Abstract
The presented seroprevalence study focused on specific antibodies to the SARS-CoV-2 virus is the second survey conducted among SAS employees. Its realization enabled monitoring of the impact of booster vaccination doses and the spread of the Omicron variant in a defined group of people. The total seropositivity of the involved SAS employees in autumn 2022 was 96.04%. In the group of vaccinated participants (1,189) the seropositivity rate was 99.5%, while among unvaccinated participants (176) it was 72.73%. By October 2022, when the study was conducted, 65.05% (888) of the participants have had a positive PCR/Ag test for SARS-CoV-2 at least once. Based on the presence of antibodies against the nucleoprotein (NCP) of SARS-CoV-2 it was proven, that 27.39% of participants (25.12% of vaccinated; 51.22% of non-vaccinated) who have never had a positive PCR/Ag test for SARS-CoV-2, overcame the COVID-19. According to self-assessment of the disease course, it was shown that a severe course occurred in 6.31% of the participants who overcame the disease without prior vaccination and in 1.44% of the participants who overcame COVID-19 after completing the baseline vaccination scheme. The most significant finding of the study is the evidence of significantly lower levels of specific antibodies after overcoming the Omicron variant of SARS-CoV-2, and thus its reduced immunogenicity compared to ancestral virus and earlier variants of concern.
Introduction
As of 11 March 2023, 3 years have passed since the declaration of the COVID-19 pandemic by the World Health Organization (WHO). During this period hundreds of millions of people have encountered the disease COVID-19 and more than 6.7 million people worldwide died. It became clear that due to the huge extent of the SARS-CoV-2 pandemic, the virus had the potential to mutate relatively effectively and create a number of variants (Carabelli et al., 2023). Among them, the so-called variants of concern (VOC1) have proven to be the most dangerous for the spreading of infection. As seen in many countries, pandemic waves were associated with the emergence of new VOC. A similar situation was also observed in Slovakia. The first wave associated with the spread of the original Wuhan-Hu-1 strain lasted from March to May 2020 and did not cause significant loss of human life, mainly thanks to the timely and stringent anti-epidemic measures of the government and the willingness of the population to follow the measures. The second wave hit the country between September 2020 and May 2021 and was caused by the spread of the B.1.1.7 (alpha) variant (Boršová et al., 2021). Unlike the first, the second wave resulted in several thousand deaths, an overload of the healthcare system, and was accompanied by a negative mood in society. In this period (December 2020), vaccination against COVID-19 began in Slovakia. The third pandemic wave hit the country in response to the spread of the B.1.617.2 VOC (delta variant) in autumn 2021 to spring 2022. Subsequently, the omicron variant (BA.1) spread gradually from December 2021, quickly displacing the delta variant. At the end of January 2022, it already dominated the sequenced samples in Slovakia (Rusňáková et al., 2022). According to the current sequencing data (March 2023) Omicron BA.5 subvariant dominated in Slovakia at the end of the year 2022. Until present days more than 21,000 COVID-19 victims were registered in the country. Such a number of deaths ranks the country fifth in the EU in terms of the number of deaths per 100,000 inhabitants. With a vaccination rate of 51.0% of the population, Slovakia, together with Romania and Bulgaria, is one of the countries with the lowest vaccination rate in the European Union2.
In general, monitoring antibody levels in response to infection or vaccination is a reasonable approach to disease surveillance. This is especially desirable in countries with a lower willingness towards vaccination, such as Slovakia, in order to better predict the population’s resistance to the COVID-19 disease. Due to the rapid spread of COVID-19, the constant emergence of SARS-CoV-2 mutations, and the lack of effective and affordable treatment, vaccination appears to be an effective way to control the disease, as it is known to be in other infectious diseases.
From available studies, it is clear that the immunological response to the virus or vaccination is mediated by both humoral and cellular immunity and protects the individual from a severe course of the disease that can eventually lead to hospitalization (Pilz et al., 2022; Spinardi and Srivastava, 2023). From an epidemiological point of view monitoring the level of antibodies in the population is of great importance. SARS-CoV-2 is a new human virus with the ability to create new variants with an increased potential to escape acquired immunity. A reliable indicator of overcoming the infection or completing the vaccination is the level of specific IgG antibodies. The most dominant glycoprotein in SARS-CoV-2 is the S-protein, which contains a receptor binding domain (RBD) to which most virus-neutralizing antibodies bind (Rashid et al., 2022). The second immunodominant protein is the viral NCP. Anti-NCP IgG antibodies are formed only in people who have overcome virus infection (Castro Dopico et al., 2022). Based on their presence, it is possible to detect the population that came into contact with the natural virus and to distinguish between uninfected people and those vaccinated with an S-protein-directed vaccine.
Since the outbreak of the pandemic, much attention has been paid to the issue of the formation and persistence of antibodies against the SARS-CoV-2 virus. Our current work follows up the study carried out in 2021 pointing to the formation and persistence of antibodies against SARS-CoV-2 in the employees of the Slovak Academy of Sciences (Kajanova et al., 2021). The additional aim of our study was to compare the suitability of two ELISA tests for the detection of antibodies produced in response to the Omicron variant of the SARS-CoV-2 infection, to indicate the immunogenicity of the Omicron variant of the virus, to identify retrospectively COVID-19 prevalence based on the presence of anti-NCP IgG, and to estimate the effect of vaccination on the severity of the subsequent COVID-19 disease.
Materials and methods
Study participants
Electronic invitation to take part in the study was sent to the employees of the research institutes/centers as well as of non-research organizations of the Slovak Academy of Sciences (SAS). After reading the information for participants and agreeing to participate in the study, the volunteers filled out the anamnestic questionnaire and received a collection set for the self-sampling of capillary blood with detailed instructions. Collection cards with dry blood spot samples were sent back to the laboratory at the Biomedical Research Center of the SAS within 2–3 days following collection. The blood samples were analyzed as described below. The study included participants regardless of their vaccination status or reported contact with the SARS-CoV-2 virus.
Collection of data and ethical approvals
The study was approved by the independent Ethics Committee of the Bratislava self-governing region, which approved the study by its decision No. 09833/2020/HF and amendment 07071/2021 from 30 June 2021. All participants provided their signed consent after reading the information about the purpose and form of the study. The anamnestic information collected from the volunteers included age, gender, height and weight, vaccine type(s), date(s) of vaccination, and/or date(s) of onset and severity of COVID-19 disease. The severity of the disease was rated on a scale from 1 to 10 by the participants themselves. All personal data provided by the volunteers were handled in compliance with the Personal Data Protection Act and other generally binding legal regulations.
Sample collection and preparation for ELISA
Dry blood spot samples were obtained by participants, who performed self-collection of capillary blood by lancet pricking of a fingertip using the in-house collection set assembled at the Biomedical Research Center of the SAS [as described in (Kajanova et al., 2021)]. Blood drops were allowed to fall on the collection card and left to dry for 3–4 h in the open air. The defined area of the card was punched out and submerged into the ELISA sample buffer for 1 h at 37°C. The extracted blood sample was then used for the serological analysis using ELISA as described below. The use of dry capillary blood spot samples was validated by parallel testing of venous blood samples of selected individuals (data not shown).
Serological analysis
Seroprevalence of antibodies to the SARS-CoV-2 virus was evaluated using an anti-SARS-CoV-2 IgG ELISA (EI 2606-9601 G, EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany), which detects IgG antibodies specifically binding to the SARS-CoV-2-encoded spike protein subunit 1 (S1) (anti-S1 IgG) containing the immunologically relevant receptor binding domain (RBD). The specificity of the anti-SARS-CoV-2 IgG ELISA of 99.6% and sensitivity of 94.4% in samples collected after day 10 post-symptoms was determined by the manufacturer. The assay was performed according to the manufacturer’s protocol and recommendations, considering a signal-to-calibrator ratio of <0.8 negative, 0.8 to <1.1 borderiline, and ≥ 1.1 positive. The presence of antibodies specific to the SARS-CoV-2 nucleoprotein (anti-NCP IgG) was determined in the Anti-SARS-CoV-2 NCP ELISA test (EI2606-9601-2 G, EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany). In the ELISA tests comparison process, we also used a test developed for the detection of antibodies created after overcoming the Omicron variant – Anti-SARS-CoV-2 Omicron ELISA (EI 2606-9601-30 G, EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany). Participants were informed about their personal test results with a comment that the result provides only a partial picture of an immune response and cannot specify an individual’s risk of subsequent infection.
Statistics
Data were categorized according to selected characteristics and subjected to statistical analysis. Each analysis included only participants who provided all necessary data. Statistical analyses of the obtained data were performed using GraphPad Prism 9.5.1. Categorical variables were expressed as numbers (%), and continuous variables as median (interquartile range, IQR) depending on the normality of their distribution. Pairwise comparisons were performed by the Student’s t-test. Group differences were analyzed by one-way ANOVA depending on the normality of the data distributions with the appropriate post hoc tests for pairwise multiple comparison procedures. The prevalence of different variables was compared using the χ2 -test. Correlations were examined using Pearson’s or Spearman’s correlation coefficient, depending on the normality of the data. A p-value of < 0.05 was considered statistically significant.
Results
Basic characteristics of the study cohort
A total of 1,365 people (912 women and 452 men) participated in the second antibody study carried out among employees of the Slovak Academy of Sciences. The median age of the participants was 46 years and the median BMI was 24.2 kg/m2. In total, at the time of the study, 87.11% of participants were vaccinated against the disease COVID-19, and 65.05% of the participants had already overcome COVID-19 confirmed by an antigen or PCR test at some point in the past. The characteristics of the participants, including the number of vaccination doses and repeated occurrences of the COVID-19 disease, are presented in Tables 1, 2.
TABLE 1
| n | % | ||
|---|---|---|---|
| Females | 912 | 66.81 | |
| Males | 452 | 33.11 | |
| Other | 1 | 0.07 | |
| Age (years), median (IQR) | 46 (21) | ||
| BMI (kg/m2), median (IQR) | 24.2 (6.06) |
Basic characteristics of the study cohort as reported by the participants in the questionnaire.
TABLE 2
| Total | n | % | % | |
|---|---|---|---|---|
| 1,365 | 100.00 | |||
| Unvaccinated | 176 | 12.89 | 100.00 | |
| No pos. Ag/PCR test | 41 | 23.30 | ||
| Pos. Ag/PCR test | 135 | 76.70 | ||
| Vaccinated | 1,189 | 87.11 | 100.00 | |
| 1 dose | 30 | 2.52 | ||
| 2 doses | 232 | 19.51 | ||
| 3 doses | 920 | 77.38 | ||
| 4 doses | 7 | 0.59 | ||
| No pos. Ag/PCR test | 436 | 36.67 | ||
| Pos. Ag/PCR test | 753 | 63.33 | ||
| No positive Ag/PCR test | 477 | 34.95 | ||
| Positive Ag/PCR test | 888 | 65.05 | 100.00 | |
| Pos. Ag/PCR test 1x | 747 | 84.12 | ||
| Pos. Ag/PCR test 2x | 136 | 15.32 | ||
| Pos. Ag/PCR test 3x | 5 | 0.56 |
Characteristics of the study cohort as reported by the participants in the questionnaire based on the vaccination status and positivity of testing for COVID-19.
Italic values represent the respective percentages with total of 100% in the respective subgroups (unvaccinated, vaccinated, positive Ag/PCR test).
Antibody levels of vaccinated and non-vaccinated participants
Since our study was performed at a time when a significant part of the population had been vaccinated against SARS-CoV-2 and/or had overcome the disease, it is not surprising that we detected specific antibodies against SARS-CoV-2 in up to 96.04% of participants. However, it was still possible to observe differences in positivity and antibody levels in vaccinated and unvaccinated persons. In the unvaccinated group, 27.27% of participants did not have specific antibodies against SARS-CoV-2. In contrast, there were only 0.50% of participants with negative antibodies in the vaccinated group. There was also a significant difference in the measured levels of antibodies between those two groups; in vaccinated participants, the median level was 9.82 S/C, whereas in unvaccinated participants the median was 2.19 S/C, p < 0.0001 (Table 3). Comparison of the seropositivity and IgG antibody levels in subjects who had vs. had never a positive Ag/PCR test for COVID-19 in the past showed a less pronounced but significant difference, which is summarized in Table 3.
TABLE 3
| Antibody status | n | % | % | Anti-S1 IgG median | |
|---|---|---|---|---|---|
| Total | 1,365 | 100.00 | 9.49 | ||
| Positive + borderline | 1,311 | 96.04 | |||
| Negative | 54 | 3.96 | |||
| Unvaccinated | 176 | 12.89 | 100.00 | 2.19 | |
| Positive + borderline | 128 | 72.73 | |||
| Negative | 48 | 27.27 | |||
| Vaccinated | 1,189 | 87.11 | 100.00 | 9.82 | |
| Positive + borderline | 1,183 | 99.50 | |||
| Negative | 6 | 0.50 | |||
| No pos. Ag/PCR test | 477 | 34.95 | 100.00 | 8.64 | |
| Positive + borderline | 446 | 93.50 | |||
| Negative | 31 | 6.50 | |||
| Pos. Ag/PCR test | 888 | 65.05 | 100.00 | 9.79 | |
| Positive + borderline | 865 | 97.41 | |||
| Negative | 23 | 2.59 |
Characteristics of the study cohort based on the seropositivity.
Italic values represents % from each group.
According to the vaccination status and overcoming the disease COVID-19, we divided the study participants into 4 groups. The first group consisted of participants who were not vaccinated and had never tested positive for SARS-CoV-2 (median 0.60 S/C), the second group consisted of unvaccinated participants who had overcome the disease COVID-19 at least once (median 3.51 S/C). The third group included vaccinated individuals who have never tested positive for COVID-19 (median 9.02 S/C) and the fourth group included vaccinated participants who have tested positive for COVID-19 either before or after vaccination (median 10.09 S/C). Our results confirm the findings of others [as reviewed in (Pilz et al., 2022)] that the levels of specific antibodies against SARS-CoV-2 were the highest in people with hybrid immunity (Figure 1A).
FIGURE 1
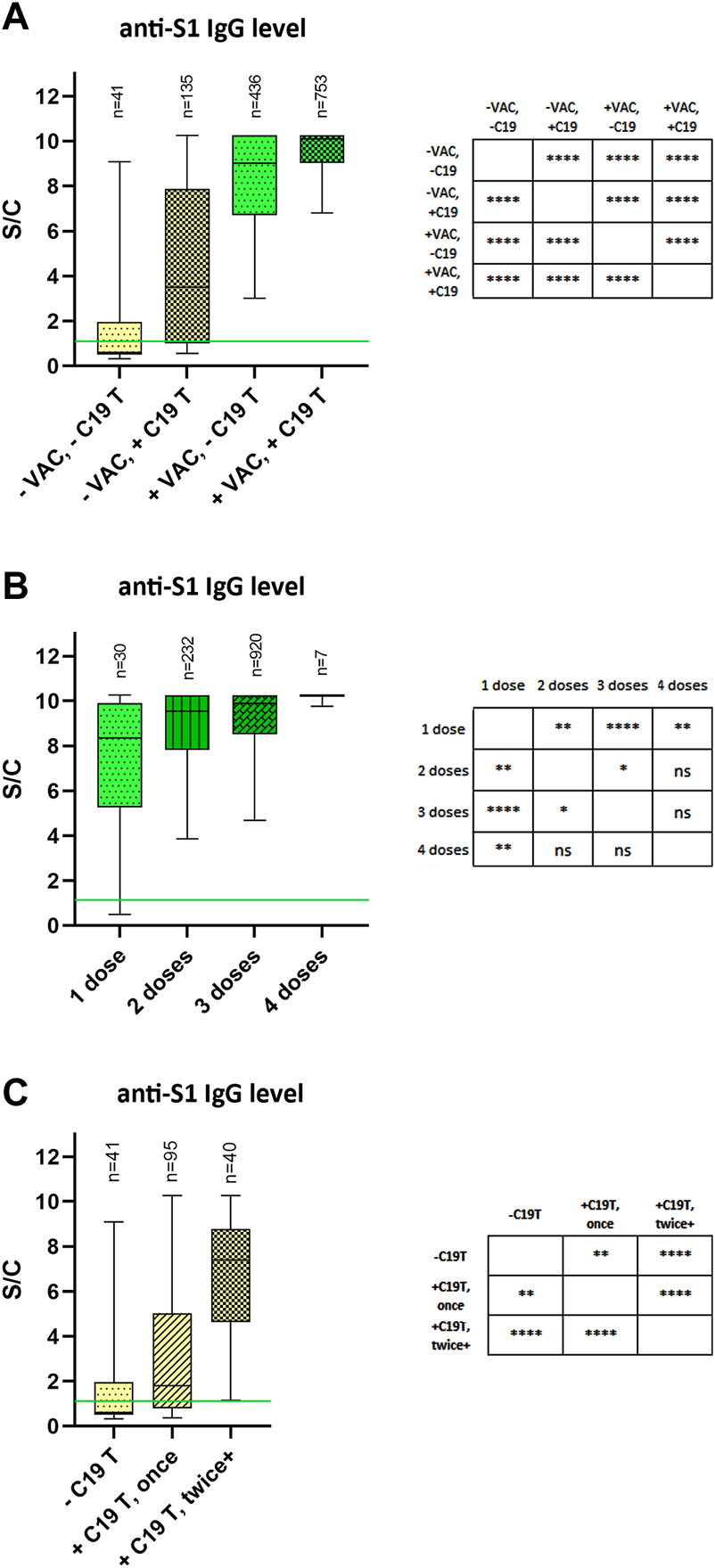
Relative SARS-CoV-2 anti-S1 IgG antibody levels (A) Participants were stratified according to the vaccination status and positivity of testing for COVID-19. (B) Vaccinated participants were stratified according to the number of received COVID-19 vaccine doses (regardless of the manufacturer). (C) Unvaccinated participants were stratified according to the number of COVID-19-positive Ag/PCR tests. The data are expressed as a ratio of signal to calibrator (S/C). The median is represented by a horizontal line within boxes. The area of boxes represents the 25th–75th percentile and whiskers the 5th–95th percentile of the measured values in the group. The number of samples in each group is shown on the top of the graphs as “n.” Green lines represent the limit of test positivity = 1.1 S/C. −VAC = did not receive any dose of the vaccine; +VAC = received at least one dose of the vaccine; −C19 T = no positive Ab/PCR test for SARS-CoV-2 in the past; +C19 T = at least one positive Ab/PCR test for SARS-CoV-2 in the past; **** = p< 0.0001, ** = p< 0.01, * = p< 0.05.
The present study also confirms the previous results of increasing levels of specific IgG antibodies against the S-protein of SARS-CoV-2 with an increasing number of vaccine doses administered, and/or with multiple instances of overcoming the disease (Figures 1B, C).
The study participants received vaccines from different manufacturers (Comirnaty, Vaxzevria, Spikevax, Jansen, SputnikV, Covishield). Since many individuals were vaccinated with vaccines from several manufacturers and we already monitored the effectiveness of individual vaccine types for the production of antibodies in our previous paper (Kajanova et al., 2021), this particular analysis was not included in this publication.
Suitability of the test to detect the antibodies after Omicron variant (B.1.1.529) SARS-CoV-2 infection
The company, whose ELISA tests were used in this study, also developed a test adapted to the Omicron variant based on the recombinant S1 domain of the spike protein of the SARS-CoV-2 Omicron variant as antigen (EI 2606-9601-30 G). We decided to compare the original ELISA test determining S-protein antibodies against SARS-CoV-2 (EI 2606–9601 G) with ELISA test EI 2606-9601-30 G. For comparison, we used only samples from those participants who positively tested for SARS-CoV-2 exclusively after 1 January 2022 (assumption of the presence of Omicron variant in Slovakia). In 54 unvaccinated volunteers who have overcome only the Omicron variant of the virus, we measured comparable antibody levels by both ELISA tests: EI 2606-9601 G = median 0.92 S/C vs. EI 2606-9601-30 G = median 0.83 S/C (Figure 2A). Although there was a significant difference (p < 0.001), the measured levels in both tests correlated robustly (r = 0.806, p < 0.0001). The higher levels seen in the original ELISA test EI 2606-9601 G suggested an underestimation of antibody levels by the new test EI 2606-9601-30 G (Figure 2B). In a randomly selected 54 samples from the vaccinated “+VAC” group, significant differences between the two tests were observed (EI 2606-9601 G = median 9.27 S/C vs. EI 2606-9601-30 G = median 8.11 S/C; p < 0.001 (Figure 2A), again with higher levels in the original EI 2606-9601 G test (Figure 2C), however with a less robust correlation of both tests used (r = 0.435, p < 0.01). The obtained results show that the ELISA test developed to measure antibodies produced in response to Omicron infection cannot detect more antibodies than the originally used test. On the contrary, it appears that the new EI 2606-9601-30 G test does not recognize all kinds of antibodies against various epitopes of the virus S-protein induced by vaccination and underestimates their actual level; however, more data are necessary to confirm this assumption. This comparison of the tests shows that the results obtained by the EI 2606-9601 G test are reliable also in participants who overcame infection by the Omicron variant of the virus.
FIGURE 2
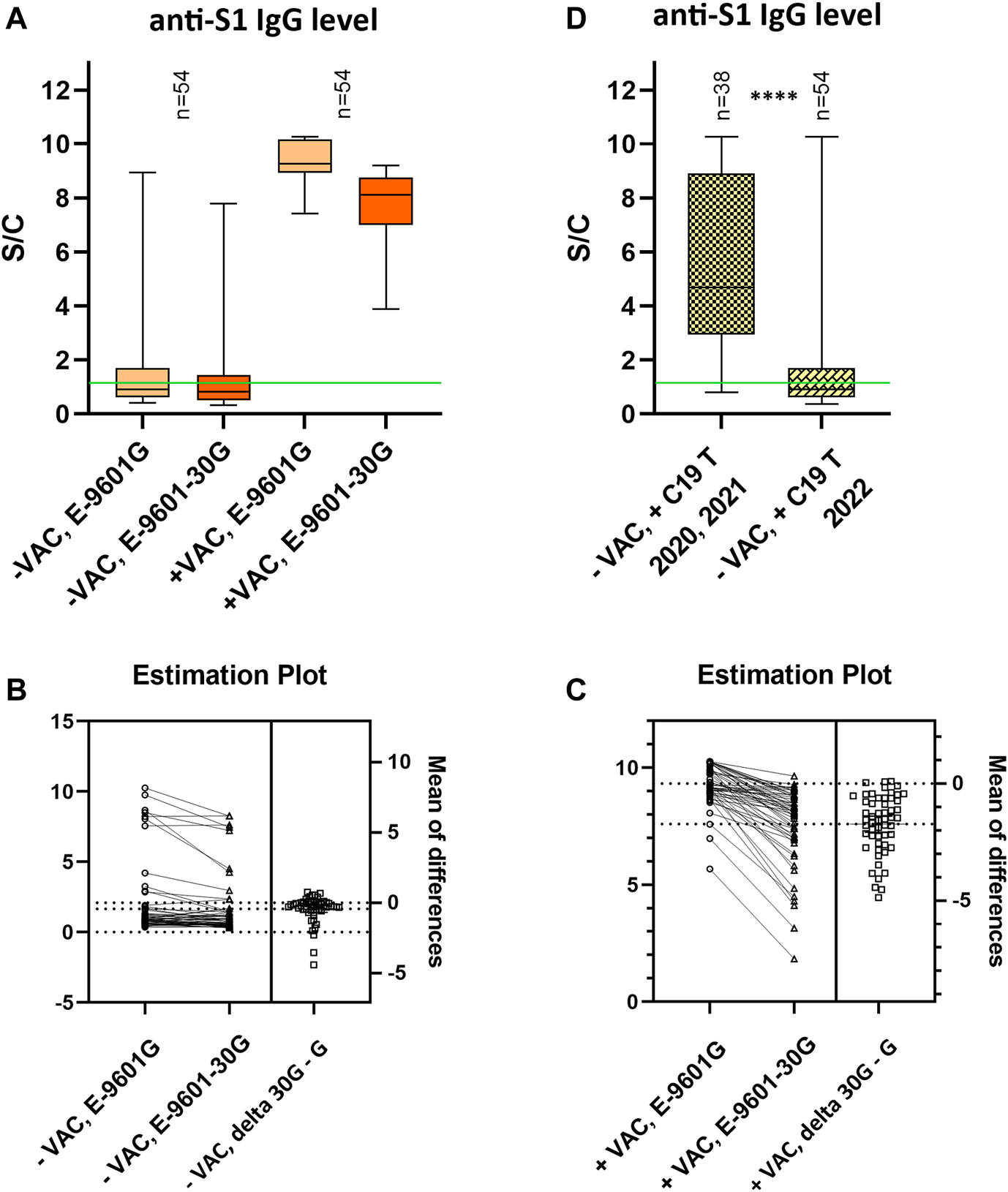
Comparison of anti-S1 IgG antibody levels determined by ELISA tests EI2606-9601 G and EI2606-9601-30 G (A–C); and anti-S1 IgG antibody levels after infections by different variants of SARS-CoV-2 virus measured by ELISA tests EI2606-9601 G (D). (A) Relative SARS-CoV-2 anti-S1 IgG antibody levels. (A) Participants were stratified according to their vaccination status. Only samples from participants who had a positive Ag/PCR test for COVID-19 exclusively after 1 January 2022 (presumption of the Omicron variant) were analyzed. (B,C) Estimation plots for (B) unvaccinated and (C) vaccinated participants present the magnitude of an effect, along with a visual representation of its precision (confidence interval). The left axis shows the data. The right axis shows the difference between the means and precision of the calculated effect size as a 95% confidence interval. Y = 0 on the right axis is aligned with the position of the mean of the first group plotted on the left axis. (D) Unvaccinated participants were stratified according to the date of positive testing for COVID-19. 2020, 2021—assumption of overcoming the original strain/Alpha/Delta variant of SARS-CoV-2; 2022—assumption of overcoming the Omicron variant of SARS-CoV-2. The data are expressed as a ratio of signal to calibrator (S/C). In the box plots, the median is represented by a horizontal line within boxes. The area of boxes represents the 25th–75th percentile and whiskers the 5th–95th percentile of the measured values in the group. The number of samples in each group is shown on the top of the graphs as “n.” Green lines represent the limit of test positivity = 1.1 S/C. −VAC = did not receive any dose of the vaccine; +VAC = receive at least one dose of the vaccine; +C19 T = at least one positive Ab/PCR test for SARS-CoV-2 in the past; E-9601G = ELISA test EI2606-9601 G; E-9601-30G = ELISA test EI2606-9601-30 G **** = p< 0.0001.
Immunogenicity of the Omicron variant (B.1.1.529) SARS-CoV-2
Unvaccinated study participants who have overcome only one SARS-CoV-2 infection represented a suitable group to compare IgG levels of antibodies generated after infection with various variants of the virus. In this group, we observed a significant difference (p < 0.0001) in antibody levels in individuals who overcame infection in 2020 and 2021 (median 4.7 S/C) compared to those who were infected with SARS-CoV-2 in 2022 (median 0.92 S/C), when evaluated using the EI2606-9601 G test. Since the dominance of the Omicron variant in the European Union was confirmed in January 2022, this result suggests that the B.1.1.529 variant is much less immunogenic than the previous SARS-CoV-2 variants. Taking into account the time that passed from the infection with the previous variants and the fact that the antibody level is gradually decreasing with time (Kajanova et al., 2021), the difference is probably even more pronounced. In the “+ C19 2022” group we detected antibody levels ≥ 3.5 S/C only in 8/54 participants. It is possible that these volunteers were infected with a different SARS-CoV-2 variant than Omicron during 2022 or that they had repeated infections without having a positive COVID-19 test previously (Figure 2D).
COVID-19 prevalence based on the presence of antibodies against the SARS-CoV-2 NCP
Almost 35% of the study participants stated in the questionnaire that they have never had a positive Ag and/or PCR test for COVID-19 in the past. In the unvaccinated subgroup of the never positively tested participants, there were cases of seropositivity for anti-S1 IgG antibodies against SARS-CoV-2 (39.02% of the group “-VAC, -C19”) (Figure 1A). This suggests that they overcame COVID-19 without being positively tested. To know what part of the population overcame the disease without evidence, we decided to test these participants (who provided a sufficient amount of sample, n = 471) also for the presence of anti-NCP IgG antibodies, which are detectable only after overcoming the disease, not after vaccination. We detected the presence of antibodies against NCP SARS-CoV-2 in up to 27.39% of participants who reported no positive Ag/PCR test. The percentage of antibody positivity differed considerably in the vaccinated (25.12%) and non-vaccinated (51.22%) groups, X2 (1, n = 471) = 12.8247, p = 0.00034. Anti-NCP IgG antibody levels in these groups were comparable (+VAC = 1.57 S/C; -VAC = 1.86 S/C). The median level of anti-NCP IgG was higher than those against S-protein in the unvaccinated group. In the group of anti-NCP IgG-positive vaccinated volunteers, the median level of anti-S1 IgG was more than 6 times higher (10.26 S/C) than the level of anti-NCP IgG (1.57 S/C). See Table 4.
TABLE 4
| anti-NCP IgG | n | % | % | anti-NCP IgG, S/C | anti-S1 IgG, S/C | |
|---|---|---|---|---|---|---|
| Total | 471 | 100.00 | ||||
| Positive + borderline | 129 | 27.39 | 1.58 | 10.19 | ||
| Negative | 342 | 72.61 | 0.43 | 7.93 | ||
| Vaccinated | 430 | 91.30 | 100.00 | |||
| Positive + borderline | 108 | 25.12 | 1.57 | 10.26 | ||
| Negative | 322 | 74.88 | 0.42 | 8.15 | ||
| Unvaccinated | 41 | 8.70 | 100.00 | |||
| Positive + borderline | 21 | 51.22 | 1.86 | 0.82 | ||
| Negative | 20 | 48.78 | 0.48 | 0.55 | ||
| COVID-19 self-assumption | 106 | 22.51 | 100.00 | |||
| Positive + borderline | 53 | 50.00 | 1.83 | 10.01 | ||
| Negative | 53 | 50.00 | 0.47 | 8.72 |
Characteristics of the never positively tested participants based on the seropositivity in anti-NCP IgG SARS-CoV-2 antibodies.
Italic values represents % from each group.
In the anamnestic questionnaire, SAS employees also answered the questions whether – based on symptoms/contact with positive persons/other circumstances – they assume overcoming the disease COVID-19, even without a positive COVID-19 test. Based on the presence of anti-NCP IgG, up to 50.00% of the participants who answered “yes” to this question overcame evidently the SARS-CoV-2 infection.
Vaccination and COVID-19 severity
One of the secondary aims of our study was to evaluate, whether previous vaccination had an effect on the severity of the disease. The study participants answered the question in the questionnaire: “If you had to rate the course of your COVID-19 disease on a scale from 1 to 10 (1 = asymptomatic course, 10 = life-threatening course including hospitalization), how would you rate it?”. It was a self-assessment, based on the individual perception of the symptoms of the disease. According to the rating, we divided the participants into two groups: group 1 included all those who had not been vaccinated (according to the baseline vaccination schema) by the date of the first positive Ag/PCR COVID-19 test (regardless of subsequent vaccination); the group 2 consisted of volunteers who completed the baseline vaccination schema before the diagnosis of SARS-CoV-2 infection. The average of numerical expressions of the disease severity in group 1 and 2 differed significantly (group 1 = 4.33; group 2 = 3.78, p < 0.0001). This difference points to a milder course of the COVID-19 disease after vaccination (Figure 3). We could also observe a substantial difference in reporting severe cases of the COVID-19 disease (rating within the scale = 8/9/10), 6.31% in group 1 vs. 1.44% in group 2, X2 (1, n = 756) = 30.17, p < 0.0001.
FIGURE 3
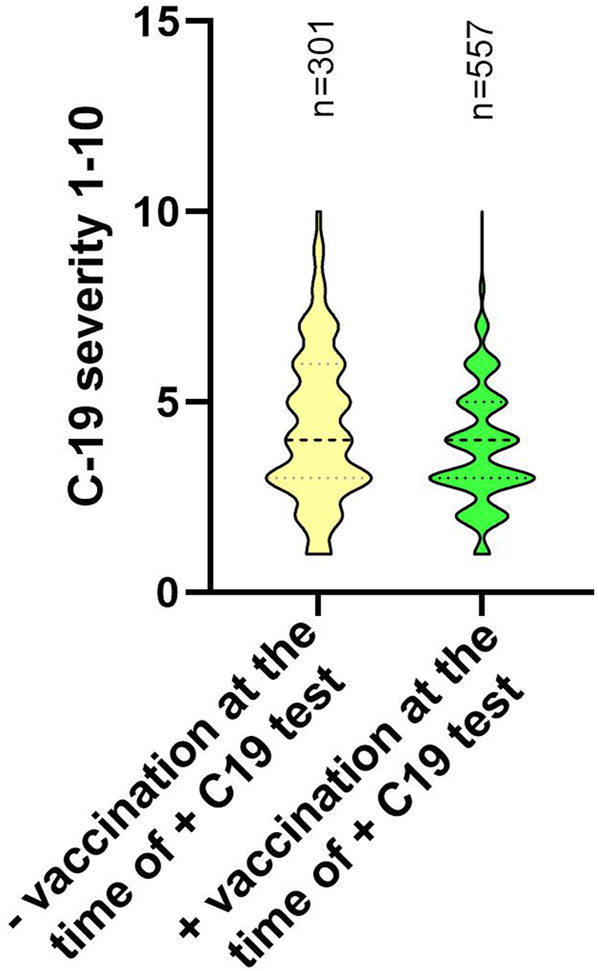
The severity of the course of the COVID-19 disease based on the self-assessment of the study participants. Participants were stratified according to their vaccination status at the time of the positive test for COVID-19. The participants rated the course of the disease on a scale from 1 to 10, according to the instructions: 1 = asymptomatic course and 10 = life-threatening course including hospitalization. Data are displayed using a truncated violin plot. The number of samples in each group is shown on the top of the graphs as “n.” −VAC = did not receive any dose of the vaccine; +VAC = received at least one dose of the vaccine; +C19 T = at least one positive Ab/PCR test for SARS-CoV-2 in the past; **** = p< 0.0001.
Changes in seropositivity and antibody levels compared to August 2021
The first serological study among SAS employees was conducted in August 2021, approximately 8 months after the start of vaccination against COVID-19 disease in Slovakia. At that time, 1,928 volunteers participated in the study, and the total seropositivity to the S-protein of the SARS-CoV-2 virus was 84.13%. Vaccinated participants made up 77.7% of all participants, and only 16.75% of the involved employees had overcame the COVID-19 disease at the time of the study (Kajanova et al., 2021). The second serological study was completed in October 2022. In the period between the studies, the administration of booster vaccine doses against COVID-19 began in Slovakia (October 2021) and new variants of SARS-CoV-2 appeared, which caused 2 significant waves of the COVID-19 disease: Delta B.1.617.2 (first confirmed case in Slovakia on 06/23/2021), and Omicron B.1.1.529 (first confirmed case in Slovakia on 12/11/2021). It is therefore not surprising that in the present study, the total seropositivity to the S-protein of the SARS-CoV-2 virus was 96.04%; 87.11% of the participating employees were vaccinated, and 65.05% of the participants had overcome the COVID-19 disease at least once.
Since 1,004 individuals participated in both studies, we compared the change in the level of specific antibodies against the SARS-CoV-2 virus (Figure 4A). In this group of volunteers, the median antibody level in 2021 was 6.43 S/C and in 2022 it was 9.43 S/C. We divided the participants of both studies into four groups: “-VAC, -C19 T”; “+VAC, -C19 T”; “-VAC, +C19 T” and “+VAC, +C19 T,” according to whether they received at least one dose of vaccination (+VAC) at any time since December 2020 or had at least one positive test for COVID-19 (+C19 T) in the period between both studies. An increase in antibody levels in all groups could be observed. In the “-VAC, -C19 T” group, the increase in median levels from 3.91 S/C to 5.41 S/C (p < 0.0001) was probably caused by infections that were not confirmed by a positive test for COVID-19. Vaccination alone (“+VAC, -C19 T”) contributed to an increase from a median of 7.11 S/C to a median of 9.16 S/C (p < 0.0001), and vaccination combined with overcoming the disease (“+VAC, +C19 T”) from a median of 6.43 S/C in 2021 to a median of IgG Ab of 10.22 S/C (p < 0.0001) in 2022. We observed the highest increase in the “-VAC, +C19 T” group, where the median level in 2021 was 2.45 S/C and in 2022 it reached a value of 7.87 S/C (p < 0.0001). See Figure 4B.
FIGURE 4
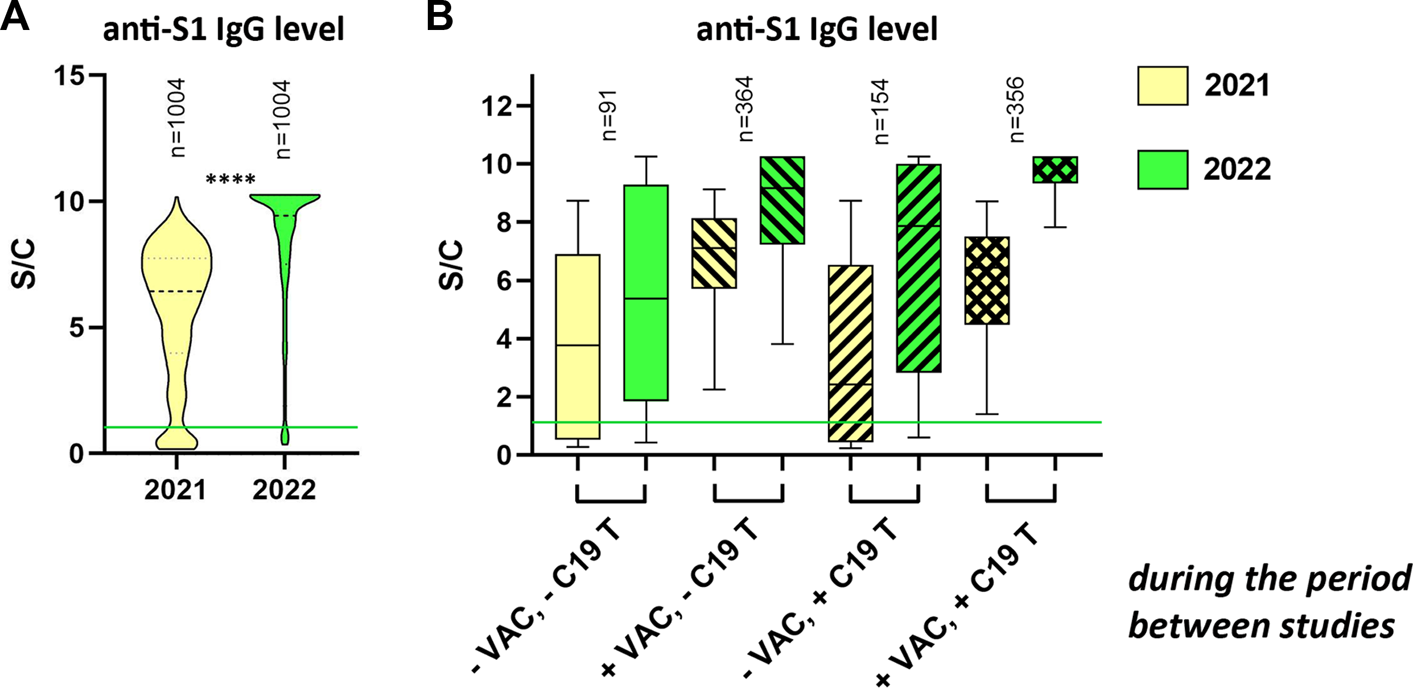
Comparison of relative levels of anti-S1 IgG in 2021 and 2022 (A) Truncated violin plot visualization of antibody levels in participants who participated in both serological studies of SAS employees in 2021 and 2022 as well. (B) Participants who participated in both serological studies of SAS employees in 2021 and 2022 were stratified according to vaccination status (one or more doses at any time since December 2020) and/or overcoming COVID-19 (one or more times) during the period between studies (September 2021–October 2022). Graph (B) shows only data from participants who filled in all dates in the correct format in the questionnaire (n = 965). The data are expressed as a ratio of signal to calibrator (S/C). The median is represented by a horizontal line within boxes. The area of boxes represents the 25th–75th percentile and whiskers the 5th–95th percentile of the measured values in the group. The number of samples in each group is shown at the top of the graphs as “n.” −VAC = did not receive any dose of the vaccine; +VAC = received at least one dose of the vaccine; −C19 T = no positive Ab/PCR test for SARS-CoV-2; +C19 T = at least one positive Ab/PCR test for SARS-CoV-2 from September 2021 to October 2022; **** = p< 0.0001.
Research areas, seroprevalence, and vaccination status
One of the goals of the seroprevalence study among SAS employees was also to obtain information about the situation in individual workplaces, which can subsequently be taken into account when implementing anti-pandemic measures. The structure of the Slovak Academy of Sciences is described in detail in our previous study (Kajanova et al., 2021). In the basic comparison of studies from the years 2021 and 2022, we found that the total participation of employees of research institutions/centers decreased in relation to the total number of employees by 16.42% (2021 – 54.2%; 2022 – 37.78%). Within the individual Scientific Sections, the decrease in participation in the study did not differ markedly: 1. Physical, Space, Earth, and Engineering Sciences – decrease by 12.9%, 2. Life, Chemical, Medical, and Environmental Sciences – decrease by 17.1%, and 3. Social Sciences, Humanities, Arts, and Culture - decrease by 13.6%. On the contrary, we noted an increase in the overall seropositivity of volunteers in all scientific departments of the academy. Seropositivity against the SARS-CoV-2 virus increased in institutions of Scientific Sections by 11.9%/9.1%/13.6%, total on average to 96.4%. It is interesting that, unlike the last study, the highest proportion of vaccinated individuals was among participants from the 3rd Scientific Section (90.1%). These data, however, can probably be somewhat distorted by the low participation in this Section, as only 26.54% of its employees participated. The overall seropositivity and participation remained the highest among researchers in natural sciences, which confirmed the persistent motivation to accept nonpharmaceutical protective measures and participate in research studies (Figure 5).
FIGURE 5
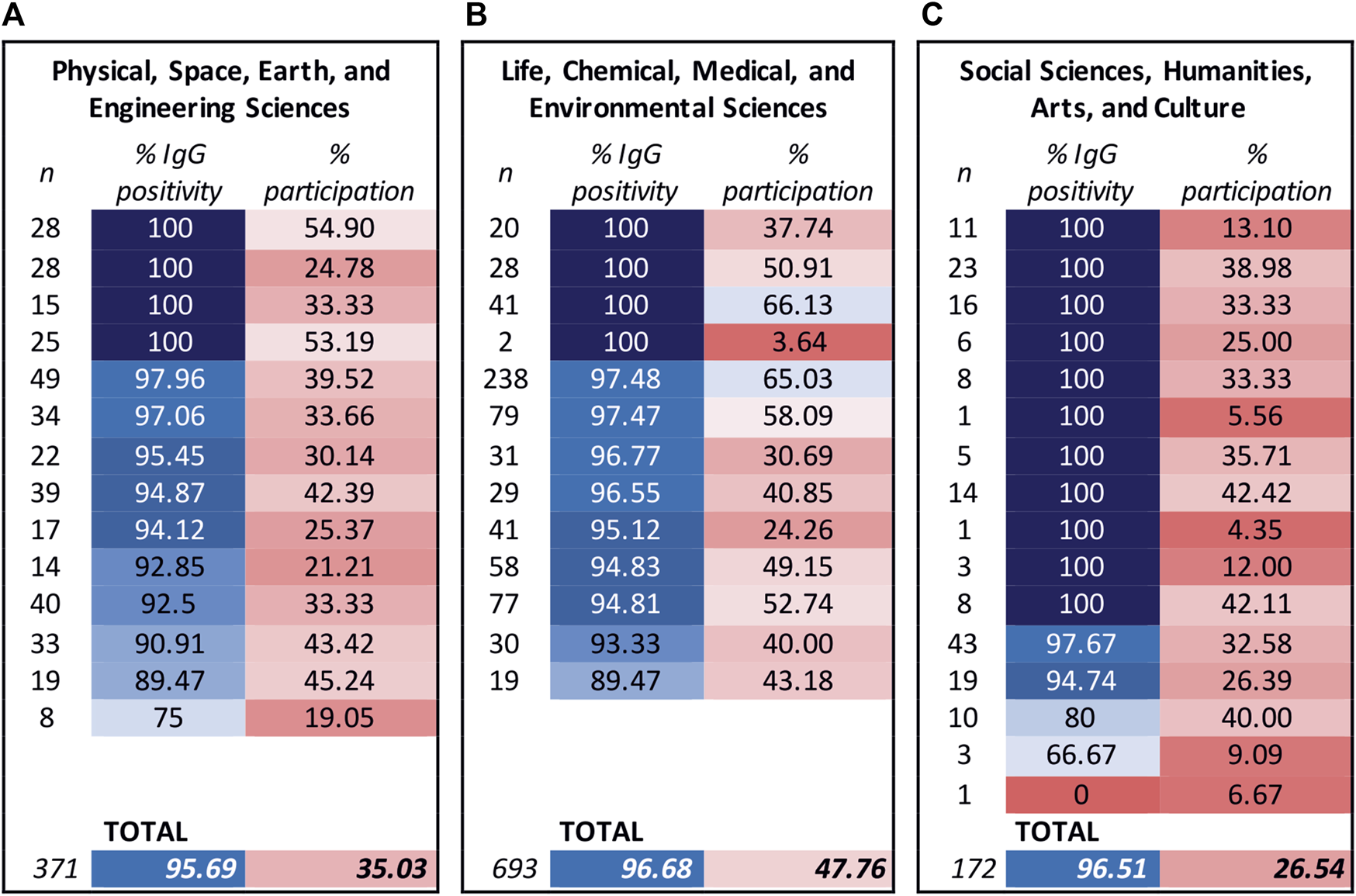
Seroprevalence and response rate of study participants in the research institutions of the Slovak Academy of Sciences. Seroprevalence (expressed as % of IgG positivity) and response rate (expressed as % of participation) were observed in the individuals participating in research institutions/centers clustered according to their respective Scientific Sections. The reported vaccination status of the study participants was 323 (87.06%) in the research organizations of the Scientific Section 1 (A), 616 (88.89%) in Section 2 (B), and 155 (90.12%) in Section 3 (C). The color scale corresponds to a percentage value, changing from shades of blue to shades of red as the value decreases. Left columns: n = number of participants of the individual workplaces.
A total of 129 employees of non-research bodies of the SAS also participated in this study, which represented 35.83% of these employees. The presence of specific IgG antibodies against SARS-CoV-2 was confirmed in 93.02% of these volunteers and 73.64% of them were vaccinated (data not shown in figure).
Discussion
In the previous 3 years from the start of the COVID-19 pandemic, hundreds of scientific seroprevalence studies have been conducted. Their results shed light on many aspects of humoral immunity induced by SARS-CoV-2 infection and/or vaccination. During this period, our research team at the Institute of Virology, Biomedical Research Center of the SAS completed 5 such studies ranging in size from 287 to 3,785 participants; two of them already published (Kajanova et al., 2021; Kajanova et al., 2022). The results of the current study are in line not only with our previous findings but with the knowledge available at the global level as well. The presented cross-sectional seroprevalence study performed among the employees of the Slovak Academy of Sciences brings original findings that are beneficial, especially with regard to a sufficiently large group of repeatedly participating volunteers and due to the timing of the analysis after a strong wave of the Omicron variant in Slovakia.
The Omicron S-protein (isolate hCoV-19/Botswana/R40B58_BHP_3321001245/2021; GISAID Accession ID: EPI_ISL_6640919) exhibits 37 mutations as compared with the Wuhan-Hu-1 spike. Thirteen of these changes are unique, while the remaining changes are known from variants of interest or concern (Hoffmann et al., 2022). It is currently a generally accepted knowledge that antibodies generated after previous SARS-CoV-2 infections and/or after vaccination are effective against the Omicron variant to a much-reduced level. Cao et al. (2022) proved that in total, over 85% of the neutralizing antibodies of different epitope groups were escaped by Omicron. Based on these facts, we have assumed that antibodies generated after Omicron infection may fundamentally differ from the antibodies that were produced after vaccination with the original COVID-19 vaccines or after infection by earlier variants of the virus. Therefore, we had doubts about whether the ELISA tests we used are still reliable and can capture all the antibodies in people who overcame infection with B.1.1.529 (Omicron variant). By comparing the standard test (EI 2606-9601 G) with the test adapted to specific IgG antibodies induced by infection with the Omicron variant (EI 2606-9601-30 G), we proved that the results obtained by the original test are reliable, and therefore we used the original test in the analyses of the antibody response. The analysis showed a surprising difference in the levels of anti-S1 IgG antibodies in the unvaccinated group depending on the overcome infection by a certain variant of SARS-CoV-2 (ancestral/Alpha/Delta vs. Omicron sub-lines). A majority of the available publications focus on breakthrough infections with the Omicron variant after vaccination. They show a significant increase in the level of specific (neutralizing) antibodies after Omicron infection, which is in line also with our results (Chowdhury et al., 2022; Pušnik et al., 2023). However, much less is known about humoral immunity after primary Omicron SARS-CoV-2 infection which is usually connected with a decrease in cross-protective immunity against other variants of the virus (Rössler et al., 2022; Stiasny et al., 2022; Suryawanshi et al., 2022). Primary infection with Omicron sub-lines, therefore, induces the production of variant-specific neutralizing antibodies, which may not provide protection against non-Omicron variants in unvaccinated individuals. We would like to point out that the humoral response to this SARS-CoV-2 variant is significantly reduced overall and in many subjects does not even reach the threshold positivity of the test used (median 0.92 S/C; EI 2606-9601 G 0.8-1.1 S/C = “borderline”). As an expected consequence, we assume that unvaccinated individuals will not be protected from further breakthrough infections even after overcoming the primary Omicron infection. This was also confirmed in the meta-analysis published recently in the Lancet journal, where the authors summarized that protection against re-infection from ancestral, alpha, and delta variants declined over time but remained at 78.6% at 40 weeks, but protection against re-infection by the omicron BA.1 variant declined more rapidly and was estimated at 36.1% (Stein et al., 2023).
Most studies aimed at monitoring the effectiveness of vaccination against the COVID-19 disease focus only on the occurrence of breakthrough infection with the SARS-CoV-2 virus or evaluate the severity of the disease based on the hospitalization or presence of specific symptoms (Feikin et al., 2022; Kumar et al., 2022). We chose a different approach and asked the volunteers who survived the COVID-19 disease how seriously they rated their personal course of the disease in a questionnaire. Such an approach allows us to estimate to what extent the patients' lives have been affected by the disease (duration and intensity of symptoms) which might be a more relevant approach. Up to 6.31% of the participants, who were infected when unvaccinated, rated the disease course as very serious (upper 30% of the scale). On the other hand, only 1.44% of volunteers, who were already vaccinated with any type of vaccine against COVID-19 at the time of infection, rated the course of COVID-19 in the same way. The fact that people with the most severe course of COVID-19 (with a fatal outcome) could not participate in the study should also be taken into account because the difference found may actually be underrated.
The uniqueness of this study is the attendance of up to 1,004 volunteers who participated in the serological studies in 2021 as well as in 2022. This presents an extraordinary opportunity to compare the change in the levels of specific anti-S1 IgG antibodies within 14 months of the pandemic. In both studies, the participants filled out a detailed anamnestic questionnaire. Therefore, we had precise information on whether and when exactly the individuals overcame COVID-19 infection or were vaccinated during this period. Since for antibody determination in both studies the same commercially available kit was used, under the same laboratory conditions and evaluated in the same way, the mutual comparison of the results is highly reliable. With regard to the course of the pandemic, the gratifying fact is that the seropositivity of the employees as well as the levels of their specific antibodies have increased to a considerable extent. In 2022 about a third of them (341 out of 1,004) even reached the upper limit (caused by the limit of measuring absorbance up to the value of O.D. = 4) of the used test. It is also interesting to note that receiving at least one dose of vaccination against the disease COVID-19 led to an increase in the levels of specific antibodies to higher values in 2022 than overcoming the infection of COVID-19 itself during this period. Receiving a booster vaccination appears to be more effective in this regard (even if we would not take into account the risks accompanying the disease itself). However, these findings are not in contradiction with the current knowledge of hybrid immunity (Pilz et al., 2022); Figure 1A, since the vaccination status or overcoming the disease before August 2021 were not taken into account in the current analysis. We assume that the effect is rather caused by decreased immunogenicity of the Omicron variant.
Despite the fact that SAS institutes are located in many regions of Slovakia, its employees cannot be considered a representative sample in terms of vaccination rate. At the time of the study, the average vaccination rate of the population throughout Slovakia was 51.13%, for the SAS employees involved it was up to 87.11%. It can therefore be assumed that the prevalence of the disease COVID-19 is presumably lower within the SAS. None of these factors, however, should have an impact on our findings regarding the immune response to the Omicron variant, the development of antibody levels over time, and the severity of the course of the disease in relation to vaccination.
After 2.5 years of the SARS-CoV-2 pandemic and 21 months of vaccination against the virus, seropositivity was detected in up to 96% of the study participants, with the highest levels of specific antibodies in those with hybrid immunity, and significantly higher levels in vaccinated than unvaccinated individuals. Among the study participants, there were up to 913 persons (67%) who had a booster dose of the vaccine against COVID-19 administered between September 2021 and October 2022. The median of specific antibodies in this group was at the level of 9.88 S/C. Therefore, the application of booster doses certainly contributed to a large extent to a significant increase in the overall average level of antibodies in the population. The Omicron variant appears to be less immunogenic than the previous VOCs. Up to 27% of never-positively tested participants overcame the COVID-19 disease. The obtained data lead to the assumption, that vaccination favorably moderates the course of the subsequent COVID-19 disease. The percentage of vaccinated participants increased in 14 months from 78% to 87% and the percentage of participants who overcame the disease increased at the same time from 17% to 65%.
In conclusion, we can prove that the realization of seroprevalence studies is of great importance even after a relatively long duration of the pandemic. The circumstances affecting the levels of specific antibodies are still evolving. The effect of administering booster doses of the vaccine is predictable and leads to an increase in the levels of specific anti-S1 IgG antibodies in the population. However, it is much more difficult to predict the impact of newly appearing variants of the SARS-CoV-2 virus, which can have a very surprising effect on the humoral immune response.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Independent Ethics Committee of the Bratislava self-governing region. The patients/participants provided their written informed consent to participate in this study.
Author contributions
IK performed the experiments, analysed and interpreted the data and wrote the manuscript; LJ, LL, KG, MB, NI, and NG performed the experiments; VZ provided expert advice; SP analysed and interpreted the data and contributed to the manuscript writing; ZR analysed and interpreted the data and contributed to the manuscript writing; JK contributed to the manuscript writing.
Acknowledgments
This publication was created thanks to the support of the Integrated Infrastructure Operational Program for the project: Development of biotechnological research potential of the SAS Biomedical Center to combat the COVID-19 pandemic in synergy with the European Virus Archive of Global Importance supported by the H2020 program, ITMS code: 313011ASU8, co-financed by the European Fund of regional development. The authors wish to thank the Presidium of the Slovak Academy of Sciences for their support and all colleagues from the Slovak Academy of Sciences for their voluntary participation in the study, to VEDA Publishing house of the SAS for printing the sample collection cards, and to Ms. Zuzana Lackovicova for the assistance in preparation and distribution of the sample collection sets.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Ag, antigen; anti-NCP IgG, immunoglobulin G antibodies against NCP protein; anti-S1 IgG, immunoglobulin G antibodies against S1 protein; BAU/mL, binding antibody units/mL; BMI, body mass index; COVID-19, Coronavirus disease 2019; IQR, interquartile range; NCP, nucleoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAS, Slovak Academy of Sciences; S-protein, SARS-CoV-2 spike protein; VOC, variant of concern.
References
1
Boršová K. Paul E. D. Kováčová V. Radvánszka M. Hajdu R. Čabanová V. et al (2021). Surveillance of SARS-CoV-2 lineage B.1.1.7 in Slovakia using a novel, multiplexed RT-qPCR assay. Sci. Rep.11 (1), 20494. 10.1038/s41598-021-99661-7
2
Cao Y. Wang J. Jian F. Xiao T. Song W. Yisimayi A. et al (2022). Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature602 (7898), 657–663. 10.1038/s41586-021-04385-3
3
Carabelli A. M. Peacock T. P. Thorne L. G. Harvey W. T. Hughes J. de Silva T. I. et al (2023). SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol.21, 162–177. 10.1038/s41579-022-00841-7
4
Castro Dopico X. Ols S. Loré K. Karlsson Hedestam G. B. (2022). Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med.291 (1), 32–50. John Wiley and Sons Inc. 10.1111/joim.13372
5
Chowdhury S. Chowdhury M. S. Chowdhury N. S. Chowdhury S. Chowdhury S. (2022). Omicron variant of SARS-CoV-2 infection elicits cross-protective immunity in people who received boosters or infected with variant strains. Int. J. Immunopathol. Pharmacol.36, 039463202211330. SAGE Publications Inc. 10.1177/03946320221133001
6
Feikin D. R. Higdon M. M. Abu-Raddad L. J. Andrews N. Araos R. Goldberg Y. et al (2022). Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet399 (10328), 924–944. 10.1016/S0140-6736(22)00152-0
7
Hoffmann M. Krüger N. Schulz S. Cossmann A. Rocha C. Kempf A. et al (2022). The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell185 (3), 447–456.e11. 10.1016/j.cell.2021.12.032
8
Kajanova I. Grossmannova K. Jelenska L. Lukacikova L. Radikova Z. Knutova N. et al (2022). Seroprevalence of SARS-CoV-2 antibodies in the county town of Slovakia - a pilot study from the Trencin city. Acta Virol.66 (3), 228–237. 10.4149/av_2022_301
9
Kajanova I. Lukacikova L. Jelenska L. Grossmannova K. Radikova Z. Vlcek M. et al (2021). Seroprevalence of SARS-CoV-2 IgG antibodies in the staff of the Slovak Academy of sciences in response to COVID-19 and/or vaccination: Situation in August 2021. Acta Virol.65 (4), 420–432. 10.4149/av_2021_407
10
Kumar S. Saikia D. Bankar M. Saurabh M. K. Singh H. Varikasuvu S. R. et al (2022). Efficacy of COVID-19 vaccines: A systematic review and network meta-analysis of phase 3 randomized controlled trials. Pharmacol. Rep.74 (6), 1228–1237. 10.1007/s43440-022-00429-1
11
Pilz S. Theiler-Schwetz V. Trummer C. Krause R. Ioannidis J. P. A. (2022). SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res.209, 112911. Academic Press Inc. 10.1016/j.envres.2022.112911
12
Pušnik J. Monzon-Posadas W. O. Zorn J. Peters K. Baum M. Proksch H. et al (2023). SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun.14 (1), 572. 10.1038/s41467-023-36250-4
13
Rashid F. Xie Z. Suleman M. Shah A. Khan S. Luo S. (2022). Roles and functions of SARS-CoV-2 proteins in host immune evasion. Front. Immunol.13, 940756. Frontiers Media S.A. 10.3389/fimmu.2022.940756
14
Rössler A. Knabl L. von Laer D. Kimpel J. (2022). Neutralization profile after recovery from SARS-CoV-2 omicron infection. N. Engl. J. Med.386 (18), 1764–1766. 10.1056/nejmc2201607
15
Rusňáková D. Sedláčková T. Radvák P. Böhmer M. Mišenko P. Budiš J. et al (2022). Systematic genomic surveillance of SARS-CoV-2 virus on illumina sequencing platforms in the Slovak republic—one year experience. Viruses14 (11), 2432. 10.3390/v14112432
16
Spinardi J. R. Srivastava A. (2023). Hybrid immunity to SARS-CoV-2 from infection and vaccination—evidence synthesis and implications for new COVID-19 vaccines. Biomedicines11 (2), 370. MDPI. 10.3390/biomedicines11020370
17
Stein C. Nassereldine H. Sorensen R. J. D. Amlag J. O. Bisignano C. Byrne S. et al (2023). Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet401, 833–842. 10.1016/S0140-6736(22)02465-5
18
Stiasny K. Springer D. Graninger M. Aberle S. Traugott M. Hoepler W. et al (2022). Human primary Omicron BA.1 and BA.2 infections result in sub-lineage-specic neutralization. 10.21203/rs.3.rs-1536794/v1
19
Suryawanshi R. K. Chen I. P. Ma T. Syed A. M. Brazer N. Saldhi P. et al (2022). Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature607 (7918), 351–355. 10.1038/s41586-022-04865-0
Summary
Keywords
SARS-CoV-2 coronavirus, COVID-19, Omicron variant, seroprevalence, antibodies
Citation
Kajanova I, Jelenska L, Lukacikova L, Grossmannova K, Belisova M, Istvanova N, Gasparovicová N, Zelnik V, Pastorekova S, Radikova Z and Kopacek J (2023) Influence of the SARS-CoV-2 Omicron (B.1.1.529) variant and booster vaccine doses on the seroprevalence of specific IgG antibodies in the staff of the Slovak Academy of Sciences. Acta Virol. 67:11637. doi: 10.3389/av.2023.11637
Received
27 March 2023
Accepted
29 May 2023
Published
28 June 2023
Volume
67 - 2023
Edited by
Katarina Polcicova, Slovak Academy of Sciences, Slovakia
Updates
Copyright
© 2023 Kajanova, Jelenska, Lukacikova, Grossmannova, Belisova, Istvanova, Gasparovicová, Zelnik, Pastorekova, Radikova and Kopacek.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juraj Kopacek, virukopa@savba.sk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.