- 1Infettare, Facultad de Medicina, Universidad Cooperativa de Colombia, Medellín, Colombia
- 2Grupo de Investigaciones Biomédicas Uniremington, Programa de Medicina, Facultad de Ciencias de la Salud, Corporación Universitaria Remington, Medellín, Colombia
- 3Universidad Cooperativa de Colombia, Campus Medellin, Envigado, Colombia
- 4Grupo Inmunovirología, Facultad de Medicina, Universidad de Antioquia UdeA, Medellín, Colombia
Respiratory infections remain a significant cause of morbidity and mortality, becoming a serious public health issue worldwide. The human respiratory syncytial virus (hRSV) is still one of the most relevant pathogenic agents involved in respiratory infections in children, the leading cause of bronchiolitis worldwide. In most cases, hRSV infection is not complicated; however, limited treatment and vaccine options increase the morbidity rates associated with bronchiolitis. The innate immune response governs the severity of the disease and controls the viral infection outcome. Current knowledge about the mechanisms involved in viral PAMPs (pathogen-associated molecular pattern molecules) recognition, genetic characteristics of the inflammatory response, and understanding of antiviral response is crucial for vaccine development and biomarker tools to predict complications and guide therapeutic management. Here, we review key concepts related to pathogenesis and immune response against hRSV, highlighting aspects that could be considered in vaccine development.
Introduction
Human respiratory syncytial virus (hRSV) is the primary cause of severe lower respiratory tract infection among newborns and young children worldwide (Shi et al., 2017). hRSV infection also includes upper respiratory tract infections, aggravation of asthma, and wheeze induced by the hRSV (Barr et al., 2019). The hRSV risk of infection is over 60%–70% in the first year of life and nearly 100% by 2 or 3 years of age (Meng et al., 2014).
In 2015, there have been an estimated 33.1 million hRSV-related lower respiratory tract infections, 3.2 million hRSV-related hospitalizations, and 59,600 deaths in children under 5 years of age, with an overall mortality of 118,200 (Shi et al., 2017). This virus is ubiquitously transmitted, being a risk factor for people with immunodeficiencies and elderly individuals. Further, hRSV is an essential nosocomial infection agent (Falsey et al., 2005; Gelfand, 2012; Checchia et al., 2017).
hRSV is an enveloped, single-stranded, and negative-sense RNA virus classified into the family Pneumoviridae, genus Orthopneumovirus, species human orthopneumovirus (Hall, 2001; Afonso et al., 2016). The viral genome contains ten genes encoding for 11 proteins: fusion protein (F); the glycoprotein (G); the small hydrophobic protein (SH); nucleoprotein (N); phosphoprotein (P); large protein (L); matrix (M), M2-1 and M2-2 regulatory proteins; non-structural (NS) proteins (NS1 and NS2). All these proteins are critical for viral replication and are involved in the innate immunity response (Cao et al., 2021).
Two hRSV subtypes (A and B) have been identified, which are phylogenetically and antigenically different. Most of the variability has been detected in the gene encoding the G protein, which is the most variable protein of the virus (Peret et al., 1998; Griffiths et al., 2017; Hu et al., 2017). Variations in hRSV-A and hRSV-B are associated with evolutionary mechanisms characterized by the induction of anti-G antibodies against primary epitopes (Botosso et al., 2009). hRSV-A and hRSV-B groups have cocirculated during specific seasonal epidemics but can circulate independently in human populations (Pangesti et al., 2018). Some studies report a higher prevalence and severe clinical course in hRSV-A (Jafri et al., 2013; Tabarani et al., 2013; Shen et al., 2022), but the evidence is contradictory (McIntosh et al., 1993; Devincenzo, 2004; Laham et al., 2017).
Pathogenesis
Inoculation of aerosol particles or direct contact facilitates virus entry via the nasopharynx, spreading to the lower respiratory tract toward the bronchioles (Piedimonte and Perez, 2014; Battles and McLellan, 2019). During the viral replication, the G-protein is responsible for the attachment to ciliated epithelial cells through the CX3CR1 receptor (Levine et al., 1987; Johnson et al., 2015; Zhivaki et al., 2017); the pre-F-protein allows the fusion of the viral envelope with the host cell membrane and hRSV enters by endocytosis (Tayyari et al., 2011). As soon as the virus is in the cytoplasmic inclusion bodies, it replicates its genome using the viral RNA-dependent RNA polymerase (RdRp) complex (large protein-L and the phosphoprotein-P) (Sourimant et al., 2015). The viral protein M2-1 is added to the complex to act as a cofactor of the transcriptional process (Sourimant et al., 2015; Braun et al., 2021).
Different receptors have been involved in the early immune response via NF-κB and IFN response factors (Liu et al., 2007; Okabayashi et al., 2011; Zeng et al., 2012), including epidermal growth factor (EGF) receptor (Weigl et al., 2001), intercellular adhesion molecule 1 (ICAM-1) (Law et al., 2004), annexin II (Fjaerli et al., 2004), calcium-dependent lectins (Fjaerli et al., 2004), and heparan sulfate proteoglycans (HSPGs) (Bradley et al., 2005). The Toll-like receptor 4 (TLR4) binds the F protein expressed on the ciliated bronchial (Marzec et al., 2019) and epithelial cells (Park et al., 2012), triggering innate immune signaling during the hRSV entry (Funchal et al., 2015). However, TLR4 activates kinases to potentiate viral entry through endocytosis (Walsh et al., 1997; Piedra et al., 2003). Another potential receptor is the CX3 chemokine receptor 1 (CX3CR1) (Anderson et al., 2020), which binds the G protein on the apical side of ciliated bronchial epithelial cells (Anderson et al., 2021). Mice deficient in CX3CR1 are less susceptible to hRSV infection, as RSV-G and CX3CR1 interaction could alter chemotaxis signaling (Anderson et al., 2020; Anderson et al., 2021). Other reports have described nucleolin binding to the F protein (Hall et al., 1986; Nielsen et al., 2003). As nucleolin is highly expressed on the surface of dividing cells, it could be pivotal in children’s lower respiratory tract infections (Parrott et al., 1973; Imaz et al., 2000; Roca et al., 2002; Ochola et al., 2009).
Innate immune response to hRSV infection
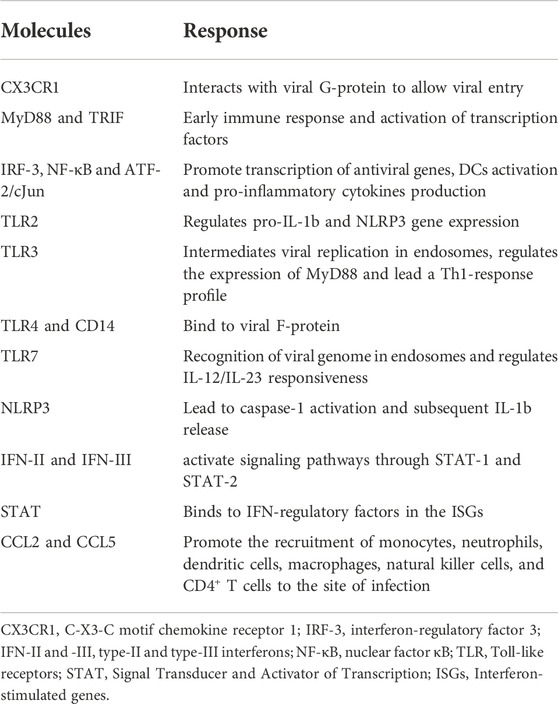
Several factors are involved in the innate immune response against hRSV infection; airway epithelial cells, dendritic cells, macrophages, monocytes, granulocytes, as well as pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs) (Yamaguchi et al., 2011; Zeng et al., 2012) (Figure 1).
FIGURE 1. Summary of the local human innate immune response to RSV. The main cell types involved in hRSV infection are shown (neutrophils, dendritic cells, macrophages, and eosinophils, among others). In addition, cytokine, chemokine, and other immune molecule responses involved in the local immune response and its production source are located according to their modulation in the infection. At the level of innate immunity, the different PRRs (TLR2, 3, 4, 7, and 9) that participate and are activated during the infection are also highlighted.
Airway epithelial cells recognize viral PAMPs through different PRRs, initiating early immune response (Lay et al., 2013). This activation occurred via MyD88 and TRIF (Kumar et al., 2011), and then, different transcription factors such as interferon-regulatory factor 3 (IRF-3), nuclear factor κB (NF-κB) and ATF-2/cJun are activated. These factors mainly promote the transcription of antiviral genes, dendritic cells activation, and the production of soluble molecules such as pro-inflammatory cytokines and chemokines by dendritic cells and alveolar macrophages (Goritzka et al., 2015; Lay et al., 2016; Feng et al., 2018).
Different TLRs have been associated with hRSV infection: TLR4 interacts with hRSV F protein using CD14 as a co-receptor leading to NF-kB activation and mediated innate immune responses and inflammation (Kurt-Jones et al., 2000). Recently, it was demonstrated that RSV-induced oxidative stress promotes enhanced activation and release of Transglutaminase 2 from human lung epithelial cells, which is mediated by Toll-like receptor (TLR)-4 and NF-κB pathways. Transglutaminase 2 is an enzyme implicated in various pathological conditions, but its role in hRSV remains unclear (Rayavara et al., 2022). TLR-2 regulates pro-IL-1β and NLRP3 gene expression during RSV infection. The TLR2 activation is the first signal necessary for the posterior formation of the NLRP3 inflammasome, leading to caspase-1 activation and subsequent IL-1β release during RSV infection (Segovia et al., 2012).
On endosomal compartments, the recognition of the genome (ssRNA) and replication intermediaries (dsRNA) occurs through TLR7 and TLR3, respectively (Aeffner et al., 2011). hRSV infection triggers the activation of the TLR3 signaling pathways that regulate the expression of MyD88-independent chemokines, such as IP-10/CXCL10 and CCL5, and further upregulate TLR3 expression in RSV-infected cells (Rudd et al., 2005). Also, it has been demonstrated that activation of TLR3 during hRSV infection promotes a predominantly Th1-type response, contributing to the establishment of the adaptive immune response (Rudd et al., 2006). TLR7 plays a crucial role in RSV detection and subsequent response immune initiation. RSV is recognized by classical and plasmacytoid DCs through TLR7, inducing the anti-RSV response and immunomodulatory effects (Smit et al., 2006; Lukacs et al., 2010). A study revealed that TLR-7 regulates IL-12/IL-23 responsiveness to RSV in dendritic cells and demonstrated that TLR7 −/− bone marrow-derived dendritic cells were significantly impaired in the induction of IL-12 in response to RSV but exhibited significantly higher production of IL-23 (Lindell et al., 2009). In addition, dendritic cells are sources of interferon-β in RSV, amplifying early antiviral responses (Kim et al., 2019).
In response to hRSV infection, the airway epithelial cells produce pro-inflammatory cytokines such as type-I and type-III interferons (IFN) that bind to its receptors (IFNRs) and activate signaling pathways through the Signal Transducer and Activator of Transcription 1 and 2 (STAT-1 and STAT-2). STAT binds to IFN-regulatory factors in the interferon-stimulated genes (ISGs). Finally, several pro-inflammatory cytokines such as IL-6, tumor necrosis factor-alpha (TNF-α) and chemokines (CXCL8, CCL3, CCL2, and CCL5) are induced and secreted. Some chemokines, such as CCL2 and CCL5, could promote the recruitment of monocytes, neutrophils, dendritic cells, macrophages, natural killer cells, and CD4+ T cells to the site of infection (Lay et al., 2016; Aronen et al., 2019) (Table 1).
In vitro, airway epithelial cells produce IL-1β, IL-6, and TNFα, macrophage inflammatory protein-1a (MIP-1a/CCL3), monocyte chemotactic protein-1 (MCP-1/CCL2), RANTES (regulated on activation, normally T cell-expressed and secreted/CCL5), eotaxin (CCL11), IL-8 (CXCL8), monokine induced by IFNγ (MIG/CXCL9), IP-10 (CXCL10), and fractalkine (CX3CL1) (Bonville et al., 1999; Miller et al., 2004; Mochizuki et al., 2009; Villenave et al., 2011). The effect of this cytokine production during hRSV infection is still controversial (Tayyari et al., 2011; Villenave et al., 2012). It has been proposed that cell lines, compared with primary cells, induced different cytokine profiles, even when exposed to the same strain of the virus (Fonceca et al., 2012). In the same way, cells from different donors significantly alter the profile. Besides, the localization of the epithelial cells in the airways seems to affect the constitutive production and cytokines upregulation (Olszewska-Pazdrak et al., 1998).
During the inflammatory process caused by the hRSV infection in the airways of infants with bronchiolitis, prominent infiltration of neutrophils is observed (Habibi et al., 2020). During severe infection, the virus interacts directly with neutrophils; these cells in BAL and the blood of infants with severe RSV infection expressed RSV N genomic RNA, indicating uptake of the whole virus (Halfhide et al., 2011). In response, neutrophils secrete cytokines, toxic proteins, and granular enzymes, including myeloperoxidase (MPO), elastase and defensins. Furthermore, ROS are released to the extracellular environment in response to viral infections. Neutrophil extracellular trap (NET) formation is active during infection, and NETs are present in BAL fluid from ventilated children. Furthermore, NETs captured RSV, precluding viral particles’ binding to target cells and preventing infection. However, excessive NETs formation contributes to the immunopathology developed by patients infected with hRSV (Cortjens et al., 2016).
In addition, superoxide production has also been observed in eosinophils after hRSV exposure. Additionally, it was observed that eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) reduced the infectivity of hRSV after exposure, suggesting their antiviral activity. On the other hand, it has been demonstrated that myelin basic protein (MBP) promotes the cell death of hRSV-infected epithelial cells (Glaser et al., 2019).
Viral evasion mechanisms
Different mechanisms have been associated with poor immune control. An optimal clearance of the hRSV requires a Th1 and Th2 balance, which promotes IFNγ production by cytotoxic CD8+ T cells (Schmidt et al., 2018). Besides, hRSV infection does not seem to engage an effective memory response that protects from future viral exposures. During convalescence, circulating RSV IgG- but not IgA-producing memory B cells are present; this deficit IgA memory may contribute to recurrent infections, especially in childhood (Russell et al., 2017).
hRSV impairs the assembly of a proper immunological synapse between the antigen-presenting cells (APC), such as the dendritic cells and T cells. The virus renders T cells unable to respond correctly, affecting the adaptive immune response and increasing the risk of reinfections (Gonzalez et al., 2008). The hRSV has been observed to infect dendritic cells and impair their maturation without affecting their ability to present antigens and prime T cells. However, the infection alters the cytokine milieu by inhibiting the formation of the immune synapse, producing inhibitory factors and affecting the viral clearance through the NS1 and NS2 proteins that hamper IFNs production (Collins and Graham, 2008; van Drunen Littel-van den Hurk and Watkiss, 2012). It is well known that an immature or hypofunctional immune system facilitates infection with RSV in neonates and young infants. In this sense, macrophages from neonatal individuals exhibit a diminished expression of PRRs or a reduced upregulation upon activation with a belittled cytokine production (Basha et al., 2014).
A limited antigenic diversity is observed in hRSV strains compared to other respiratory viruses. However, reinfections with hRSV occur throughout life, and this can result from an incomplete and short-lived protective immunity. Nonetheless, hRSV has developed several mechanisms of evasion which could explain the impairing of the immune response (van Drunen Littel-van den Hurk and Watkiss, 2012). The G and F proteins are heavily glycosylated antigens that produce neutralizing antibodies, which could interfere with antibody recognition (García-Beato and Melero, 2000; Palomo et al., 2000). In the central conserved domain of the G protein, a CX3C motif resembles the one in the chemokine fractalkine; this motif shows in vitro a similar leukocyte chemotactic activity (Jartti et al., 2009). It impairs the activation of the NF-κB and the production of inflammatory cytokines in human monocytes, suggesting an immune inhibitory role (Levine et al., 2004). It has been described that the secreted form of G protein inhibits TLR3/4 mediated IFN-beta in vitro (Shingai et al., 2008), probably suppressing the innate immune response.
The F protein responsible for the fusion and subsequent syncytia formation (Sun et al., 2013)is critical in the viral attachment, interacting with the hRSV receptor: nucleolin (Resch et al., 2007). This protein induces the innate immune response through TLR4 on leukocytes (Freymuth et al., 1997) and epithelial cells, facilitating p53-dependent apoptosis (Freymuth et al., 2006). The SH protein has been implicated in inhibiting apoptosis by the TNF-α pathway (Stockman et al., 2012; Zhou et al., 2012).
On the other hand, the production of G protein occurred in a full-length membrane-bound form and truncated secreted form. The secreted protein seems to decoy the neutralizing antibodies (van Drunen Littel-van den Hurk and Watkiss, 2012). Besides, its CX3C motif allows the signaling through the CX3CR1 receptor and confers a chemotactic activity similar to fractalkine (CX3CL1). It is unclear if this capacity affects the leukocyte recruitment to the infected lungs in vivo (Collins and Graham, 2008). Some studies have reported how the G protein can induce specific T cell clones to produce IL-4 and IL-10, while the T cells specific to the F protein induce a Th1 immune response that mimics the answer raised by whole hRSV (Jackson and Scott, 1996). These data suggest that the G protein could downregulate cellular immunity and induce a negative immune regulation, antagonizing TLRs signaling (Collins and Graham, 2008; van Drunen Littel-van den Hurk and Watkiss, 2012).
TH1-TH2 immune response in hRSV infection
Variable results have been obtained when children’s cytokine profiles are analyzed in primary hRSV infection. Some studies found no change or decreased IFNγ levels in mitogen-stimulated peripheral blood mononuclear cells (PBMC) (Bendelja et al., 2000; Pinto et al., 2006). This cytokine’s production was also low compared with other respiratory infection agents (Aberle et al., 2004).
Some conflicting results around the concurrent upregulation of Th2 cytokines suggest a Th2 dominance (Bendelja et al., 2000; Gut et al., 2013), while other researchers could not find an IL-4 or IL-5 production (Pinto et al., 2006). Besides, the specific role of IFNγ production by PBMC and the positive correlation with disease severity appears to be difficult to establish even when other data propose a protective effect for this cytokine (Bendelja et al., 2000). A study suggests a combined Th1 and Th2 immune response, RSV stimulation of PBMC from RSV-hospitalized patients results in Th1 and Th2 cytokine expression, accompanied by enhanced production of IL-2, IL-4, and IL-13 (Tripp et al., 2002). Another study reported a predominant production of IFN-gamma and low levels of IL-4 and IL-10, but any association of clinical severity with T cell profile was not observed (Brandenburg et al., 2000).
Although hRSV RNA has been detected in blood monocytes and neutrophils, it is well known that the hRSV infection is restricted to the respiratory tract (O'Donnell et al., 1998; Halfhide et al., 2011). Consequently, the analysis of PBMC could not reflect the local immune responses occurring in the respiratory tract; besides, hRSV-specific CD8+ T cells are identified and show quantitative differences between blood and the respiratory location, indicating active recruitment into the tissue (Heidema et al., 2008). Compared with the lower respiratory tract, cytokine levels in the upper respiratory tract have a strong or moderate correlation in children with ventilation (van Schaik et al., 1999; Semple et al., 2007). However, these data are controversial; increased IFNγ production is observed during the acute hRSV infection compared to healthy controls (Garofalo et al., 2001; Kim et al., 2012) or bronchiolitis patients unrelated to hRSV (Bennett et al., 2007). In contrast, in other studies, a low or undetectable IFNγ production has been reported (Kristjansson et al., 2005).
In the same way, although increased levels of Th2 cytokines have been described (Legg et al., 2003), other data report low or undetectable levels, similar to controls (Bont et al., 2001; Pinto et al., 2006; Kim et al., 2012). The Th2-skewing has been found in patients with ambulatory lower respiratory tract infection compared to those with upper respiratory tract infection (Legg et al., 2003). However, other studies propose a Th2-skewing in upper respiratory tract infection and hypoxic bronchiolitis (Garofalo et al., 2001). Virus-induced wheezing is characterized by an immunologic imbalance, resulting in excessive release of IFN-γ in the airway of patients with bronchiolitis (van Schaik et al., 1999). A significant inter-individual disparity in the expression of Th1 and Th2 cytokines and the dominant cytokine profile has been found in cytokine mRNA response analysis (Mobbs et al., 2002). Another study evaluated nasopharyngeal secretions in infected infants, reporting that hRSV infection promotes Th2-like response in the nose with local production of IL-4, IL-5, macrophage inflammatory protein 1b, and infiltration and activation of eosinophils (Kristjansson et al., 2005).
A noticeable age-dependence has been observed in IFNγ and IL-12 production with the responsiveness to IL-12 (type 1 immune responses inductor) (Krampera et al., 1999; Buck et al., 2002; Motegi et al., 2006; Yerkovich et al., 2007). IL-12 and IL-18 have independent effects on their role in the induction of IFNγ production, and it seems to protect from bronchiolitis (van Benten et al., 2003). It is unclear if the higher IL-4 production and lower IFNγ production are relevant in the hRSV infections, which seem to be related to the age in mice models (Culley et al., 2002; Dakhama et al., 2005). In the same way, the disease severity impacts on the lower respiratory tract after the first infection and the immune response in secondary infections remains unclear. The IL-10 levels in different specimens are higher in some cohorts (Sheeran et al., 1999; Chung et al., 2005) and similar in others (van Schaik et al., 1999; Joshi et al., 2003; Pinto et al., 2006). During acute infection, some studies suggest a link between marked IL-10 production and atopy (Chung et al., 2005). However, other reports deny this association (Joshi et al., 2003).
Moreover, the nasopharyngeal or tracheal IL-10 levels strongly correlate with severity (Sheeran et al., 1999; Bennett et al., 2007; Vieira et al., 2010). The pro-inflammatory and regulatory profile of IL-10 could be partially responsible for the discrepancies that the researchers report. Besides, the timing and intensity of production may influence its role as a protective or detrimental factor. On the other hand, methodological issues and the differential capacity to induce IL-10 that the specific strains of hRSV exhibit could influence the results (Lukacs et al., 2006).
Independently of Th1/Th2 response, robust production of IL-13 has been observed during the early phase of RSV infection in a murine model. This production of IL-13 in several other pulmonary diseases is mediated for Group 2 innate lymphoid cells and can contribute to immunopathology during RSV infection (Stier et al., 2016).
Immunity in animal models
In the search for treatments and vaccines, animal models have been necessary to understand the immune response against hRSV. Still, no animal can widely represent the immune response in humans, so it has been necessary to use different animal models (Taylor, 2017). Several animals have been used as models, including cotton rats, mice, ferrets, guinea pigs, hamsters, chinchillas, neonatal lambs, bovines, and non-human primates like chimpanzees, African green monkeys, and macaques (Taylor, 2017).
Replicating the hRSV infection in animals is difficult because these are not entirely permissive to the virus, and clinical signs are not present in some cases; chimpanzees are the only non-human primate permissive to the virus with symptom development on the respiratory tract (Teng et al., 2000). Green monkeys are less permissive to the virus than chimpanzees, but the viral load is a reliable parameter to determine in this model (Ispas et al., 2015).
Despite differences between humans and animals, the mice model is one of the most used for testing vaccine development and elucidation of immunopathogenic mechanisms in the hRSV infection context (Mazur et al., 2018; Andersen and Winter, 2019). For example, the Th2 immune response polarization has been observed in this model, where Th2 cytokines (IL-4, IL-10, IL-13, and CCL5) were upregulated (Sawada and Nakayama, 2016).
The use of animal models exhibits great benefits in understanding the pathology of the infection and is also essential for preclinical studies to achieve vaccine development. In the same way, different studies emphasize that there is no reliable animal model to approximate the morbidity and mortality of hRSV infection in humans. For this reason, the study of infected humans is very important (Altamirano-Lagos et al., 2019).
Current information on vaccines against hRSV
Target populations for hRSV vaccination include infants, pregnant women, and older adults. Despite there being no approved vaccines against hRSV for all target populations available, there are several candidates in different clinical trial phases. Currently, scientists have dedicated their efforts to 33 vaccine candidates using 6 hRSV vaccine platforms, 9 candidates are in pivotal phase III clinical trials, and 2 are approved by US Food and Drug Administration (FDA). These platforms are particle-based vaccines, vector-based vaccines, live-attenuated viral particles or chimeric vaccines, subunit vaccines, mRNA vaccines, and monoclonal antibodies (Qiu et al., 2022; Mazur et al., 2023).
There are three target populations: pediatric, maternal and older adults. For pediatrics, strategies include passive immunoprophylaxis with monoclonal antibodies, live-attenuated vaccines and passive immunization with maternal antibodies; for maternal subunit vaccines are in late-stage studies to protect infants and; for older adults, strategies include vector, subunit, and nucleic acid approaches (Mazur et al., 2023).
GSK’s landmark positive pivotal AReSVi-006 (Adult Respiratory Syncytial Virus) phase III trial data showed that adults aged 60 years or older receiving a single dose of an AS01E-adjuvanted RSV prefusion F protein-based vaccine (RSVPreF3 OA) exhibited an efficacy against hRSV of 82.6% (96.95% confidence interval [CI], 57.9–94.1) (Papi et al., 2023), making this, in May 2023, the first hRSV vaccine approved by FDA to be used in adults aged 60 years or older (U.S. Food and Drug Administration, 2023). Recently, the FDA has approved the Pfizer vaccine ABRISVOTM for older adults, a RSV A and RSV B prefusion F protein, employed in the RENOIR phase 3 clinical trial (NCT05035212) which enrolled 35.971 participants and exhibited a vaccine efficacy of 85.7% (96.66% confidence interval [CI], 32.0–98.7) (Walsh et al., 2023). These current vaccines are the result of years of research, and have the potential to reduce the incidence, morbidity, mortality, and economic burden of hRSV infections worldwide.
Conclusion
The innate immune response has a crucial role during hRSV infection. Its modulation has been widely demonstrated by hRSV in which activation of PRRs, induction of pro-inflammatory cytokines, induction of antiviral response, and shape of adaptive immune response have been described. Local and systemic immune responses induced during viral infection have been associated with pathogenesis, and the contribution of Th1 and Th2 immune responses is variable. Understanding the immune response generated during hRSV infection is necessary for developing new therapeutics that can modulate the immunopathogenesis of hRSV infection. These advances would also be valuable for developing an effective and safe vaccine necessary for all target populations and ameliorate the burden of RSV infection in public health.
Author contributions
DC, DMG and SG: Writing—Original Draft. NAT and JCH: Conceptualization; Writing—Review & Editing. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by Universidad Cooperativa de Colombia and Corporación Universitaria Remington. The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aberle, J. H., Aberle, S. W., Rebhandl, W., Pracher, E., Kundi, M., and Popow-Kraupp, T. (2004). Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin. Exp. Immunol. 137, 146–150. doi:10.1111/j.1365-2249.2004.02504.x
Aeffner, F., Traylor, Z. P., Yu, E. N., and Davis, I. C. (2011). Double-stranded RNA induces similar pulmonary dysfunction to respiratory syncytial virus in BALB/c mice. Am. J. Physiol. Lung Cell Mol. Physiol. 301, L99–L109. doi:10.1152/ajplung.00398.2010
Afonso, C. L., Amarasinghe, G. K., Bányai, K., Bào, Y., Basler, C. F., Bavari, S., et al. (2016). Taxonomy of the order mononegavirales: update 2016. Arch. Virol. 161, 2351–2360. doi:10.1007/s00705-016-2880-1
Altamirano-Lagos, M. J., Diaz, F. E., Mansilla, M. A., Rivera-Perez, D., Soto, D., Mcgill, J. L., et al. (2019). Current animal models for understanding the pathology caused by the respiratory syncytial virus. Front. Microbiol. 10, 873. doi:10.3389/fmicb.2019.00873
Andersen, M. L., and Winter, L. M. F. (2019). Animal models in biological and biomedical research - experimental and ethical concerns. Acad Bras Cienc 91, e20170238. doi:10.1590/0001-3765201720170238
Anderson, C. S., Chu, C. Y., Wang, Q., Mereness, J. A., Ren, Y., Donlon, K., et al. (2020). CX3CR1 as a respiratory syncytial virus receptor in pediatric human lung. Pediatr. Res. 87, 862–867. doi:10.1038/s41390-019-0677-0
Anderson, C. S., Chirkova, T., Slaunwhite, C. G., Qiu, X., Walsh, E. E., Anderson, L. J., et al. (2021). CX3CR1 engagement by respiratory syncytial virus leads to induction of nucleolin and dysregulation of cilia-related genes. J. Virol. 95, e00095-21. doi:10.1128/JVI.00095-21
Aronen, M., Viikari, L., Kohonen, I., Vuorinen, T., Hameenaho, M., Wuorela, M., et al. (2019). Respiratory tract virus infections in the elderly with pneumonia. BMC Geriatr. 19, 111. doi:10.1186/s12877-019-1125-z
Barr, R., Green, C. A., Sande, C. J., and Drysdale, S. B. (2019). Respiratory syncytial virus: diagnosis, prevention and management. Ther. Adv. Infect. Dis. 6, 204993611986579. doi:10.1177/2049936119865798
Basha, S., Surendran, N., and Pichichero, M. (2014). Immune responses in neonates. Expert Rev. Clin. Immunol. 10, 1171–1184. doi:10.1586/1744666X.2014.942288
Battles, M. B., and Mclellan, J. S. (2019). Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 17, 233–245. doi:10.1038/s41579-019-0149-x
Bendelja, K., Gagro, A., Bace, A., Lokar-Kolbas, R., Krsulovic-Hresic, V., Drazenovic, V., et al. (2000). Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin. Exp. Immunol. 121, 332–338. doi:10.1046/j.1365-2249.2000.01297.x
Bennett, B. L., Garofalo, R. P., Cron, S. G., Hosakote, Y. M., Atmar, R. L., Macias, C. G., et al. (2007). Immunopathogenesis of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 195, 1532–1540. doi:10.1086/515575
Bont, L., Heijnen, C. J., Kavelaars, A., Van Aalderen, W. M., Brus, F., Draaisma, J. M., et al. (2001). Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J. Infect. Dis. 184, 355–358. doi:10.1086/322035
Bonville, C. A., Rosenberg, H. F., and Domachowske, J. B. (1999). Macrophage inflammatory protein-1α and RANTES are present in nasal secretions during ongoing upper respiratory tract infection: chemokines induced by URIs. Pediatr. Allergy Immunol. 10, 39–44. doi:10.1034/j.1399-3038.1999.101005.x
Botosso, V. F., Zanotto, P. M., Ueda, M., Arruda, E., Gilio, A. E., Vieira, S. E., et al. (2009). Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog. 5, e1000254. doi:10.1371/journal.ppat.1000254
Bradley, J. P., Bacharier, L. B., Bonfiglio, J., Schechtman, K. B., Strunk, R., Storch, G., et al. (2005). Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics 115, e7–e14. doi:10.1542/peds.2004-0059
Brandenburg, A. H., Kleinjan, A., Van Het Land, B., Moll, H. A., Timmerman, H. H., De Swart, R. L., et al. (2000). Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J. Med. Virol. 62, 267–277. doi:10.1002/1096-9071(200010)62:2<267:AID-JMV20>3.0.CO;2-8
Braun, M. R., Noton, S. L., Blanchard, E. L., Shareef, A., Santangelo, P. J., Johnson, W. E., et al. (2021). Respiratory syncytial virus M2-1 protein associates non-specifically with viral messenger RNA and with specific cellular messenger RNA transcripts. PLoS Pathog. 17, e1009589. doi:10.1371/journal.ppat.1009589
Buck, R. H., Cordle, C. T., Thomas, D. J., Winship, T. R., Schaller, J. P., and Dugle, J. E. (2002). Longitudinal study of intracellular T cell cytokine production in infants compared to adults. Clin. Exp. Immunol. 128, 490–497. doi:10.1046/j.1365-2249.2002.01851.x
Cao, D., Gao, Y., and Liang, B. (2021). Structural insights into the respiratory syncytial virus RNA synthesis complexes. Viruses 13, 834. doi:10.3390/v13050834
Checchia, P. A., Paes, B., Bont, L., Manzoni, P., Simões, E. A., Fauroux, B., et al. (2017). Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with congenital heart disease. Infect. Dis. Ther. 6, 37–56. doi:10.1007/s40121-016-0142-x
Chung, H. L., Kim, W. T., Kim, J. K., Choi, E. J., Lee, J. H., Lee, G. H., et al. (2005). Relationship between atopic status and nasal interleukin 10 and 11 levels in infants with respiratory syncytial virus bronchiolitis. Ann. Allergy Asthma Immunol. 94, 267–272. doi:10.1016/S1081-1206(10)61307-5
Collins, P. L., and Graham, B. S. (2008). Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82, 2040–2055. doi:10.1128/JVI.01625-07
Cortjens, B., De Boer, O. J., De Jong, R., Antonis, A. F., Sabogal Pineros, Y. S., Lutter, R., et al. (2016). Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 238, 401–411. doi:10.1002/path.4660
Culley, F. J., Pollott, J., and Openshaw, P. J. (2002). Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196, 1381–1386. doi:10.1084/jem.20020943
Dakhama, A., Park, J. W., Taube, C., Joetham, A., Balhorn, A., Miyahara, N., et al. (2005). The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 175, 1876–1883. doi:10.4049/jimmunol.175.3.1876
Devincenzo, J. P. (2004). Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr. Res. 56, 914–917. doi:10.1203/01.PDR.0000145255.86117.6A
Falsey, A. R., Hennessey, P. A., Formica, M. A., Cox, C., and Walsh, E. E. (2005). Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352, 1749–1759. doi:10.1056/NEJMoa043951
Feng, S., Zeng, D., Zheng, J., and Zhao, D. (2018). MicroRNAs: mediators and therapeutic targets to airway hyper reactivity after respiratory syncytial virus infection. Front. Microbiol. 9, 2177. doi:10.3389/fmicb.2018.02177
Fjaerli, H. O., Farstad, T., and Bratlid, D. (2004). Hospitalisations for respiratory syncytial virus bronchiolitis in akershus, Norway, 1993-2000: a population-based retrospective study. BMC Pediatr. 4, 25. doi:10.1186/1471-2431-4-25
Fonceca, A. M., Flanagan, B. F., Trinick, R., Smyth, R. L., and Mcnamara, P. S. (2012). Primary airway epithelial cultures from children are highly permissive to respiratory syncytial virus infection. Thorax 67, 42–48. doi:10.1136/thoraxjnl-2011-200131
Freymuth, F., Vabret, A., Galateau-Salle, F., Ferey, J., Eugene, G., Petitjean, J., et al. (1997). Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin. Diagn Virol. 8, 31–40. doi:10.1016/s0928-0197(97)00060-3
Freymuth, F., Vabret, A., Cuvillon-Nimal, D., Simon, S., Dina, J., Legrand, L., et al. (2006). Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J. Med. Virol. 78, 1498–1504. doi:10.1002/jmv.20725
Funchal, G. A., Jaeger, N., Czepielewski, R. S., Machado, M. S., Muraro, S. P., Stein, R. T., et al. (2015). Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One 10, e0124082. doi:10.1371/journal.pone.0124082
García-Beato, R., and Melero, J. A. (2000). The C-terminal third of human respiratory syncytial virus attachment (G) protein is partially resistant to protease digestion and is glycosylated in a cell-type-specific manner. J. Gen. Virol. 81, 919–927. doi:10.1099/0022-1317-81-4-919
Garofalo, R. P., Patti, J., Hintz, K. A., Hill, V., Ogra, P. L., and Welliver, R. C. (2001). Macrophage inflammatory protein–1α (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 184, 393–399. doi:10.1086/322788
Gelfand, E. W. (2012). Development of asthma is determined by the age-dependent host response to respiratory virus infection: therapeutic implications. Curr. Opin. Immunol. 24, 713–719. doi:10.1016/j.coi.2012.08.011
Glaser, L., Coulter, P. J., Shields, M., Touzelet, O., Power, U. F., and Broadbent, L. (2019). Airway epithelial derived cytokines and chemokines and their role in the immune response to respiratory syncytial virus infection. Pathogens 8, 106. doi:10.3390/pathogens8030106
Gonzalez, P. A., Prado, C. E., Leiva, E. D., Carreno, L. J., Bueno, S. M., Riedel, C. A., et al. (2008). Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 105, 14999–15004. doi:10.1073/pnas.0802555105
Goritzka, M., Makris, S., Kausar, F., Durant, L. R., Pereira, C., Kumagai, Y., et al. (2015). Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 212, 699–714. doi:10.1084/jem.20140825
Griffiths, C., Drews, S. J., and Marchant, D. J. (2017). Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev. 30, 277–319. doi:10.1128/CMR.00010-16
Gut, W., Pancer, K., Abramczuk, E., Czescik, A., Dunal-Szczepaniak, M., Lipka, B., et al. (2013). RSV respiratory infection in children under 5 y. o. -dynamics of the immune response Th1/Th2 and IgE. Przegl Epidemiol. 67, 17–22. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23745370 (Accessed November 23, 2022).
Habibi, M. S., Thwaites, R. S., Chang, M., Jozwik, A., Paras, A., Kirsebom, F., et al. (2020). Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 370, eaba9301. doi:10.1126/science.aba9301
Halfhide, C. P., Flanagan, B. F., Brearey, S. P., Hunt, J. A., Fonceca, A. M., Mcnamara, P. S., et al. (2011). Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J. Infect. Dis. 204, 451–458. doi:10.1093/infdis/jir280
Hall, C. B., Powell, K. R., Macdonald, N. E., Gala, C. L., Menegus, M. E., Suffin, S. C., et al. (1986). Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315, 77–81. doi:10.1056/NEJM198607103150201
Hall, C. B. (2001). Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344, 1917–1928. doi:10.1056/NEJM200106213442507
Heidema, J., Rossen, J. W., Lukens, M. V., Ketel, M. S., Scheltens, E., Kranendonk, M. E., et al. (2008). Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J. Immunol. 181, 5551–5559. doi:10.4049/jimmunol.181.8.5551
Hu, P., Zheng, T., Chen, J., Zhou, T., Chen, Y., Xu, X., et al. (2017). Alternate circulation and genetic variation of human respiratory syncytial virus genotypes in Chengdu, West China, 2009-2014. J. Med. Virol. 89, 32–40. doi:10.1002/jmv.24603
Imaz, M. S., Sequeira, M. D., Videla, C., Veronessi, I., Cociglio, R., Zerbini, E., et al. (2000). Clinical and epidemiologic characteristics of respiratory syncytial virus subgroups A and B infections in Santa Fe, Argentina. J. Med. Virol. 61, 76–80. doi:10.1002/(sici)1096-9071(200005)61:1<76:aid-jmv12>3.0.co;2-p
Ispas, G., Koul, A., Verbeeck, J., Sheehan, J., Sanders-Beer, B., Roymans, D., et al. (2015). Antiviral activity of TMC353121, a respiratory syncytial virus (RSV) fusion inhibitor, in a non-human primate model. PLoS One 10, e0126959. doi:10.1371/journal.pone.0126959
Jackson, M., and Scott, R. (1996). Different patterns of cytokine induction in cultures of respiratory syncytial (RS) virus-specific human TH cell lines following stimulation with RS virus and RS virus proteins. J. Med. Virol. 49, 161–169. doi:10.1002/(SICI)1096-9071(199607)49:3<161:AID-JMV2>3.0.CO;2-2
Jafri, H. S., Wu, X., Makari, D., and Henrickson, K. J. (2013). Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr. Infect. Dis. J. 32, 335–340. doi:10.1097/INF.0b013e318282603a
Jartti, T., Lehtinen, P., Vuorinen, T., and Ruuskanen, O. (2009). Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr. Infect. Dis. J. 28, 311–317. doi:10.1097/INF.0b013e31818ee0c1
Johnson, S. M., Mcnally, B. A., Ioannidis, I., Flano, E., Teng, M. N., Oomens, A. G., et al. (2015). Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 11, e1005318. doi:10.1371/journal.ppat.1005318
Joshi, P., Shaw, A., Kakakios, A., and Isaacs, D. (2003). Interferon-gamma levels in nasopharyngeal secretions of infants with respiratory syncytial virus and other respiratory viral infections. Clin. Exp. Immunol. 131, 143–147. doi:10.1046/j.1365-2249.2003.02039.x
Kim, C. K., Callaway, Z., Koh, Y. Y., Kim, S. H., and Fujisawa, T. (2012). Airway IFN-γ production during RSV bronchiolitis is associated with eosinophilic inflammation. Lung 190, 183–188. doi:10.1007/s00408-011-9349-5
Kim, T. H., Oh, D. S., Jung, H. E., Chang, J., and Lee, H. K. (2019). Plasmacytoid dendritic cells contribute to the production of IFN-beta via TLR7-MyD88-dependent pathway and CTL priming during respiratory syncytial virus infection. Viruses 11, 730. doi:10.3390/v11080730
Krampera, M., Vinante, F., Tavecchia, L., Morosato, L., Chilosi, M., Romagnani, S., et al. (1999). Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration. Clin. Exp. Immunol. 117, 291–297. doi:10.1046/j.1365-2249.1999.00977.x
Kristjansson, S., Bjarnarson, S. P., Wennergren, G., Palsdottir, A. H., Arnadottir, T., Haraldsson, A., et al. (2005). Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J. Allergy Clin. Immunol. 116, 805–811. doi:10.1016/j.jaci.2005.07.012
Kumar, H., Kawai, T., and Akira, S. (2011). Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34. doi:10.3109/08830185.2010.529976
Kurt-Jones, E. A., Popova, L., Kwinn, L., Haynes, L. M., Jones, L. P., Tripp, R. A., et al. (2000). Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1, 398–401. doi:10.1038/80833
Laham, F. R., Mansbach, J. M., Piedra, P. A., Hasegawa, K., Sullivan, A. F., Espinola, J. A., et al. (2017). Clinical profiles of respiratory syncytial virus subtypes A and B among children hospitalized with bronchiolitis. Pediatr. Infect. Dis. J. 36, 808–810. doi:10.1097/INF.0000000000001596
Law, B. J., Langley, J. M., Allen, U., Paes, B., Lee, D. S., Mitchell, I., et al. (2004). The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr. Infect. Dis. J. 23, 806–814. doi:10.1097/01.inf.0000137568.71589.bd
Lay, M. K., González, P. A., León, M. A., Céspedes, P. F., Bueno, S. M., Riedel, C. A., et al. (2013). Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 15, 230–242. doi:10.1016/j.micinf.2012.11.012
Lay, M. K., Bueno, S. M., Galvez, N., Riedel, C. A., and Kalergis, A. M. (2016). New insights on the viral and host factors contributing to the airway pathogenesis caused by the respiratory syncytial virus. Crit. Rev. Microbiol. 42, 800–812. doi:10.3109/1040841X.2015.1055711
Legg, J. P., Hussain, I. R., Warner, J. A., Johnston, S. L., and Warner, J. O. (2003). Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 168, 633–639. doi:10.1164/rccm.200210-1148OC
Levine, S., Klaiber-Franco, R., and Paradiso, P. R. (1987). Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68 (9), 2521–2524. doi:10.1099/0022-1317-68-9-2521
Levine, D. A., Platt, S. L., Dayan, P. S., Macias, C. G., Zorc, J. J., Krief, W., et al. (2004). Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics 113, 1728–1734. doi:10.1542/peds.113.6.1728
Lindell, D. M., Morris, S., Mukherjee, S., and Lukacs, N. W. (2009). TLR-7 mediated responses to respiratory syncytial virus (RSV). J. Immunol. 182 (1_Supplement), 133.45. doi:10.4049/jimmunol.182.Supp.133.45
Liu, P., Jamaluddin, M., Li, K., Garofalo, R. P., Casola, A., and Brasier, A. R. (2007). Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 81, 1401–1411. doi:10.1128/JVI.01740-06
Lukacs, N. W., Moore, M. L., Rudd, B. D., Berlin, A. A., Collins, R. D., Olson, S. J., et al. (2006). Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 169, 977–986. doi:10.2353/ajpath.2006.051055
Lukacs, N. W., Smit, J. J., Mukherjee, S., Morris, S. B., Nunez, G., and Lindell, D. M. (2010). Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 185, 2231–2239. doi:10.4049/jimmunol.1000733
Marzec, J., Cho, H. Y., High, M., Mccaw, Z. R., Polack, F., and Kleeberger, S. R. (2019). Toll-like receptor 4-mediated respiratory syncytial virus disease and lung transcriptomics in differentially susceptible inbred mouse strains. Physiol. Genomics 51, 630–643. doi:10.1152/physiolgenomics.00101.2019
Mazur, N. I., Higgins, D., Nunes, M. C., Melero, J. A., Langedijk, A. C., Horsley, N., et al. (2018). The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 18, e295–e311. doi:10.1016/S1473-3099(18)30292-5
Mazur, N. I., Terstappen, J., Baral, R., Bardaji, A., Beutels, P., Buchholz, U. J., et al. (2023). Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 23, e2–e21. doi:10.1016/S1473-3099(22)00291-2
Mcintosh, E. D., De Silva, L. M., and Oates, R. K. (1993). Clinical severity of respiratory syncytial virus group A and B infection in Sydney, Australia. Pediatr. Infect. Dis. J. 12, 815–819. doi:10.1097/00006454-199310000-00004
Meng, J., Stobart, C. C., Hotard, A. L., and Moore, M. L. (2014). An overview of respiratory syncytial virus. PLoS Pathog. 10, e1004016. doi:10.1371/journal.ppat.1004016
Miller, A. L., Bowlin, T. L., and Lukacs, N. W. (2004). Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 189, 1419–1430. doi:10.1086/382958
Mobbs, K. J., Smyth, R. L., O'hea, U., Ashby, D., Ritson, P., and Hart, C. A. (2002). Cytokines in severe respiratory syncytial virus bronchiolitis. Pediatr. Pulmonol. 33, 449–452. doi:10.1002/ppul.10101
Mochizuki, H., Todokoro, M., and Arakawa, H. (2009). RS virus-induced inflammation and the intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation 32, 252–264. doi:10.1007/s10753-009-9128-0
Motegi, A., Kinoshita, M., Sato, K., Shinomiya, N., Ono, S., Nonoyama, S., et al. (2006). An in vitro Shwartzman reaction-like response is augmented age-dependently in human peripheral blood mononuclear cells. J. Leukoc. Biol. 79, 463–472. doi:10.1189/jlb.0705396
Nielsen, H. E., Siersma, V., Andersen, S., Gahrn-Hansen, B., Mordhorst, C. H., Nørgaard-Pedersen, B., et al. (2003). Respiratory syncytial virus infection-risk factors for hospital admission: a case-control study. Acta Paediatr. 92, 1314–1321. doi:10.1111/j.1651-2227.2003.tb00502.x
Ochola, R., Sande, C., Fegan, G., Scott, P. D., Medley, G. F., Cane, P. A., et al. (2009). The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 4, e8088. doi:10.1371/journal.pone.0008088
O'donnell, D. R., Mcgarvey, M. J., Tully, J. M., Balfour-Lynn, I. M., and Openshaw, P. J. (1998). Respiratory syncytial virus RNA in cells from the peripheral blood during acute infection. J. Pediatr. 133, 272–274. doi:10.1016/s0022-3476(98)70234-3
Okabayashi, T., Kojima, T., Masaki, T., Yokota, S., Imaizumi, T., Tsutsumi, H., et al. (2011). Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 160, 360–366. doi:10.1016/j.virusres.2011.07.011
Olszewska-Pazdrak, B., Casola, A., Saito, T., Alam, R., Crowe, S. E., Mei, F., et al. (1998). Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J. Virol. 72, 4756–4764. doi:10.1128/JVI.72.6.4756-4764.1998
Palomo, C., Cane, P. A., and Melero, J. A. (2000). Evaluation of the antibody specificities of human convalescent-phase sera against the attachment (G) protein of human respiratory syncytial virus: influence of strain variation and carbohydrate side chains. J. Med. Virol. 60, 468–474. doi:10.1002/(sici)1096-9071(200004)60:4<468:aid-jmv16>3.0.co;2-e
Pangesti, K. N. A., Abd El Ghany, M., Walsh, M. G., Kesson, A. M., and Hill-Cawthorne, G. A. (2018). Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virology 28, e1968. doi:10.1002/rmv.1968
Papi, A., Ison, M. G., Langley, J. M., Lee, D. G., Leroux-Roels, I., Martinon-Torres, F., et al. (2023). Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med. 388, 595–608. doi:10.1056/NEJMoa2209604
Park, H. W., Lee, B. S., Kim, A. R., Yoon, H. S., Kim, B. I., Song, E. S., et al. (2012). Epidemiology of respiratory syncytial virus infection in infants born at less than thirty-five weeks of gestational age. Pediatr. Infect. Dis. J. 31, e99–e104. doi:10.1097/INF.0b013e318257f619
Parrott, R. H., Kim, H. W., Arrobio, J. O., Hodes, D. S., Murphy, B. R., Brandt, C. D., et al. (1973). Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am. J. Epidemiol. 98, 289–300. doi:10.1093/oxfordjournals.aje.a121558
Peret, T. C., Hall, C. B., Schnabel, K. C., Golub, J. A., and Anderson, L. J. (1998). Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79 (9), 2221–2229. doi:10.1099/0022-1317-79-9-2221
Piedimonte, G., and Perez, M. K. (2014). Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 35, 519–530. doi:10.1542/pir.35-12-519
Piedra, P. A., Jewell, A. M., Cron, S. G., Atmar, R. L., and Glezen, W. P. (2003). Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21, 3479–3482. doi:10.1016/s0264-410x(03)00355-4
Pinto, R. A., Arredondo, S. M., Bono, M. R., Gaggero, A. A., and Díaz, P. V. (2006). T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 117, e878–e886. doi:10.1542/peds.2005-2119
Qiu, X., Xu, S., Lu, Y., Luo, Z., Yan, Y., Wang, C., et al. (2022). Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 68, 37–53. doi:10.1016/j.cytogfr.2022.10.001
Rayavara, K., Kurosky, A., and Hosakote, Y. M. (2022). Respiratory syncytial virus infection induces the release of transglutaminase 2 from human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 322, L1–L12. doi:10.1152/ajplung.00013.2021
Resch, B., Gusenleitner, W., and Mueller, W. D. (2007). Risk of concurrent bacterial infection in preterm infants hospitalized due to respiratory syncytial virus infection. Acta Paediatr. 96, 495–498. doi:10.1111/j.1651-2227.2007.00226.x
Roca, A., Abacassamo, F., Loscertales, M. P., Quintó, L., Gómez-Olivé, X., Fenwick, F., et al. (2002). Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J. Med. Virol. 67, 616–623. doi:10.1002/jmv.10148
Rudd, B. D., Burstein, E., Duckett, C. S., Li, X., and Lukacs, N. W. (2005). Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79, 3350–3357. doi:10.1128/JVI.79.6.3350-3357.2005
Rudd, B. D., Smit, J. J., Flavell, R. A., Alexopoulou, L., Schaller, M. A., Gruber, A., et al. (2006). Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176, 1937–1942. doi:10.4049/jimmunol.176.3.1937
Russell, C. D., Unger, S. A., Walton, M., and Schwarze, J. (2017). The human immune response to respiratory syncytial virus infection. Clin. Microbiol. Rev. 30, 481–502. doi:10.1128/CMR.00090-16
Sawada, A., and Nakayama, T. (2016). Experimental animal model for analyzing immunobiological responses following vaccination with formalin-inactivated respiratory syncytial virus. Microbiol. Immunol. 60, 234–242. doi:10.1111/1348-0421.12365
Schmidt, M. E., Knudson, C. J., Hartwig, S. M., Pewe, L. L., Meyerholz, D. K., Langlois, R. A., et al. (2018). Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 14, e1006810. doi:10.1371/journal.ppat.1006810
Segovia, J., Sabbah, A., Mgbemena, V., Tsai, S. Y., Chang, T. H., Berton, M. T., et al. (2012). TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 7, e29695. doi:10.1371/journal.pone.0029695
Semple, M. G., Dankert, H. M., Ebrahimi, B., Correia, J. B., Booth, J. A., Stewart, J. P., et al. (2007). Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One 2, e1038. doi:10.1371/journal.pone.0001038
Sheeran, P., Jafri, H., Carubelli, C., Saavedra, J., Johnson, C., Krisher, K., et al. (1999). Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 18, 115–122. doi:10.1097/00006454-199902000-00007
Shen, Z., Zhang, Y., Li, H., and Du, L. (2022). Rapid typing diagnosis and clinical analysis of subtypes A and B of human respiratory syncytial virus in children. Virol. J. 19, 15. doi:10.1186/s12985-022-01744-y
Shi, T., Mcallister, D. A., O'brien, K. L., Simoes, E. A. F., Madhi, S. A., Gessner, B. D., et al. (2017). Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958. doi:10.1016/S0140-6736(17)30938-8
Shingai, M., Azuma, M., Ebihara, T., Sasai, M., Funami, K., Ayata, M., et al. (2008). Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int. Immunol. 20, 1169–1180. doi:10.1093/intimm/dxn074
Smit, J. J., Rudd, B. D., and Lukacs, N. W. (2006). Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203, 1153–1159. doi:10.1084/jem.20052359
Sourimant, J., Rameix-Welti, M. A., Gaillard, A. L., Chevret, D., Galloux, M., Gault, E., et al. (2015). Fine mapping and characterization of the L-polymerase-binding domain of the respiratory syncytial virus phosphoprotein. J. Virol. 89, 4421–4433. doi:10.1128/JVI.03619-14
Stier, M. T., Bloodworth, M. H., Toki, S., Newcomb, D. C., Goleniewska, K., Boyd, K. L., et al. (2016). Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 138, 814–824.e11. doi:10.1016/j.jaci.2016.01.050
Stockman, L. J., Curns, A. T., Anderson, L. J., and Fischer-Langley, G. (2012). Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr. Infect. Dis. J. 31, 5–9. doi:10.1097/INF.0b013e31822e68e6
Sun, Z., Pan, Y., Jiang, S., and Lu, L. (2013). Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses 5, 211–225. doi:10.3390/v5010211
Tabarani, C. M., Bonville, C. A., Suryadevara, M., Branigan, P., Wang, D., Huang, D., et al. (2013). Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 32, e437–e442. doi:10.1097/INF.0b013e3182a14407
Taylor, G. (2017). Animal models of respiratory syncytial virus infection. Vaccine 35, 469–480. doi:10.1016/j.vaccine.2016.11.054
Tayyari, F., Marchant, D., Moraes, T. J., Duan, W., Mastrangelo, P., and Hegele, R. G. (2011). Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 17, 1132–1135. doi:10.1038/nm.2444
Teng, M. N., Whitehead, S. S., Bermingham, A., St Claire, M., Elkins, W. R., Murphy, B. R., et al. (2000). Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74, 9317–9321. doi:10.1128/jvi.74.19.9317-9321.2000
Tripp, R. A., Moore, D., Barskey, A., Jones, L., Moscatiello, C., Keyserling, H., et al. (2002). Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J. Infect. Dis. 185, 1388–1394. doi:10.1086/340505
U.S. Food and Drug Administration (2023). FDA approves first respiratory syncytial virus (RSV) vaccine. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine (Accessed May 30, 2023).
Van Benten, I. J., Van Drunen, C. M., Koopman, L. P., Kleinjan, A., Van Middelkoop, B. C., De Waal, L., et al. (2003). RSV-induced bronchiolitis but not upper respiratory tract infection is accompanied by an increased nasal IL-18 response. J. Med. Virol. 71, 290–297. doi:10.1002/jmv.10482
van Drunen Littel-van den Hurk, S., and Watkiss, E. R. (2012). Pathogenesis of respiratory syncytial virus. Curr. Opin. Virol. 2, 300–305. doi:10.1016/j.coviro.2012.01.008
Van Schaik, S. M., Tristram, D. A., Nagpal, I. S., Hintz, K. M., and Welliver, R. C. (1999). Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J. Allergy Clin. Immunol. 103, 630–636. doi:10.1016/s0091-6749(99)70235-6
Vieira, R. A., Diniz, E. M., and Ceccon, M. E. (2010). Correlação entre mediadores inflamatórios na secreção nasofaríngea e no soro de crianças com infecção do trato respiratório inferior por vírus sincicial respiratório e a gravidade da doença. J. Bras. Pneumol. 36, 59–66. doi:10.1590/s1806-37132010000100011
Villenave, R., O'donoghue, D., Thavagnanam, S., Touzelet, O., Skibinski, G., Heaney, L. G., et al. (2011). Differential cytopathogenesis of respiratory syncytial virus prototypic and clinical isolates in primary pediatric bronchial epithelial cells. Virol. J. 8, 43. doi:10.1186/1743-422X-8-43
Villenave, R., Thavagnanam, S., Sarlang, S., Parker, J., Douglas, I., Skibinski, G., et al. (2012). In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. U. S. A. 109, 5040–5045. doi:10.1073/pnas.1110203109
Walsh, E. E., Mcconnochie, K. M., Long, C. E., and Hall, C. B. (1997). Severity of respiratory syncytial virus infection is related to virus strain. J. Infect. Dis. 175, 814–820. doi:10.1086/513976
Walsh, E. E., Perez Marc, G., Zareba, A. M., Falsey, A. R., Jiang, Q., Patton, M., et al. (2023). Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 388, 1465–1477. doi:10.1056/NEJMoa2213836
Weigl, J. A., Puppe, W., and Schmitt, H. J. (2001). Incidence of respiratory syncytial virus-positive hospitalizations in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 20, 0452–0459. doi:10.1007/s100960100527
Yamaguchi, M., Sano, Y., Dapat, I. C., Saito, R., Suzuki, Y., Kumaki, A., et al. (2011). High frequency of repeated infections due to emerging genotypes of human respiratory syncytial viruses among children during eight successive epidemic seasons in Japan. J. Clin. Microbiol. 49, 1034–1040. doi:10.1128/JCM.02132-10
Yerkovich, S. T., Wikström, M. E., Suriyaarachchi, D., Prescott, S. L., Upham, J. W., and Holt, P. G. (2007). Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 62, 547–552. doi:10.1203/PDR.0b013e3181568105
Zeng, R., Cui, Y., Hai, Y., and Liu, Y. (2012). Pattern recognition receptors for respiratory syncytial virus infection and design of vaccines. Virus Res. 167, 138–145. doi:10.1016/j.virusres.2012.06.003
Zhivaki, D., Lemoine, S., Lim, A., Morva, A., Vidalain, P. O., Schandene, L., et al. (2017). Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 46, 301–314. doi:10.1016/j.immuni.2017.01.010
Keywords: human respiratory syncytial virus (hRSV), bronchiolitis, immune response, inflammation, pathogenesis
Citation: Correa D, Giraldo DM, Gallego S, Taborda NA and Hernandez JC (2023) Immunity towards human respiratory syncytial virus. Acta Virol. 67:11887. doi: 10.3389/av.2023.11887
Received: 08 December 2022; Accepted: 28 June 2023;
Published: 24 August 2023.
Edited by:
Katarina Polcicova, Slovak Academy of Sciences, SlovakiaCopyright © 2023 Correa, Giraldo, Gallego, Taborda and Hernandez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan C. Hernandez, anVhbmtoZXJuYW5kZXpAZ21haWwuY29t
 Dahiana Correa1
Dahiana Correa1 Diana M. Giraldo
Diana M. Giraldo Salomon Gallego
Salomon Gallego Natalia A. Taborda
Natalia A. Taborda Juan C. Hernandez
Juan C. Hernandez