Despite considerable efforts, the scientific community has not yet succeeded to define uniform correlate of protection (CoP) against SARS-CoV-2 infections based on the level of specific (receptor-binding domain (RBD)-targeting or neutralizing) antibodies (Perry et al., 2022). Obviously, this is because of the high complexity and great interindividual variability of the immune response. Although the share of cellular immunity in the response to SARS-CoV-2 infection is considerable, it would not be appropriate to underestimate the protective function of specific antibodies. Increase in their level generally correlates with a decrease in the probability of infection as well as the probability of a more severe course of the disease (Khoury et al., 2021). Determining the quantity of specific antibodies providing CoP is, however, challenging precisely in view of the individual characteristics of the T-cell branch of the immune system. However, that does not diminish the significance of studying the protective function of antibodies generated after vaccination or infection with different variants of SARS-CoV-2 against breakthrough infections with new variants of the virus. Antibodies against the RBD domain of the S1 subunit of the Spike protein have the greatest importance in the neutralization of viral particles (Dolscheid-Pommerich et al., 2022). At the same time, the Spike protein is the viral component with the highest mutation potential, therefore the neutralizing capacity of specific antibodies is constantly evolving hand in hand with the appearance of new variants of concern (VoCs). Of the VoCs known so far, the Omicron variant sublineages (B.1.1.529) are the source of the highest number of mutations. This is necessarily related to a change in the effectiveness of the antibodies present in the host before its confrontation with the Omicron variant (Andrews et al., 2022; Pajon et al., 2022; Planas et al., 2022).
When investigating CoP of antibodies, it is ideal to know their levels before a person has been exposed to the risk of infection. We have extensive experience with seroepidemiological studies of SARS-CoV-2 (Kajanova et al., 2021; Kajanova et al., 2022). Employees of the Biomedical Research Center of the Slovak Academy of Sciences (BMC SAS) are largely vaccinated against COVID-19, often with a booster dose of vaccines. Moreover, at the turn of 2021–2022, it was very likely that the wave of the Omicron variant would hit our region in the near future. We decided to take advantage of these factors in the investigation of CoP of the S1-specific antibodies against the Omicron variant. The study involved 263 BMC SAS employees who took two self-collected samples of capillary blood from the finger on 14–17 January 2022 (1st sampling, before the start of the Omicron BA.1/2 wave in Slovakia, hereinafter marked “BEFORE”) and on 26–28 April 2022 (2nd sampling, after the end of the Omicron BA.1/2 wave in Slovakia, hereinafter marked “AFTER”). In that period, the vast majority of samples collected in Slovakia and tested positive for SARS-CoV-2 by RT PCR or Ag test were confirmed as Omicron BA.1/2 by sequencing (Rusňáková et al., 2022). Participants filled out a detailed anamnestic questionnaire. Of the volunteers involved, there were 200 women (76%) and 63 men (24%) with a median age of 45 years (IQR 23) and a median BMI of 23.6 kg/m2 (IQR 5.92). At the time of the study, 90.5% of participants (n = 238) were vaccinated against COVID-19, 79.4% (n = 189) of them also received a booster dose of the vaccine. We determined the levels of specific antibodies against the S1 protein of SARS-CoV-2 (anti-S1 IgG; marker of post-vaccination and/or post-infection response) using the quantitative Anti-SARS-CoV-2 QuantiVac ELISA (IgG) test (EI 2606-9601-10 G, Euroimmun) in international units BAU/mL (<25.6 = negative; 25.6–35.2 = borderline; >35.2 = positive) and the level of specific antibodies to SARS-CoV-2 nuclear protein (NCP) (anti-NCP IgG; marker of a post-infection response only) in the semiquantitative Anti-SARS-CoV-2 NCP ELISA (IgG) test (EI 2606-9601-2 G, Euroimmun) in ratio units S/C (sample/calibrator; <0.8 = negative; 0.8–1.1 = borderline; >1.1 = positive). Outliers identified by Grubb’s test (n = 4) were excluded from all statistical analyses. We designated the overcoming of infection with the SARS-CoV-2 virus on the basis of an increase in the level of NCP antibodies in the “AFTER” sample compared to the “BEFORE” sample, while simultaneously fulfilling the condition that the final NCP antibody levels were >0.8 S/C. Based on these criteria, 41.3% of the involved volunteers (n = 107) overcame SARS-CoV-2 infection during the monitored period (”+OMI”), of which 66.4% (n = 71) had the disease confirmed in 2022 by the PCR/Ag test (according to the data in the questionnaire).
The key result of this study is the finding of a significant difference (p = 0.005) in the median levels of anti-S1 IgG antibodies in the group of participants who were subsequently infected with the Omicron variant of SARS-CoV-2 (“+OMI, BEFORE” = 2478 BAU/mL; IQR = 4047 BAU/mL; n = 107) versus the group in which no breakthrough infection occurred during the monitored period (“-OMI, BEFORE” = 3803 BAU/mL; IQR = 7266 BAU/mL; n = 152). The results also naturally showed an increase in antibody levels after overcoming the infection (”+OMI AFTER” = 4813 BAU/mL; IQR = 5373 BAU/mL; p < 0.0001) and an expected decrease in antibody levels caused by the effect of waning immunity in the “-OMI” group (”-OMI AFTER” = 2097 BAU/mL; IQR = 3486 BAU/mL; p < 0.001) (Figure 1A). At the same time, we observed a significant difference in the probability of infection based on the measured level of antibodies. Due to a not normal distribution of the anti-S1 IgG antibodies, for statistical analysis we divided all our participants into four groups based on the quartiles of the anti-S1 IgG before the Omicron wave. In the lowest 3 quartiles was the portion of “+OMI” and “-OMI” comparable, however the group in the highest quartile (anti-S1 IgG >6201.5 BAU/mL) consisted of only 24.6% of Omicron-infected and 75.4% of uninfected individuals. A Yates’s chi-square test was performed to examine the relation between the Omicron infection and the level of incipient anti-S1 IgG. The relation between these variables was significant, Χ2 (1, n = 259) = 9.081, p = 0.003. Subjects in the most upper quartile of anti-S1 IgG levels were more likely to avoid Omicron infection than subjects in the lower three quartiles. (Figure 1B).
FIGURE 1
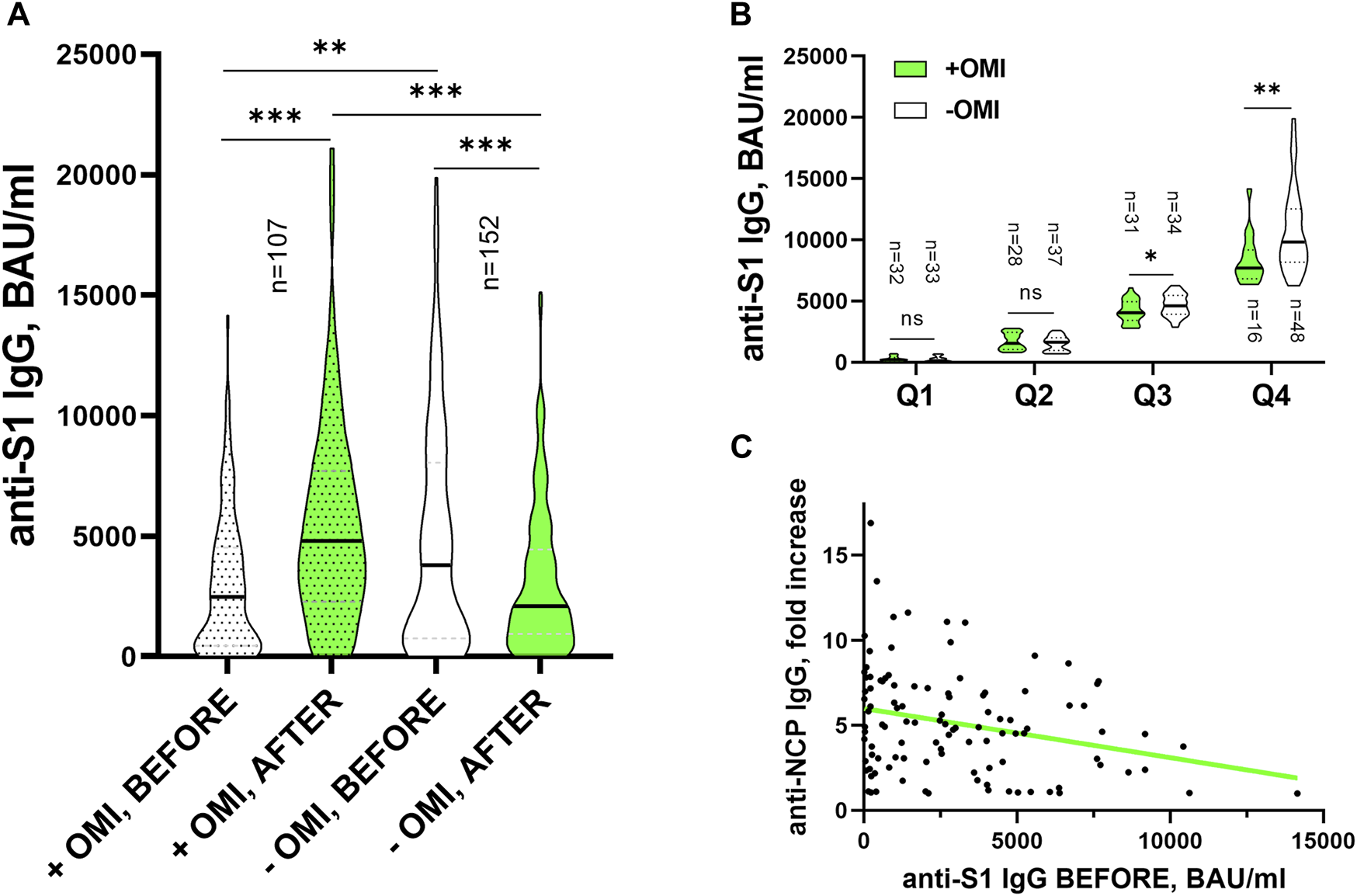
(A) Levels of anti-S1 IgG (BAU/mL) before and after Omicron wave in those who got infected with the Omicron variant (“+OMI”) and those who did not (“-OMI”), compared by Mann-Whitney U test between the “+OMI” and “-OMI” groups and by Wilcoxon Signed Rank test “BEFORE” and “AFTER” within the OMI groups. **p < 0.01; ***p < 0.001. (B) The levels of anti-S1 IgG (BAU/mL) before Omicron wave were compared between the OMI groups in 4 subgroups divided according to quartiles of the antibodies by the Mann-Whithey U test (*p = 0.046; **p = 0.006). (C) Linear regression of the anti-NCP IgG -fold increase following infection and anti-S1 IgG BEFORE levels. The simple linear regression was used to test if initial levels of anti-S1 IgG (BAU/mL) significantly predict the fold increase of NCP following the infection. The fitted regression model was: NCP fold = 5.99 - (0.000289 x BAU/mL). The overall regression was statistically significant (R2 = 0.075 F(1,105)= 8.506, p = 0.004) and it was found that initial levels of anti-S1 IgG (BAU/mL) significantly predicted following increase of NCP antibodies (β = −0.000289, p = 0.004).
As mentioned above, for the most accurate identification of participants who overcame SARS-CoV-2 infection during the monitored period, we also tested the levels of specific antibodies against the viral nucleoprotein. This allowed us to investigate the relationship between the levels of anti-S1 IgG and anti-NCP IgG antibodies. By analyzing the obtained data, we found that the level of anti-S1 IgG antibodies before the infection is in a significant negative correlation with the fold increase of NCP antibodies caused by the infection (r = −0.252; p = 0.0089). This finding could indicate that individuals with higher anti-S1 IgG concentrations at the time of infection produce fewer copies of the SARS-CoV-2 virus during infection inducing lower levels of anti-NCP antibodies (Figure 1C). This result is in line with the findings of a study where authors observed a reduced/delayed increase or complete absence of NCP antibodies after breakthrough infection (Vu et al., 2022).
It is of particular interest that the presence of anti-NCP IgG antibodies in vaccinated participants in the “BEFORE” sampling had no effect on the probability of becoming infected during the Omicron wave, X2 (1, n = 234) = 0.9957, p = 0.3183. This means that the hybrid immunity (vaccination + overcoming COVID-19) did not guarantee increased protection against infection with Omicron variant sublineages in comparison to vaccination alone. The degree of protection depended on the level of specific anti-S1 IgG antibodies preceding the subsequent infection, which represent the majority of antibodies with a virus-neutralizing function. The strong positive relationship of anti-S1 IgG levels and their virus-neutralizing capacity was described by others (Mendrone-Junior et al., 2021; Möhlendick et al., 2022).
In the anamnestic questionnaire, the participants had to answer a question on their perception of the severity of the COVID-19 (self-evaluation). BMC employees were invited to rate the course of the COVID-19 on a scale from 1 to 10 (1 = asymptomatic course, 10 = life-threatening course including hospitalization). From the participants who apparently overcame infection with the Omicron variant (positive PCR/Ag test between January and April 2022), answers on a scale from 1 up to 7 were obtained. It is not surprising, since more than 90% of volunteers were vaccinated against COVID-19. In line with the known fact, that there is a positive correlation between the severity of the COVID-19 and the level of antibodies produced in response to the SARS-CoV-2 infection (Kajanova et al., 2021; Kajanova et al., 2022), we expected similar results in this study as well. Even though it was possible to observe the same trend, there was no statistically significant relationship between the levels of anti-S1 IgG before (p > 0.5), after (p = 0.137) or their change (p = 0.09) in response to the infection on one hand and the severity of symptoms on the other hand. Neither relationship of anti-NCP IgG (“BEFORE” (p = 0.339), “AFTER” (p > 0.5) or the change (p = 0.25) in response to the infection) with the severity of the symptoms of the Omicron infection could be observed. Although there was a tendency towards more severe symptoms with lower levels of antibodies measured before the Omicron infection and on the contrary, with increased levels measured after the infection, this tendency did not reach statistical significance, probably due to the lower sample size, or due to different features and properties of the Omicron variant of the virus.
Only few studies specifically investigating the issue of CoP for the Omicron variant of SARS-CoV-2 have been published since the accomplishment of the research described here. A team from Essen, Germany monitored the levels of S1 specific antibodies and their neutralizing capacity in 1391 healthcare workers. They conclude that in participants with low pre-infection anti-spike antibodies (≤2641.0 BAU/mL) and weaker neutralization capacity (≤65.9%) against Omicron 1 month after the booster vaccination was the risk for developing an Omicron infection 10-fold increased (Möhlendick et al., 2022). Dimeglio et al. in their short letter to the editor state that in a cohort of 1169 people vaccinated with a booster dose of the mRNA vaccine, 90% of the Omicron infections occurred in individuals whose total antibody concentration was less than or equal to 6967 BAU/mL (Dimeglio et al., 2022). Both of these works point to a mutual relationship between the level of specific antibodies and the risk of infection with the B.1.1.529 variant. In opposition to them, as well as to the results of our study, stands the publication of the Spanish team, which followed a specific group of nursing home residents. Its results show that neutralization antibodies titers may not reliably predict the risk of contracting COVID‐19 due to the Omicron variant (Torres et al., 2022). In agreement with other authors dealing with the issue, we must state that more work is needed in this area to establish a SARS-CoV-2 CoP as the pandemic continues. The difficulty of defining CoP lies not only in the complexity of the individual immune response, but also in the prevalence of antibodies at the population level (degree of collective immunity), because it determines the possibilities of virus spreading (and with it the viral load), not forgetting the properties of the virus itself - infectivity, pathogenicity and the ability to escape existing immunity.
Based on the results of our study, we can summarize, that the probability of subsequent infection with Omicron decreased with increasing level of specific anti-S1 IgG antibodies before the contact with the virus variant. The relevant antibody levels to prevent Omicron infection or to overcome the disease with mild symptoms are an order of magnitude higher than those concerning the previous variants (Goldblatt et al., 2022), however, these levels can be achieved after administration of a booster dose of a vaccine. The percentual increase of anti-NCP IgG antibodies after overcoming the infection with Omicron depended inversely on the prior levels of specific anti-S1 IgG antibodies; the initially higher levels of anti-S1 IgG may be responsible for more efficient immune response limiting the virus replication and leading therefore subsequently to lower production of NCP antibodies.
Statements
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the independent Ethics Committee of the Bratislava self-governing region approved the study by its decision No. 09833/2020/HF and amendment 07071/2021 from June 30, 2021. The participants provided written informed consent to participate in this study.
Author contributions
IK performed the experiments, analysed and interpreted the data and wrote the manuscript; ZR analysed and interpreted the data and contributed to the manuscript writing; KG, LJ, LL, and MB performed the experiments; JK contributed to the manuscript writing; SP designed the study, analysed and interpreted the data and contributed to the manuscript writing.
Acknowledgments
This publication was created thanks to the support of the Integrated Infrastructure Operational Program for the project: Development of biotechnological research potential of the SAS Biomedical Center to combat the COVID-19 pandemic in synergy with the European Virus Archive of Global Importance supported by the H2020 program, ITMS code: 313011ASU8, co-financed by the European Fund of regional development. The authors wish to thank all colleagues from the BMC SAS for their voluntary participation in the study and to Zuzana Lackovicova for the assistance in preparation and distribution of the sample collection sets.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Ag, antigen; BAU/mL, binding antibody units/mL; BMI, body mass index; CoP, correlate of protection; COVID-19, Coronavirus disease 2019; IQR, interquartile range; NCP, nucleoprotein; anti-NCP IgG, immunoglobulin G antibodies against NCP protein; RBD, receptor binding domain; S1, SARS-CoV-2 spike protein S1 subunit; anti-S1 IgG, immunoglobulin G antibodies against S1 protein; SAS, Slovak Academy of Sciences; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VoC, variant of concern.
References
1
Andrews N. Stowe J. Kirsebom F. Toffa S. Rickeard T. Gallagher E. et al (2022). Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med.386 (16), 1532–1546. 10.1056/nejmoa2119451
2
Dimeglio C. Migueres M. Mansuy J. M. Saivin S. Miedougé M. Chapuy-Regaud S. et al (2022). Antibody titers and breakthrough infections with Omicron SARS-CoV-2. J. Infect.84 (4), e13–e15. 10.1016/j.jinf.2022.01.044
3
Dolscheid-Pommerich R. Bartok E. Renn M. Kümmerer B. M. Schulte B. Schmithausen R. M. et al (2022). Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol.94 (1), 388–392. 10.1002/jmv.27287
4
Goldblatt D. Fiore-Gartland A. Johnson M. Hunt A. Bengt C. Zavadska D. et al (2022). Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine40 (2), 306–315. 10.1016/j.vaccine.2021.12.006
5
Kajanova I. Grossmannova K. Jelenska L. Lukacikova L. Radikova Z. Knutova N. et al (2022). Seroprevalence of SARS-CoV-2 antibodies in the county town of Slovakia - a pilot study from the Trencin city. Acta Virol.66 (3), 228–237. 10.4149/av_2022_301
6
Kajanova I. Lukacikova L. Jelenska L. Grossmannova K. Radikova Z. Vlcek M. et al (2021). Seroprevalence of SARS-CoV-2 IgG antibodies in the staff of the Slovak Academy of Sciences in response to COVID-19 and/or vaccination: Situation in august 2021. Acta Virol.65 (4), 420–432. 10.4149/av_2021_407
7
Khoury D. S. Cromer D. Reynaldi A. Schlub T. E. Wheatley A. K. Juno J. A. et al (2021). Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med.27 (7), 1205–1211. 10.1038/s41591-021-01377-8
8
Mendrone-Junior A. Dinardo C. L. Ferreira S. C. Nishya A. Salles N. A. Almeida Neto C. et al (2021). Correlation between SARS-COV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfus. Paris.61 (4), 1181–1190. 10.1111/trf.16268
9
Möhlendick B. Čiučiulkaitė I. Elsner C. Anastasiou O. E. Trilling M. Wagner B. et al (2022). Individuals with weaker antibody responses after booster immunization are prone to Omicron breakthrough infections. Front. Immunol.13, 907343. 10.3389/fimmu.2022.907343
10
Pajon R. Doria-Rose N. A. Shen X. Schmidt S. D. O’Dell S. McDanal C. et al (2022). SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med.386 (11), 1088–1091. 10.1056/nejmc2119912
11
Perry J. Osman S. Wright J. Richard-Greenblatt M. Buchan S. A. Sadarangani M. et al (2022). Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One17 (4), e0266852. 10.1371/journal.pone.0266852
12
Planas D. Saunders N. Maes P. Guivel-Benhassine F. Planchais C. Buchrieser J. et al (2022). Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature602 (7898), 671–675. 10.1038/s41586-021-04389-z
13
Rusňáková D. Sedláčková T. Radvák P. Böhmer M. Mišenko P. Budiš J. et al (2022). Systematic genomic surveillance of SARS-CoV-2 virus on illumina sequencing platforms in the Slovak republic—one year experience. Viruses14 (11), 2432. 10.3390/v14112432
14
Torres I. Giménez E. Albert E. Zulaica J. Álvarez‐Rodríguez B. Burgos J. S. et al (2022). SARS-CoV-2 Omicron BA.1 variant breakthrough infections in nursing home residents after an homologous third dose of the Comirnaty® COVID-19 vaccine: Looking for correlates of protection. J. Med. Virol.94 (9), 4216–4223. 10.1002/jmv.27867
15
Vu L. D. Wallace S. Phan A. T. Q. Christofferson R. C. T urner E. Parker S. et al (2022). Absence of antibody responses to SARS-CoV-2 N protein in COVID-19 vaccine breakthrough cases. Exp. Biol. Med.247 (21), 1923–1936. 10.1177/15353702221134097
Summary
Keywords
anti-S1 IgG, SARS-CoV-2, Omicron variant, anti-NCP IgG, correlate of protection, COVID19 vaccination
Citation
Kajanova I, Radikova Z, Lukacikova L, Jelenska L, Grossmannova K, Belisova M, Kopacek J and Pastorekova S (2023) Study of anti-S1-protein IgG antibody levels as potential correlates of protection against breakthrough infection with SARS-CoV-2 Omicron BA.1 and BA.2 variants. Acta Virol. 67:11652. doi: 10.3389/av.2023.11652
Received
04 April 2023
Accepted
25 April 2023
Published
22 June 2023
Volume
67 - 2023
Edited by
Katarina Polcicova, Slovak Academy of Sciences, Slovakia
Updates
Copyright
© 2023 Kajanova, Radikova, Lukacikova, Jelenska, Grossmannova, Belisova, Kopacek and Pastorekova.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Pastorekova, virusipa@savba.sk
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.