Sado Kamdem, S. L., Serrazanetti, D. I., Etoa, F. X., and Guerzoni, M. E. (2010). Synthesis of cyclopropane fatty acids in lactobacillus helveticus and lactobacillus sanfranciscensis and their cellular fatty acids changes following short term acid and cold stresses. Food Microbiol. 27 (4), 493–502. doi:10.1016/j.fm.2009.12.003
Murga, M. L. F., de Valdez, G. F., and Disalvo, E. (2001). Effect of lipid composition on the stability of cellular membranes during freeze–thawing of lactobacillus acidophilus grown at different temperatures. Archives Biochem. Biophysics 388 (2), 179–184. doi:10.1006/abbi.2001.2274
Nacef, M., Chevalier, M., Chollet, S., Drider, D., and Flahaut, C. (2017). MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a French cheese: the maroilles. Int. J. Food Microbiol. 247 (kwiecień), 2–8. doi:10.1016/j.ijfoodmicro.2016.07.005
Ogawa, J., Kishino, S., Ando, A., Sugimoto, S., Mihara, K., and Shimizu, S. (2005). Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 100 (4), 355–364. doi:10.1263/jbb.100.355
Parsons, J. B., and Rock, C. O. (2013). Bacterial lipids: metabolism and membrane homeostasis. Prog. Lipid Res. 52 (3), 249–276. doi:10.1016/j.plipres.2013.02.002
Partanen, L., Marttinen, N., and Alatossava, T. (2001). Fats and fatty acids as growth factors for lactobacillus delbrueckii. Syst. Appl. Microbiol. 24 (4), 500–506. doi:10.1078/0723-2020-00078
Pawar, K., and Aranha, C. (2022). Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr. Res. Toxicol. 3 (styczeń), 100088. doi:10.1016/j.crtox.2022.100088
Reitermayer, D., Kafka, T. A., Lenz, C. A., and Vogel, R. F. (2018). Interrelation between Tween and the membrane properties and high pressure tolerance of Lactobacillus plantarum. BMC Microbiol. 18 (1), 72. doi:10.1186/s12866-018-1203-y
Rizzo, A. F., Korkeala, H., and Mononen, I. (1987). Gas chromatography analysis of cellular fatty acids and neutral monosaccharides in the identification of lactobacilli. Appl. Environ. Microbiol. 53 (12), 2883–2888. doi:10.1128/aem.53.12.2883-2888.1987
Rodrigues, D., Rocha-Santos, T. A. P., Gomes, A. M., Goodfellow, B. J., and Freitas, A. C. (2012). Lipolysis in probiotic and synbiotic cheese: the influence of probiotic bacteria, prebiotic compounds and ripening time on free fatty acid profiles. Food Chem. 131 (4), 1414–1421. doi:10.1016/j.foodchem.2011.10.010
Rodríguez-Alcalá, L. M., Braga, T., Xavier Malcata, F., Gomes, A., and Fontecha, J. (2011). Quantitative and qualitative determination of CLA produced by Bifidobacterium and lactic acid bacteria by combining spectrophotometric and Ag+-HPLC techniques. Food Chem. 125 (4), 1373–1378. doi:10.1016/j.foodchem.2010.10.008
Rouch, D., Hillier, A., and Britz, M. (2002). NSLAB in cheddar: A stressful life. Aust. J. Dairy Technol. 57 (2), 107.
Rozès, N., and Peres, C. (1998). Effects of phenolic compounds on the growth and the fatty acid composition of lactobacillus plantarum. Appl. Microbiol. Biotechnol. 49 (1), 108–111. doi:10.1007/s002530051145
Russell, N. J., Evans, R. I., ter Steeg, P. F., Hellemons, J., Verheul, A., and Abee, T. (1995). Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28 (2), 255–261. doi:10.1016/0168-1605(95)00061-5
Santivarangkna, C., Kulozik, U., and Foerst, P. (2008). Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 105 (1), 1–13. doi:10.1111/j.1365-2672.2008.03744.x
Schujman, G. E., and de Mendoza, D. (2008). Regulation of type II fatty acid synthase in Gram-positive bacteria. Curr. Opin. Microbiol. 11 (2), 148–152. doi:10.1016/j.mib.2008.02.002
Tan, W. S., Budinich, M. F., Ward, R., Broadbent, J. R., and Steele, J. L. (2012). Optimal growth of Lactobacillus casei in a Cheddar cheese ripening model system requires exogenous fatty acids. J. Dairy Sci. 95 (4), 1680–1689. doi:10.3168/jds.2011-4847
Taranto, M. P., Perez-Martinez, G., and Font de Valdez, G. (2006). Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res. Microbiol. 157 (8), 720–725. doi:10.1016/j.resmic.2006.04.002
Terraf, M. C. L., Juárez Tomás, M. S., Nader-Macías, M. E. F., and Silva, C. (2012). Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 113 (6), 1517–1529. doi:10.1111/j.1365-2672.2012.05429.x
Weeks, G., and Wakil, S. J. (1970). Studies on the control of fatty acid metabolism: II. The inhibition of fatty acid synthesis in lactobacillus plantarum by exogenous fatty acid. J. Biol. Chem. 245 (8), 1913–1921. doi:10.1016/S0021-9258(18)63185-0
Wu, P., An, J., Chen, L., Zhu, Q., Li, Y., Mei, Y., et al. (2020). Differential analysis of stress tolerance and transcriptome of probiotic Lacticaseibacillus casei zhang produced from solid-state (SSF-SW) and liquid-state (LSF-MRS) fermentations. Microorganisms 8 (11), 1656. doi:10.3390/microorganisms8111656
Zhang, G., Liu, L., and Li, C. (2020). Effects of ccpA gene deficiency in Lactobacillus delbrueckii subsp. bulgaricus under aerobic conditions as assessed by proteomic analysis. Microb. Cell Factories 19 (1), 9. doi:10.1186/s12934-020-1278-7
Zhu, L., Zou, Q., Cao, X., and Cronan, J. E. (2019). Enterococcus faecalis encodes an atypical auxiliary acyl carrier protein required for efficient regulation of fatty acid synthesis by exogenous fatty acids. mBio 10 (3), e00577-19. doi:10.1128/mBio.00577-19
Zotta, T., Parente, E., and Ricciardi, A. (2017a). Aerobic metabolism in the genus lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 122 (4), 857–869. doi:10.1111/jam.13399
Keywords: lactic acid bacteria, lactobacilli, fatty acid profile, functionality, Tween 80TM
Citation: Zaręba D and Ziarno M (2024) Tween 80™-induced changes in fatty acid profile of selected mesophilic lactobacilli. Acta Biochim. Pol 71:13014. doi: 10.3389/abp.2024.13014
Received: 20 March 2024; Accepted: 19 June 2024;
Published: 04 July 2024.
Edited by:
Arun S. Kharat, Jawaharlal Nehru University, IndiaReviewed by:
Margaret Lorraine Britz, University of Tasmania, AustraliaGhada A. Mohammad, University of Mosul, Iraq
Copyright © 2024 Zaręba and Ziarno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Małgorzata Ziarno, bWFsZ29yemF0YV96aWFybm9Ac2dndy5lZHUucGw=
 Dorota Zaręba1
Dorota Zaręba1 Małgorzata Ziarno
Małgorzata Ziarno