Abstract
Serological testing is a powerful tool for analyzing the infectious disease burden landscape. Therefore, this study aimed to determine the seroprevalence against SARS-CoV-2 in the population of the municipality of Kragujevac, Serbia, with a particular reference to silent infections. A total of 4,804 participants over 19 years of age were randomly sampled for population-based seroprevalence research. Anti-N IgG antibodies were measured using rapid serological tests (UNscience®). The population was divided into four Cohorts, according to the history of SARS-CoV-2 infection and vaccination status with the whole inactivated virus vaccine BBIBP-CorV (Vero Cell®, Sinopharm), as follows: Cohort I—confirmed SARS-CoV-2 infection, not vaccinated with the BBIBP-CorV vaccine; Cohort II—without confirmed SARS- CoV-2 infection, vaccinated with the BBIBP-CorV vaccine; Cohort III—confirmed SARS-CoV-2 infection, vaccinated with the BBIBP-CorV vaccine; Cohort IV—without confirmed SARS-CoV-2 infection, not vaccinated with the BBIBP-CorV vaccine (silent immunization). Cohorts I and IV included patients vaccinated with vaccines other than the BBIBP-CorV vaccine. The results showed that the overall prevalence of anti-N IgG antibodies was 56.5%, with the highest seroprevalence in Cohort III at 85.8%. In Cohort IV, the prevalence of anti-N IgG antibodies was 40.7%, attributed to silent immunization. The results also suggest that the prevalence of anti-N IgG antibodies decreased over time but remained detectable for more than 12 months in Cohort I. Since currently, there is no data on silent infection frequency in our country, these findings may provide insight into the extent of silent infections in the Serbian population.
Introduction
The outbreak of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has led to serious global health and economic burden, resulting in a state of emergency with appropriate preventive, protective, and therapeutic measures to cope with the COVID-19 pandemic. From the pandemic’s beginning in the Republic of Serbia until December 2022, 2,439,730 cases of SARS-CoV-2 virus infections were registered1, of which 540,242 in central Serbia2.
SARS-CoV-2 virus is a single-stranded RNA virus from the Coronaviridae family. It is transmitted by respiratory droplets during close personal contact (Leao et al., 2022). In addition to respiratory affection, an infected person may have symptoms and signs from other organs and organ systems, such as cardiovascular, neurological, digestive, urinary, and hematological (Rabaan et al., 2020). Symptomatic patients infected with the SARS-CoV-2 virus can exhibit a wide range of clinical features, but the infection is asymptomatic in up to 40% of cases (Sah et al., 2021). Such asymptomatic patients and those with mild symptoms often remain unidentified. In addition, the limited sensitivity of laboratory tests could negatively affect the number of laboratory-confirmed cases, especially if we test only patients with COVID-19 symptoms (Buitrago-Garcia et al., 2020). Therefore, there is a possibility that a significant number of infections may not be detected (Reese et al., 2021).
Laboratory detection of SARS-CoV-2 is carried out by nucleic acid amplification test and immunoassay. Real-time RT-PCR assays are used to detect viral nucleic acids. These tests are designed to detect protein-coding sequences of interest and represent the gold standard in laboratory diagnostics. The immunological test measures the proteins of the COVID-19 virus (rapid antigen tests) and the antibodies produced by the host’s immune response to the virus infection (serological tests). Rapid antigen tests are designed to detect one or more of the structural proteins encoded by the coronavirus genome [spike (S), membrane (M), envelope (E), nucleocapsid (N)] (Boson et al., 2021). Serological tests are used to monitor the humoral immune response to SARS-CoV-2 infection and vaccination. These tests detect antibodies against the virus found in the host’s circulation. Given that antibodies are detected in people who had an infection without or with mild symptoms, it is possible to determine the frequency of asymptomatic cases without the confirmed disease. That is why these tests have an epidemiological significance in the spread of infection.
The SARS-CoV-2 virus triggers specific humoral and cellular immune responses. Humoral immune response implies the production of different classes of antibodies, of which IgM and IgG are significant for us as markers of acute infection or prior exposure to the virus, either through infection or vaccination (Long et al., 2020a; Munster et al., 2020; World Health Organization, 2022). It is not yet fully known which antibodies at what concentration could protect the population from primary or re-infection. In addition, there are conflicting views on the persistence of a specific humoral immune response to the SARS-CoV-2 virus over time, ranging from stable, persistent immunity through its rapid decline to late onset of low antibody levels or complete absence of long-term antibodies, especially in vaccinated patients (Jeyanathan et al., 2020). Several studies have shown that neutralizing antibodies circulate in the peripheral circulation for up to 8 months after the disease (Castro Dopico et al., 2022). Another study showed that after 10 months, 13% of subjects lost their IgG antibody titer (Vanshylla et al., 2021). Asymptomatic infections are known to give lower titer of antibodies and remain in the circulation for a shorter period (Long et al., 2020b).
The aim of this study was to assess the seroprevalence of anti-N-IgG antibodies against the SARS-CoV-2 virus in the population of the municipality of Kragujevac, Serbia. Since there is no data from previous research in Serbia, these findings may provide insight into the extent of silent infections in the population. In addition, this prevalence study aimed to determine the time interval for maintaining anti-N IgG seropositivity after confirmed infection and/or administration of COVID-19 vaccines available in Serbia.
Materials and methods
Study design, setting, and participants
This descriptive cross-sectional study was conducted in the city of Kragujevac, Serbia. It included a sample of residents from the territory covered by the Kragujevac Health Centre (also known as the Dom zdravlja Kragujevac), which provides primary healthcare for approximately 170,000 health-insured inhabitants of all ages. A representative, “convenient” sample was used, which means that each person available for seroassay for SARS-CoV-2 at the Kragujevac Health Centre was included in the study. There were no other exclusion criteria besides patients with currently confirmed COVID-19 infection. A total of 5,085 adult patients (at least 18 years old) of both sexes with different clinical characteristics were enrolled. Each participant was referred by his/her own chosen general practitioner for routine laboratory blood tests. In rare cases, if the participant was serologically tested several times, only the first test’s results were taken in further data processing and analysis.
The research was approved by the Ethics Committee of the Kragujevac Health Centre on 06.12.2021. (Decision No. 01-8625/3). Samples were collected and tested between 10 January and 17 February 2022. All patients gave their written informed consent.
IgG detection
Anti-SARS-CoV-2 IgG antibodies specific for protein N were determined using rapid serological tests. Anti-SARS-CoV-2 antibodies were detected using UNscience® (UNscience Biotechnology, Wuhan, China). It is a qualitative membrane-based immunoassay based on a combination of particles coated with SARS-CoV-2 antigens which allow the detection of IgG antibodies for the protein of the SARS-CoV-2 virus. In order to run the test, a small volume of the sample (20 µL or one drop) is placed in the cassette, and then one drop of the buffer is added. Ten to twenty minutes later, the results are read. If a colored line emerges in the IgG test section and a colored line in the control region, the result is positive. When a colored line emerges in the control region but not in the test region for IgG, the result is negative. When the control line does not display, the result is invalid. This point-of-care (POC) serology test has a sensitivity and specificity of 98.81% and 98.02%, respectively.
Data collection
Outcome data (i.e., serological test results) were observed as a categorical dichotomous variable depending on whether antibodies to the N protein of the SARS-CoV-2 virus were detected or not. The following independent variables were observed: 1) Ascertained vaccine status of the subjects, including the type of vaccine received as well as the date it was received, which allowed the length of the time interval from the date of administration of the last dose of vaccine to sampling to be determined (in months); 2) Data on the previous COVID-19 infection; this made it possible to determine the length of the time from last positive SARS-CoV-2 PCR and/or rapid Ag test to sampling (in months). Vaccine status and data on the previous COVID-19 infection were taken from the Institute of Public Health of Serbia database and the Republic Fund of Health Insurance database, respectively. Confounding factors, such as age and sex, were collected from patients’ electronic medical records in primary care and observed as continuous and categorical variables, respectively. According to the observed age, a total sample was divided into two age categories (younger than 60 and older than 60). Regarding the vaccination status, there were participants vaccinated with one, two, or three doses who received all types of vaccines available in Serbia [RNA-based BNT162b2 (Comirnaty
®, Pfizer-BioNTech), inactivated BBIBP-CorV (Vero Cell
®, Sinopharm), and vector-based Gam-COVID-Vac (Sputnik V
®, Gamaleya Institute) and ChAdOx1-n CoV-19 (Vaxzevria
®, University of Oxford/AstraZeneca)]. Patients vaccinated with different vaccines were excluded from the total sample. The distribution of vaccine types by received doses is shown in
Supplementary Table S1. From the total number of persons included in the study (
n= 5,085), participants who were vaccinated with a combination of different vaccines were excluded from the study (
n= 281). Given the fact that the test we used detects only anti-N IgG antibodies produced either during natural infection or vaccination with the BBIBP-CorV vaccine, we classified the population into four Cohorts formed according to the history of COVID-19 infection and BBIBP-CorV vaccination status, as follows:
⁃ Cohort I (n = 1,075): patients who tested positive for virus infection and were not vaccinated with the BBIBP-CorV vaccine;
⁃ Cohort II (n = 1,357): patients who did not test positive for virus infection and were vaccinated with the BBIBP-CorV vaccine;
⁃ Cohort III (n = 691): patients who tested positive for virus infection and were vaccinated with the BBIBP-CorV vaccine;
⁃ Cohort IV (n = 1,681): patients who did not test positive for virus infection and were not vaccinated with the BBIBP-CorV vaccine (silent immunization).
Cohorts I and IV included patients vaccinated with vaccines other than the BBIBP-CorV vaccine (Supplementary Table S2).
Statistical analysis
Statistical software SPSS version 22.0 (IBM Corporation, Armonk, NY, United States) was used to perform all statistical analyses. Based on its findings, continuous variables were summarized as median and interquartile range, while the significance of the difference between them was determined using Mann-Whitney test. Absolute and relative frequencies were used to summarize categorical variables, while the Chi-square test was performed to determine differences in the frequencies of individual categories. The probability of statistical significance for all analyses was set at p < 0.05 (5%).
Results
Our study examined a total sample of participants aged 19–94 years. A higher prevalence of seropositivity (58%) was observed in individuals under the age of 60 [χ2 (1) = 6.298, p = 0.012]. The female sex was slightly more represented in our study, accounting for 63.8% of the participants. Among the respondents, a lesser percentage had a positive SARS-CoV-2 virus test outcome (36.8%), while a greater percentage had been vaccinated with one of the vaccines available in Serbia (58.8%). Notably, 56.5% of the samples showed presence of anti-N IgG antibodies (Table 1).
TABLE 1
| Study population | n |
|---|---|
| Overall | 4,804 |
| Age, median (IQR) [years] | 55 (40–68) |
| Age groups | |
| <60 years | 2,786 |
| ≥60 years | 2,018 |
| Sex | |
| Male | 1,740 |
| Female | 3,064 |
| History of positive testing for SARS-CoV-2 | |
| Yes | 1,766 |
| No | 3,038 |
| History of vaccinationwith any vaccine for SARS-CoV-2 | |
| Yes | 2,825 |
| No | 1,979 |
| Seropositive for SARS-CoV-2 anti-N IgG antibodies | |
| Yes | 2,714 |
| No | 2,090 |
Characteristics of the study participants.
n, number; IQR, interquartile range.
As shown in Figure 1, our results demonstrated significant differences in anti-N IgG seropositivity among the four studied Cohorts [χ2 (3) = 469.376, p < 0.001]. In Cohorts I, II, and III, over 50% of participants displayed IgG seropositivity to N antibodies. Cohort III, comprising participants who received the BBIBP-CorV vaccine and tested positive for SARS-CoV-2, exhibited the highest seropositivity rate (85.8%). No notable influence of sex or age on anti-N IgG seropositivity was observed in Cohorts I and III. Nevertheless, in Cohort II, which involved individuals vaccinated with BBIBP-CorV but lacking confirmed infection, a greater rate of seropositive individuals was observed within the younger age group [χ2 (1) = 19.697, p < 0.001].
FIGURE 1
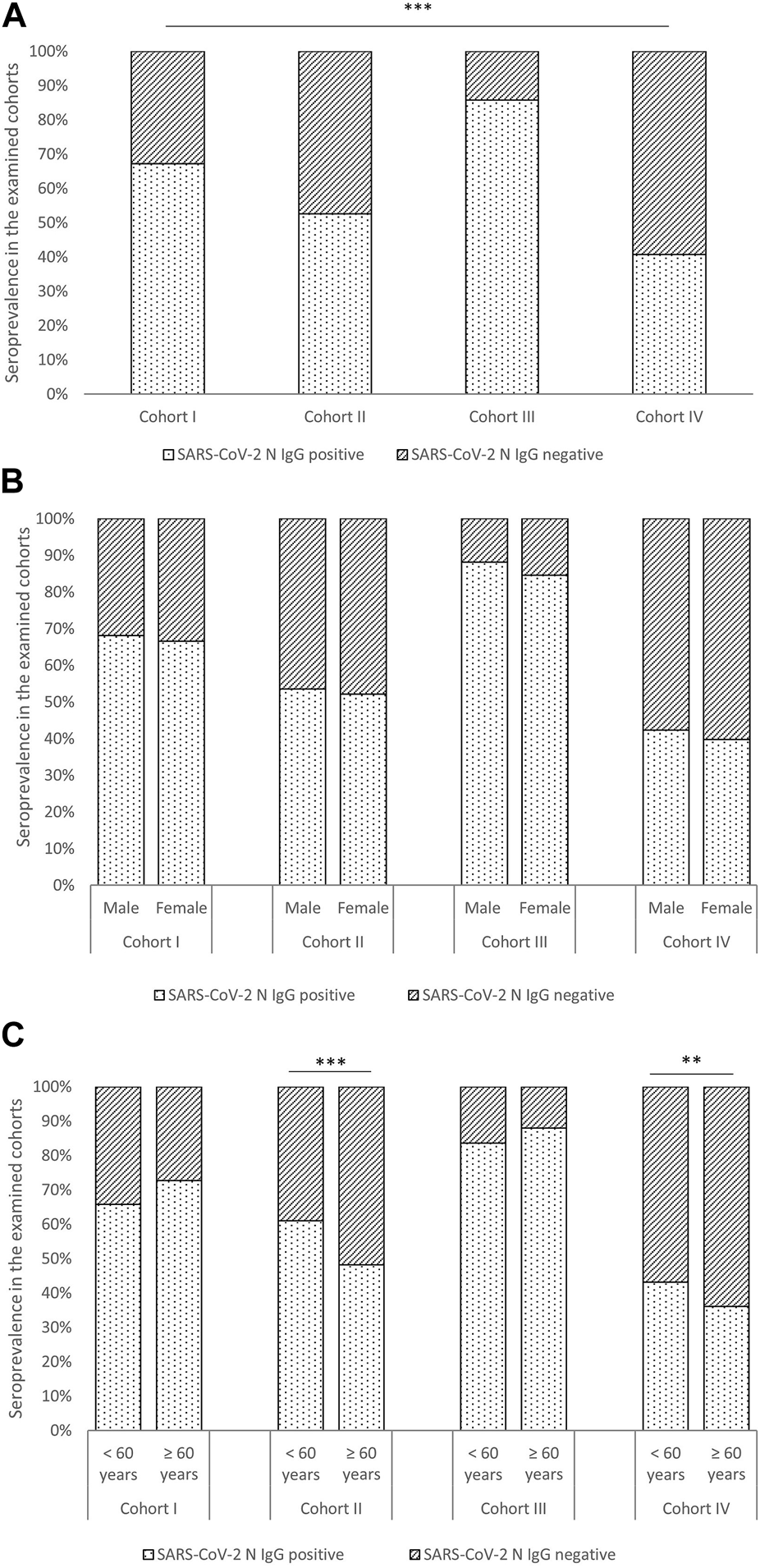
Seroprevalence among Cohorts (A) and effects of sex (B) and age (C) on seropositivity. Legend: **—statistical significance level p < 0.01; ***—statistical significance level p < 0.001.
In Cohort IV, which included participants with unconfirmed SARS-CoV-2 infection and without previous BBIBP-CorV vaccination, the rate of anti-N IgG seropositivity was 40.7% (Figure 1). It can be assumed that seropositivity in these patients is an effect of asymptomatic or undetected infection. In this Cohort, a statistically significant difference in seropositivity was observed based on age groups, with participants under 60 exhibiting a higher prevalence of anti-N IgG seropositivity than those over 60 [χ2 (1) = 7.206, p = 0.006]. Additionally, participants in Cohort IV who were vaccinated with a vaccine other than BBIBP-CorV had lower seropositivity than those who were not vaccinated [χ2 (1) = 11.032, p = 0.001].
Our analysis suggests that the prevalence of anti-N IgG seropositivity decreased over time in Cohorts I-III (as shown in Figure 2). Specifically, a continuous decline in the prevalence of seropositivity was observed in Cohorts II and III, while in Cohort I, there was a decrease in the prevalence of seropositivity up to 7–9 months, followed by a period of sustained seropositivity that lasted for more than 12 months.
FIGURE 2
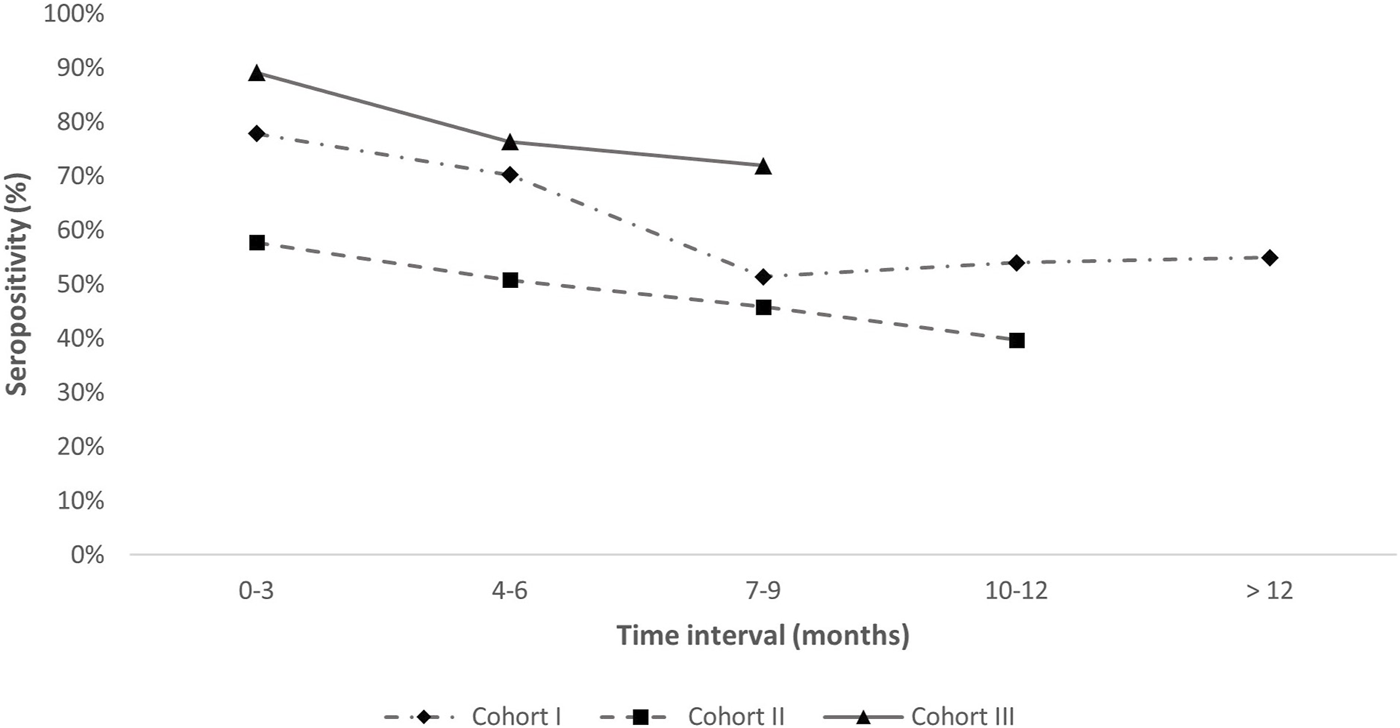
Maintenance of anti-N IgG antibodies in Cohorts I–III. Legend: Cohort I-time since prior infection; Cohort II-time since vaccination; Cohort III-time since prior infection or vaccination. X-axis represents time intervals in months. The frequency of IgG seropositive participants in a given Cohorts is presented on the y-axis. Cohort IV is not included since there is no known time of infection.
Discussion
The SARS-CoV-2 virus possesses a major structural protein, known as the nucleocapsid protein, which plays a significant role in various processes. These processes include the transcription and replication of viral RNA, the packaging of the genome into infectious particles, and the interference with the host cell cycle (Cubuk et al., 2021). Studies have demonstrated this protein is highly immunogenic and plentiful, with antibodies directed against it being more sensitive to early infection detection than antibodies targeting the spike protein (Burbelo et al., 2020). In addition, detecting anti-N antibodies allows differentiating individuals vaccinated with S-directed vaccines based on the history of infection (Demmer et al., 2021). IgG directed against the N protein can indicate both symptomatic and asymptomatic SARS-CoV-2 infection and vaccination with the BBIBP-CorV vaccine. Anti-N IgG antibodies are particularly important because their seroconversion has had a significant place in defining asymptomatic cases of COVID-19 (Jones et al., 2021). As there is a lack of data on this relevant issue in our country, the population of central Serbia was examined for the presence of anti-SARS-CoV-2 nucleocapsid IgG antibodies. The main finding of our research indicates that slightly more than half of the tested population developed IgG antibodies to the N antigen of the SARS-CoV-2 virus due to prior infection and/or vaccination with the BBIBP-CorV vaccine. Previous studies have shown that the seroprevalence of COVID-19 in general populations varies worldwide (Long et al., 2020a; Jeyanathan et al., 2020; Vanshylla et al., 2021; Castro Dopico et al., 2022). According to a newly published meta-analysis, global SARS-CoV-2 seroprevalence from infection or vaccination was 59.2% (Bergeri et al., 2022). In addition, the seroprevalence differed across the different regions and continents, with the higher prevalence present in the high-income European countries (95.9%) (Bergeri et al., 2022). Although our results follow the global seroprevalence, they are significantly lower than European countries.
In order to determine the seroprevalence depending on the history of SARS-CoV-2 infection and vaccination status, the studied population was divided into four groups (Cohort I–IV). It was observed that the frequency of anti-N IgG antibodies is higher among the vaccinated and infected population than the population that is only vaccinated or has a history of previous COVID-19 disease. These results are consistent with the assumption that vaccination and infection mutually enhance the immune response regardless of whether the person was first vaccinated or infected with the SARS-CoV-2 virus (Karachaliou et al., 2022).
Silent infections, as defined by Segen’s Medical Dictionary (Segen, 1992 February 15), are characterized by a lack of overt clinical symptoms and signs but can be identified through the presence of specific antibodies. Serological testing is a powerful epidemiological tool in identifying silent SARS-CoV-2 infections, as these infections may not be detectable through other methods. Both the BBIBP-CorV vaccine and natural SARS-CoV-2 infection result in the production of anti-S and -N antibodies, but the test used in the present study only detected anti-N IgG antibodies. Therefore, the silent SARS-CoV-2 infection Cohort (Cohort IV) included individuals who had never tested positive for SARS-CoV-2 and were not vaccinated with the BBIBP-CorV vaccine. The prevalence of asymptomatic and unconfirmed SARS-CoV-2 infections varies widely. A study conducted in Malawi found that approximately 45% of SARS-CoV-2 infections were asymptomatic (Meinus et al., 2022), while another study conducted in Brazil found a prevalence of asymptomatic infection of around 8% (Borges et al., 2020). In contrast, a study conducted in the United States found a much lower prevalence of asymptomatic infection, at just 4% (Kalish et al., 2021). Our analyzes found that 40.7% of patients in Cohort IV had developed anti-N IgG antibodies due to an asymptomatic or unconfirmed SARS-CoV-2 infection. Many factors may contribute to these variations, including differences in the populations studied, the methods used to detect infection, and the overall prevalence of the virus in the community. In addition, there may be social, psychological, and economic barriers to testing for SARS-CoV-2, such as fear, anxiety, and the consequences of absenteeism from work (McDermott and Newman, 2020; Rubin, 2020).
Various comorbidities (obesity, diabetes mellitus, respiratory system disease, and cardiovascular diseases) as variable factors had an impact on seroprevalence (Ejaz et al., 2020; Yang et al., 2020; Gasmi et al., 2021). In our work, only the effects of non-variable factors (sex, age) were evaluated in the tested population. Consistent with global findings on sex differences in seroconversion (Lai et al., 2020; Pollan et al., 2020; Tess et al., 2021; Varona et al., 2021; Wiens et al., 2021), we found no significant effect of sex on seroprevalence. On the other hand, age was a substantial determinant of seroconversion. Anti-N IgG antibody seroprevalence was significantly higher in those under 60 following BBIBP-CorV vaccination (Cohort II). The same age pattern in anti-S IgG antibody levels after vaccination with BBIBP-CorV and other available vaccines was observed in another Serbian population (Petrovic et al., 2022) as well as in other populations (Badano et al., 2022; Chiarella et al., 2022). In addition, individuals over 60 exhibited a lower occurrence of silent SARS-CoV-2 infection. The possible explanation may be that older individuals may be more likely to follow guidelines for social distancing, and wearing masks, which can help reduce the risk of contracting and spreading SARS-CoV-2 (Anderson-Carpenter and Tacy, 2022). On the other hand, older individuals may have preexisting medical conditions. People with underlying medical conditions, such as diabetes, heart disease, and lung disease, are more likely to experience severe illness COVID-19 form (Pera et al., 2015; Thakur et al., 2021). The higher prevalence of these conditions among older individuals might elucidate the relatively less pronounced occurrence of silent infections in this age group. At least, aging is associated with immunosenescence, a well-known phenomenon defined by alterations in the distribution and functionality of cells engaged in cellular and humoral adaptive immunity.
Persistence of antibodies against the N protein of SARS-CoV-2 differs depending on the individual factors and whether they were produced in response to infection or vaccination. Studies have shown that antibodies against the N protein of SARS-CoV-2 are generally detectable within 6 months after infection (Chansaenroj et al., 2021; Brlic et al., 2022), whereas one-fourth had detectable antibodies after 9–12 months (Chansaenroj et al., 2021). However, our results showed a decrease in seropositivity prevalence around 7–9 months after infection, with more than 60% of participants having detectable anti-N IgG antibodies after 12 months. Moreover, we found that the anti-N IgG elicited by the BBIBP-CorV vaccine remained detectable for up to 10–12 months after vaccination, slightly longer than the 9 months of detectability found in the report of Wang et al. (2022). Significant seroprevalence 10–12 months after BBIBP-CorV vaccination in Cohort II could, to some extent, be explained by an asymptomatic or unconfirmed infection.
There are several limitations in our study. Firstly, we detected only the presence of antibodies to the SARS-CoV-2 N protein and not to the S protein, to which the most available vaccines elicit a humoral immune response. The accuracy and reliability of used POC serological tests are limited. Moreover, the humoral immune response was determined qualitatively. Finally, no data were available on comorbidities, the severity of COVID-19 in those who had previously been infected, and other relevant confounders.
Conclusion
Our study has established that more than half of the tested population had encountered the SARS-CoV-2 virus either through infection or vaccination. Notably, our findings indicate that 40.7% of patients without confirmed SARS-CoV-2 infection and not vaccinated with the BBIBP-CorV vaccine (Cohort IV) were seropositive, suggesting that they had experienced asymptomatic or undetected infections. This observation is particularly noteworthy, due to the lack of evidence in our country, where no previous study has focused on silent infections. This matter may have important implications for understanding the true extent of the pandemic and public health efforts to control the spread of COVID-19. Understanding the prevalence and factors contributing to silent infections can inform public health efforts to protect vulnerable populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Kragujevac Health Centre on 06.12.2021. (Decision No. 01-8625/3). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: DB and SM. Data collection: NC, VC, MS, BR, and MR. Methodology and formal analysis: NC, VC, SM, and DB. Data curation and validation: NC, VC, MS, SS, and VA. Literature review: NC, VC, SM, DB, SP, DT, and ND. Original draft of the manuscript: NC, VC, DB, and SM. All authors contributed to the article and approved the submitted version.
Funding
This report is independent research supported by Primary Health Centre Kragujevac (PHCK), Serbia.
Acknowledgments
We kindly thank Labena DOO for donation of UNscience® assays. We also thank PHCK team, all staff supporting study delivery at participating sites and all participants for their ongoing commitment and contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/av.2023.11996/full#supplementary-material
Abbreviations
COVID-19, Coronavirus Disease 2019; IgG, immunoglobulin G; IgM, immunoglobulin M; RNA, ribonucleic acid; RT-PCR, reverse transcription PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
References
1
Anderson-Carpenter K. D. Tacy G. S. (2022). Predictors of social distancing and hand washing among adults in five countries during COVID-19. PLoS One17, e0264820. 10.1371/journal.pone.0264820
2
Badano M. N. Sabbione F. Keitelman I. Pereson M. Aloisi N. Colado A. et al (2022). Humoral response to the BBIBP-CorV vaccine over time in healthcare workers with or without exposure to SARS-CoV-2. Mol. Immunol.143, 94–99. 10.1016/j.molimm.2022.01.009
3
Bergeri I. Whelan M. G. Ware H. Subissi L. Nardone A. Lewis H. C. et al (2022). Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: a systematic review and meta-analysis of standardized population-based studies. PLoS Med.19, e1004107. 10.1371/journal.pmed.1004107
4
Borges L. P. Martins A. F. de Melo M. S. de Oliveira M. G. B. Neto J. M. R. Dosea M. B. et al (2020). Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev. Panam. Salud Publica44, 1. 10.26633/RPSP.2020.108
5
Boson B. Legros V. Zhou B. Siret E. Mathieu C. Cosset F. L. et al (2021). The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem.296, 100111. 10.1074/jbc.RA120.016175
6
Brlic P. K. Pavletic M. Lerga M. Krstanovic F. Matesic M. P. Miklic K. et al (2022). SARS-CoV-2 spike and nucleocapsid antibody response in vaccinated Croatian healthcare workers and infected hospitalized patients: a single center Cohort study. Viruses14, 1966. 10.3390/v14091966
7
Buitrago-Garcia D. Egli-Gany D. Counotte M. J. Hossmann S. Imeri H. Ipekci A. M. et al (2020). Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med.17, e1003346. 10.1371/journal.pmed.1003346
8
Burbelo P. D. Riedo F. X. Morishima C. Rawlings S. Smith D. Das S. et al (2020). Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J. Infect. Dis.222, 206–213. 10.1093/infdis/jiaa273
9
Castro Dopico X. Ols S. Lore K. Karlsson Hedestam G. B. (2022). Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern Med.291, 32–50. 10.1111/joim.13372
10
Chansaenroj J. Yorsaeng R. Posuwan N. Puenpa J. Wanlapakorn N. Sudhinaraset N. et al (2021). Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci. Rep.11, 23216. 10.1038/s41598-021-02659-4
11
Chiarella S. E. Jenkins S. M. Smith C. Y. Prasad V. Shakuntulla F. Ahluwalia V. et al (2022). Predictors of seroconversion after coronavirus disease 2019 vaccination. Ann. Allergy Asthma Immunol.129, 189–193. 10.1016/j.anai.2022.05.026
12
Cubuk J. Alston J. J. Incicco J. J. Singh S. Stuchell-Brereton M. D. Ward M. D. et al (2021). The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun.12, 1936. 10.1038/s41467-021-21953-3
13
Demmer R. T. Baumgartner B. Wiggen T. D. Ulrich A. K. Strickland A. J. Naumchik B. M. et al (2021). Identification of natural SARS-CoV-2 infection in seroprevalence studies among vaccinated populations. medRxiv. Available from: https://www.medrxiv.org/content/10.1101/2021.04.12.21255330v1 (Accessed April 12, 2021).
14
Ejaz H. Alsrhani A. Zafar A. Javed H. Junaid K. Abdalla A. E. et al (2020). COVID-19 and comorbidities: deleterious impact on infected patients. J. Infect. Public Health13, 1833–1839. 10.1016/j.jiph.2020.07.014
15
Gasmi A. Peana M. Pivina L. Srinath S. Gasmi Benahmed A. Semenova Y. et al (2021). Interrelations between COVID-19 and other disorders. Clin. Immunol.224, 108651. 10.1016/j.clim.2020.108651
16
Jeyanathan M. Afkhami S. Smaill F. Miller M. S. Lichty B. D. Xing Zs (2020). Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol.20, 615–632. 10.1038/s41577-020-00434-6
17
Jones J. M. Stone M. Sulaeman H. Fink R. V. Dave H. Levy M. E. et al (2021). Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA326, 1400–1409. 10.1001/jama.2021.15161
18
Kalish H. Klumpp-Thomas C. Hunsberger S. Baus H. A. Fay M. P. Siripong N. et al (2021). Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci. Transl. Med.13, eabh3826. 10.1126/scitranslmed.abh3826
19
Karachaliou M. Moncunill G. Espinosa A. Castano-Vinyals G. Rubio R. Vidal M. et al (2022). SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: a Cohort study in Catalonia. BMC Med.20, 347. 10.1186/s12916-022-02547-2
20
Lai C. C. Wang J. H. Hsueh P. R. (2020). Population-based seroprevalence surveys of anti- SARS-CoV-2 antibody: an up-to-date review. Int. J. Infect. Dis.101, 314–322. 10.1016/j.ijid.2020.10.011
21
Leao J. C. Gusmao T. P. L. Zarzar A. M. Leao Filho J. C. Barkokebas Santos de Faria A. Morais Silva I. H. et al (2022). Coronaviridae—old friends, new enemy!Oral Dis.28 (1), 858–866. 10.1111/odi.13447
22
Long Q. X. Liu B. Z. Deng H. J. Wu G. C. Deng K. Chen Y. K. et al (2020a). Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med.26, 845–848. 10.1038/s41591-020-0897-1
23
Long Q. X. Tang X. J. Shi Q. L. Li Q. Deng H. J. Yuan J. et al (2020b). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med.26, 1200–1204. 10.1038/s41591-020-0965-6
24
McDermott J. H. Newman W. G. (2020). Refusal of viral testing during the SARS-CoV-2 pandemic. Clin. Med. (Lond)20, e163–e164. 10.7861/clinmed.2020-0388
25
Meinus C. Singer R. Nandi B. Jagot O. Becker-Ziaja B. Karo B. et al (2022). SARS-CoV-2 prevalence and immunity: a hospital-based study from Malawi. Int. J. Infect. Dis.116, 157–165. 10.1016/j.ijid.2021.12.336
26
Munster V. J. Koopmans M. van Doremalen N. van Riel D. de Wit E. (2020). A novel coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med.382, 692–694. 10.1056/nejmp2000929
27
Pera A. Campos C. Lopez N. Hassouneh F. Alonso C. Tarazona R. et al (2015). Immunosenescence: implications for response to infection and vaccination in older people. Maturitas82, 50–55. 10.1016/j.maturitas.2015.05.004
28
Petrovic V. Vukovic V. Patic A. Markovic M. Ristic M. (2022). Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS- CoV-2 infection-A comparative study from Novi Sad, Serbia. PLoS One17, e0263468. 10.1371/journal.pone.0263468
29
Pollan M. Perez-Gomez B. Pastor-Barriuso R. Oteo J. Hernan M. A. Perez-Olmeda M. et al (2020). Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet396, 535–544. 10.1016/S0140-6736(20)31483-5
30
Rabaan A. A. Al-Ahmed S. H. Haque S. Sah R. Tiwari R. Malik Y. S. et al (2020). SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez. Med.28, 174–184.
31
Reese H. Iuliano A. D. Patel N. N. Garg S. Kim L. Silk B. J. et al (2021). Estimated incidence of coronavirus disease 2019 (COVID-19) illness and hospitalization- United States, February-September 2020. Clin. Infect. Dis.72, e1010–e1017. 10.1093/cid/ciaa1780
32
Rubin Rs (2020). First it was masks; now some refuse testing for SARS-CoV-2. JAMA324, 2015–2016. 10.1001/jama.2020.22003
33
Sah P. Fitzpatrick M. C. Zimmer C. F. Abdollahi E. Juden-Kelly L. Moghadas S. M. et al (2021). Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc. Natl. Acad. Sci. U. S. A.118, e2109229118. 10.1073/pnas.2109229118
34
Segen J. C. (1992). The dictionary of modern medicine. United States: CRC Press.
35
Tess B. H. Granato C. F. H. Alves M. Pintao M. C. T. Nunes M. C. Rizzatti E. G. et al (2021). Assessment of initial SARS-CoV-2 seroprevalence in the most affected districts in the municipality of Sao Paulo, Brazil. Braz. J. Infect. Dis.25, 101604. 10.1016/j.bjid.2021.101604
36
Thakur B. Dubey P. Benitez J. Torres J. P. Reddy S. Shokar N. et al (2021). A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci. Rep.11, 8562. 10.1038/s41598-021-88130-w
37
Vanshylla K. Di Cristanziano V. Kleipass F. Dewald F. Schommers P. Gieselmann L. et al (2021). Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe29, 917–929.e4. 10.1016/j.chom.2021.04.015
38
Varona J. F. Madurga R. Penalver F. Abarca E. Almirall C. Cruz M. et al (2021). Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int. J. Epidemiol.50, 400–409. 10.1093/ije/dyaa277
39
Wang Q. Ning J. Chen Y. Li B. Shi L. He T. et al (2022). The BBIBP-CorV inactivated COVID-19 vaccine induces robust and persistent humoral responses to SARS-CoV-2 nucleocapsid, besides spike protein in healthy adults. Front. Microbiol.13, 1008420. 10.3389/fmicb.2022.1008420
40
Wiens K. E. Mawien P. N. Rumunu J. Slater D. Jones F. K. Moheed S. et al (2021). Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Juba, South Sudan: a population-based study. medRxiv. Available from: https://www.medrxiv.org/content/10.1101/2021.03.08.21253009v1 (Accessed March 08, 2021).
41
World Health Organization (2022). Coronavirus disease (COVID-19). Available from: https://www.who.int (Accessed August 29, 2023).
42
Yang J. Zheng Y. Gou X. Pu K. Chen Z. Guo Q. et al (2020). Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis.94, 91–95. 10.1016/j.ijid.2020.03.017
Summary
Keywords
SARS-CoV-2, COVID-19, seroprevalence, anti-N IgG antibodies, silent infections
Citation
Cicaric N, Canovic V, Stojkovic M, Matic S, Stefanovic S, Popovic S, Todorovic D, Djordjevic N, Radenkovic B, Radenkovic M, Antic V and Baskic D (2023) Silent SARS-CoV-2 infection: seroprevalence study of SARS-CoV-2 anti- nucleocapsid IgG antibodies in Kragujevac, Serbia. Acta Virol. 67:11996. doi: 10.3389/av.2023.11996
Received
24 January 2023
Accepted
16 August 2023
Published
26 September 2023
Volume
67 - 2023
Edited by
Katarina Polcicova, Slovak Academy of Sciences, Slovakia
Updates
Copyright
© 2023 Cicaric, Canovic, Stojkovic, Matic, Stefanovic, Popovic, Todorovic, Djordjevic, Radenkovic, Radenkovic, Antic and Baskic.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanja Matic, sanjad.matic@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.