Abstract
Humankind has witnessed increased frequency of emerging and re-emerging viral diseases in the past few decades. The major categories of pathogenic emerging and re-emerging viral infections include respiratory, arthropod-borne and bat-borne zoonotic viruses. These viral infections are notorious for causing immune dysregulation and have the potential to mount excessive immune reaction, causing immunopathology that includes tissue injury, systemic inflammation, multi-organ failure and even death. A better understanding of the emerging or re-emerging viral-mediated immunomodulation is necessary for controlling the virus, while preventing severity of the disease associated with exaggerated immune response. In this article, we review the current understanding of emerging and re-emerging respiratory, arboviral and bat-borne zoonotic viruses; and consequent immune dysregulation or immunopathology associated with these viral infections.
Introduction
Emerging and re-emerging infectious diseases are a never-ending threat and it continues to wreak havoc on population health and the economy worldwide. Emerging infections have been defined as infections that have never been recognized previously and re-emerging infections are those that have come back in a different form or a location after previous decline in the incidence (Fauci, 2005). Several factors are involved in contributing to emergence of “new infection” or re-emergence of “past infection,” including ecological destruction, increasing population, increased regional and global connectivity, social and behavior changes. As per the WHO list of top emerging and re-emerging diseases having potential of causing major epidemics include different viral diseases, such as severe acute respiratory syndrome corona virus-2 (SARS-CoV-2), Middle East respiratory syndrome coronavirus (MERS-CoV), Nipah, Ebola, etc., (Mukherjee, 2017).
The clinical features of patients infected with emerging or re-emerging viruses ranges from asymptomatic infection to severe symptoms and even death. The host immune response against the virus should be appropriate to control the disease without causing self-injury (Gogoi et al., 2021). However, the emerging or re-emerging viral infections are mostly associated with an exaggerated immune response, resulting in immunopathology in the infected individuals (Yang and Yang, 2021). In this review, we discuss about our current understanding of immune dysregulation and immunopathology associated with emerging and re-emerging viral infections of the twenty-first century with a focus on respiratory virus, arbovirus and bat-borne zoonotic viral infections.
Emerging and re-emerging respiratory virus mediated immune dysregulation
One of the most fatal emerging viral disease discovered in the early twentieth century was the “Spanish flu.” In the last decades, there has been increased incidence of the emergence of several new respiratory viral infections (Jefferson et al., 2023). Currently, the novel SARS-CoV-2, a positive-sense RNA virus from the Coronaviridae family, is responsible for considerable morbidity and mortality throughout the World. Corona virus disease-2019 (COVID-19) is the disease caused by the SARS-CoV-2 virus. Most of the deaths in critically ill COVID-19 positive patients have been reported to be the outcome of Acute Respiratory Disease Syndrome (ARDS) (Yang et al., 2020). Initial reports in symptomatic severe COVID-19 patients showed increased concentrations of TNF-α, GCSF, IP10, MCP1, and MIP1A, suggesting association of hyperinflammation with the disease severity (Huang et al., 2020). With respect to status of T cells, SARS-CoV-2 infection leads to a significantly decrease in the counts of circulating CD4+ and CD8+ T cells, but they were highly activated (HLADR positive) along with increased proportion of proinflammatory Th17-enriched CCR6+CD4+ T cells and increased levels of cytotoxic granules (perforin and granulysin) in CD8+ T cells (Xu et al., 2020). Over a decade ago (in the year 2009), pandemic H1N1 influenza A virus (pH1N1), a negative-sense RNA virus belonging to the family Ortomyxoviridae, emerged that had spread rapidly, contributing to momentous morbidity and between 105700–395600 deaths during the first 1 year of the viral circulation (Dawood et al., 2012). Early secretion of copious amount of Th17 and Th1 cytokines were observed in pH1N1 infected patients with severe symptoms as well as in the experimental mouse model, indicating hyperimmune response mediated pathogenicity of the disease (Bermejo-Martin et al., 2009; Liu et al., 2019). The proinflammatory cytokine-IL-1 signaling against pH1N1 has been shown to induce lung immunopathology by mediating the recruitment of neutrophils to the infected lung (Guo et al., 2017). Another notable negative-sense RNA virus of the family Orthomyxoviridae is avian H5N1 virus, commonly known as “bird flu” and since 1997, this virus has caused several outbreaks in poultry and have occasionally transmitted to humans, leading to case fatality rate of more than 50 percent (Neumann et al., 2010). Infection with H5N1 has been shown to cause a significant depletion of peripheral blood CD4+ and CD8+ T cells and apoptosis of dendritic cells in the lungs and draining lymphnodes (Baskin et al., 2009). In addition, high concentrations of cytokines-IL-6, IFN-γ, IL-10, and chemokines-interferon induced protein-10 (IP-10) along with monokine induced by interferon-γ (MIG) have been detected in the serum of H5N1 positive patients, and an increased expression of TNF-α was observed in the lung tissue of H5N1 autopsy (Peiris et al., 2004; de Jong et al., 2006). The most cause of death due to H5N1 infection is cytokine-induced inflammation and subsequent lung pathology (ARDS) (Peiris et al., 2009). Although avian to human or human to human transmission efficiency of H5N1 is low, but it is extremely pathogenic due to its ability to induce disproportionate cytokines and chemokines production that results in pulmonary tissue damage. One more important virus of the 21st century that was first reported in 2012 is MERS-CoV, a positive-sense RNA virus belonging to the family Coronaviridae that infected huge numbers of dromedary camels and occasional transmission from the infected camels to exposed humans and rarely from the infected human to human transmission occurred (Mackay and Arden, 2015). In the infected human, MERS-CoV cause severe respiratory disease and accounts for mortality rate of more than 35 percent (Chafekar and Fielding, 2018). This virus has been reported to efficiently infect and kill T cells by inducing both intrinsic and extrinsic apoptotic pathways (Chu et al., 2016). Dysregulated serum levels of cytokines such as TGF-β, EGF, IL-6, IL-7, IFN-γ, IL-15, and IL-10 and chemokines such as CXCL8, CCL3 and CCL5 have been observed in severe and fatal cases of MERS-CoV, indicating the potential involvement of impaired cytokine levels as well as recruitment of leukocytes to the site of infection and consequently in immune mediated pathogenicity (Min et al., 2016; Alhetheel et al., 2023).
Overall, the severity of the disease associated with these emerging and re-emerging respiratory viral infections is directly linked to hypercytokinemia and inefficient cell mediated immune response (Table 1). The initial recognition of respiratory virus in the lungs by innate immune cells through pathogen associated molecular patterns (PAMPs) initiate secretion of myriad of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α which are required for setting up the stage for immune response, but excessive production of these cytokines can cause acute lung injury (Goodman et al., 2003). The injured tissue of the lungs in turn releases damage associated molecular patterns (DAMPs) such as mitochondrial DNA, formyl peptides and cardiolipin that impairs lung structure and functions (Zhang et al., 2010b; Ray et al., 2010). Additionally, chemokine such as IL-8 secreted by the innate immune cells, stimulated lung fibroblasts and epithelial cells recruit neutrophils and macrophages to the lung and becomes activated by direct viral recognition though PAMPs and also by mitochondrial DNA produced by the cellular injury leading to release of reactive oxygen species, thereby contributing to acute lung injury (Kunkel et al., 1991; Zhang et al., 2010a; Foo et al., 2023). This sequence of events which lead to cytokine storm and consequent immune dysregulation in pathogenic respiratory viral infections play a crucial role in the development of ARDS or other pathological conditions such as sepsis or multiple organ failure and even death (Figure 1A).
TABLE 1
| Virus group | Pathologic cytokine mediator | Reference |
| Respiratory | ||
| SARS-CoV-2 | TNF-α, GCSF, IP10, MCP1, MIP1A, IL17 | Huang et al. (2020), Xu et al. (2020) |
| H1N1 | IL-12, IFN-γ, IL-6, TNF-α, IL17, IL1β | Bermejo-Martin et al. (2009), Liu et al. (2019) |
| H5N1 | IL-6, IFN-γ, IL-10, IP10, TNF-α | Peiris et al. (2004), de Jong et al. (2006) |
| MERS-CoV | TGF-β, EGF, IL-6, IL-7, IFN-γ, IL-15 and IL-10 | Min et al. (2016), Alhetheel et al. (2023) |
| Arbovirus | ||
| DENV | MIF, TNF-α, IFN-γ | Duangchinda et al. (2010), Chen et al. (2016) |
| JEV | TNF-α, IL-6, IFN-α, IL-8 | Burke and Morill (1987), Winter et al. (2004), Kalita et al. (2010) |
| CHIKV | IL-1β, IL-6, TNF-α | Suhrbier and Mahalingam (2009) |
| Bat-borne virus | ||
| NiV | IL-6, CXCL-10 | Lo et al. (2010), Prescott et al. (2012) |
| Ebola | TNF-α, IFN-γ, IFN-α | Villinger et al. (1999) |
Cytokines involved in immunopathogenesis during emerging and re-emerging viral infections.
FIGURE 1
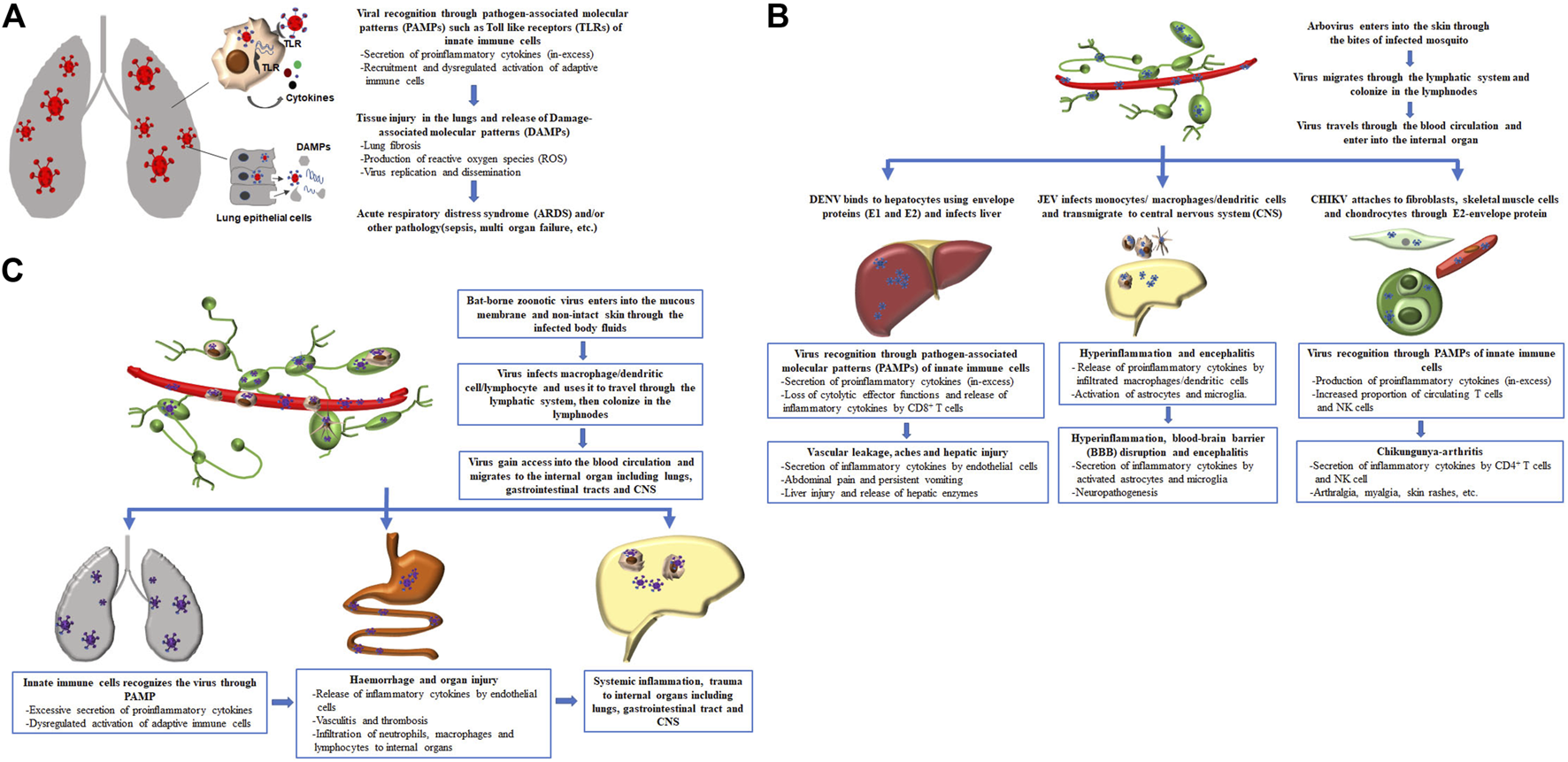
Sequence of events which lead to immune dysregulation and consequent disease severity in pathogenic emerging and re-emerging viral infections. (A) Cascade of events associated with pathogenic respiratory viral infection, (B) entry of arbovirus into the body and subsequent pathological events associated with different types of arbovirus, and (C) pathological events associated with invasion of bat-borne zoonotic virus and its clinical manifestation.
Emerging and re-emerging arbovirus mediated immune dysregulation
The emergence and re-emergence of arthropod-borne viruses (arboviruses) such as dengue virus (DENV), Japanese encephalitis virus (JEV) and Chikungunya virus (CHIKV) are on the rise, particularly in Southeast Asia region. These three diseases occur through the bite of infected mosquito and usually take place during warm and wet season, when the arthropods (mosquitoes) are active. DENV is an RNA virus (positive-sense) belonging to the Flaviviridae family that can be transmitted to humans through the bites of infected Aedes aegypti mosquito. Infection with DENV causes release of macrophage migratory inhibitory factor (MIF) from the endothelial cells and secretion of TNF-α by macrophages, resulting in vascular leakage and haemorrhage (Wu-Hsieh et al., 2009; Chen et al., 2016). The sign of liver damage with increased plasma levels of liver enzymes have been detected in the symptomatic serious patients, and it was experimentally shown in mice model that DENV infection causes activation and recruitment of NK cells to the liver resulting in hepatic injury (Kalayanarooj et al., 1997; Sung et al., 2012). In a recent study, higher level of proinflammatory NK cells and an increased expression of Fc receptor-CD64 on myeloid cells have been shown to be responsible for the pathogenesis of severe dengue in human (Robinson et al., 2023). Although CD8+ T cells play a protective role against DENV but during severe dengue infection, CD8+ T cells do not undergo degranulation while secrete higher level of TNF-α and IFN-γ cytokines that further enhances immunopathology in the host (Duangchinda et al., 2010). The next important positive-sense RNA virus in the family Flaviviridae is JEV. The reservoir hosts of this virus are pigs and water birds, and an infected mosquito, primarily Culex species (Culex tritaeniorhyncus and Culex vishnui), can transmit the virus to human (mostly children), who are incidental or dead-end hosts; often leaving survivors with permanent neuropsychiatric sequalae (Solomon et al., 1998). The virus after getting entry into the human through the bite of an infected mosquito replicates primarily in the macrophages and dendritic cells leading to a transient low viremia (Aleyas et al., 2009). Following evasion of the immune surveillance in the periphery, JEV-infected cells transmigrate to the central nervous system (CNS) resulting in inflammatory demyelination and encephalitis (Wang et al., 2022). One of the earlier studies had shown that TNF-α is locally produced in the CNS contributing to the severity of symptoms in Japanese encephalitis (JE) patients (Burke and Morill, 1987). Subsequent studies have reported an association of higher levels of proinflammatory cytokines-IL-6, IFN-α and chemokine-IL-8 in CNS with poor outcome in patients with JE (Kalita et al., 2010; Winter et al., 2004). The amount of chemokine-CCL5 (RANTES) which is required for migration of activated T cells was found to be elevated in both plasma and CNS of JE patients (Winter et al., 2004). CHIKV is another pathogenic arbovirus with a positive sense RNA genome, belonging to the Togaviridae family (alphavirus), that has re-emerged to cause a major epidemic outbreak in Asia, Africa and United States, affecting millions of people globally with only 5%–25% reported asymptomatic cases of CHIKV (Morrison, 2014). Although, the natural host of CHIKV is wild primates, but it is majorly transmitted from human to human by the bites of infected Aedes species mosquitoes (Aedes aegypti or Aedes albopictus) and perinatal mother-to-child transmission can also occur (Runowska et al., 2018). The most common symptom of CHIKV-infected patients is arthralgia, primarily affecting peripheral joints, including knees, ankles, wrists and the small joints of the hand; this symptom can persist in the patients for months or years (Suhrbier et al., 2012). Other clinical manifestations of acute CHIKV disease includes headache, back pain, generalised myalgia, fatigue and skin rashes; the severe course of disease in adults and neonates has been associated with neurological, cardiovascular and respiratory complications that can be fatal (Runowska et al., 2018). CHIKV after entering into the human host infects macrophage leading to the production of copious amount of proinflammatory cytokines like IL-1β, IL-6, and TNF-α, and also activation of platelets that releases inflammatory factors such as cleaved IL-1β, NLR-3, and caspase-4 which play a key role in inflammation and arthritis (Suhrbier and Mahalingam, 2009; Gomes de Azevedo-Quintanilha et al., 2022). Infection with CHIKV also causes increased in the frequency of circulating T cells and NK cells in symptomatic patients that have been suggested to play a detrimental role in the host (Petitdemange et al., 2011; Miner et al., 2015). Indeed, depletion of CD4+ T cells or NK cells has been shown to diminish joint pathology in a mouse model, indicating their pathogenic role during CHIKV infection (Teo et al., 2013; Teo et al., 2015). Thus, disease manifestation of these three arboviruses (DENV, JEV and CHIKV) ranges from no symptoms or a mild fever, headache, joint or muscle ache and or a skin rash to severe febrile disease, liver injury, neurological or cardiac complications, coma and even death.
Initially, the infected arthropod in the process of blood feeding, inject saliva that carries the virus into the skin of human. Immunomodulatory factors present in the saliva of arthropods prevent localized inflammation, and it also polarize the host immune response towards a Th2 response characterized by heightened secretion of cytokines such as IL-4 and IL-10 that protects the virus (Titus et al., 2006). The arbovirus then colonizes the lymphnodes and after a brief incubation period, the virus travels in the bloodstream to internal organs, including the liver, CNS and peripheral joints where it initiates immunopathology by inducing abberent immune responses and cytokine production resulting in host tissue damage (Table 1). The ideal site of infection or replication for different arbovirus varies due to their dependence on cell surface specific receptors to enter into the organ. For instance, the envelope protein of DENV is required for binding to the hepatocytes and heparin sulphate present on the cell membrane facilitates its entry into the liver; JEV takes the help of peripheral macrophage to induce inflammation that disrupt the blood-brain-barrier and then the virus cross into CNS; and binding between CHIKV-E2 protein and cell surface-Mxra8 receptor expressed on fibroblasts, skeletal muscle cells, and chondrocytes is essential for CHIKV infection (Chen et al., 1996; Thepparit and Smith, 2004; Lannes et al., 2017; Zhang et al., 2018). The immunopathological consequence of arboviral infections at their respective anatomic sites of infection with or without systemic inflammation causes significant morbidity and mortality (Figure 1B).
Emerging and re-emerging bat-borne virus mediated immune dysregulation
The best exemplified bat-borne viruses that have been found to infect humans and have come into prominent notice in the recent past are Nipah (NiV) and Ebola viruses. Both NiV and Ebola viruses enter into mucous membrane or non-intact skin and uses macrophage, dendritic cells or lymphocytes as a vehicle to travel to the lymphnodes and nodal chains, where viral replication occurs (Mathieu et al., 2011; Baseler et al., 2017). After a few days of inoculation in lymph glands, the virus enters the bloodstream and reaches internal organ such as CNS and lungs inducing excessive immune response leading to immunopatholgy and extensive tissue damage (Figure 1C). NiV is an a negative-sense, single-stranded RNA virus, belonging to Paramyxoviridae family, was first detected during an outbreak of unknown respiratory and neurological disease in swine (intermediate host) and humans in West Malaysia in 1998 and had re-emerged in Kerela, India in 2018 and on September 2023, a fresh Nipah outbreak has taken place in the same State of India (Thiagarajan, 2023). Transmission of NiV to humans can occur through the consumption of fruit that is contaminated by saliva or urine of bats, or through contact with contaminated body fluids of infected pigs or humans. The clinical manifestation of NiV-positive patients ranges from asymptomatic infection to severe respiratory illness, meningitis and encephalitis with overall case fatality rate of 61% (Kenmoe et al., 2019). NiV infection has been shown to trigger the endothelial cells to produce IL-6 and chemoattractant-CXCL-10 that have been described in contributing to the widespread vasculitis and thrombosis (Table 1) (Lo et al., 2010; Prescott et al., 2012). Indeed, the main clinical and autopsy findings from NiV-infected humans revealed a vasculitis in the CNS and lungs, characterized by inflammation and infiltration of neutrophils, macrophages and lymphocytes (Wong et al., 2002). Another deadliest bat-transmitted negative-sense single-stranded RNA virus is Ebola virus, belonging to Filoviridae family, was first reported in remote village in Democratic Republic of Congo (formerly, Zaire) in 1976 and re-emergence of several Ebola virus outbreaks have been observed in rural regions of West and Central Africa during the past few years. Humans are initially infected with Ebola virus through direct contact with an infected bat and then the virus spread in the community through the infected body fluids or contaminated objects used by a person who is sick or has died from the Ebola virus. An estimated case fatality rate of 65.4% was observed and varied significantly among the Ebola outbreaks (Lefebvre et al., 2014). The major symptoms of Ebola disease include fever, aches, gastrointestinal symptoms, weakness, hemorrhage and bleeding. Early studies of the Ebola virus disease (EVD) showed significant increased levels of TNF-α, IFN-γ, and IFN-α in the serum samples of fatal EVD cases, suggesting a contribution of soaring immune activation in lethal outcome of Ebola disease (Table 1) (Villinger et al., 1999). Ebola could potentially target mononuclear phagocytes as the primary cells affected by the virus. This targeting could lead to the release of various substances, such as cytokines (e.g., TNF-α, IL-1β), chemotactic chemokines (e.g., MIP-1), reactive oxygen, and nitrogen species (Feldmann et al., 1996; Baseler et al., 2017). This response to the infection might result in the breakdown of endothelial barriers, causing symptoms like edema and hypovolemic shock. Moreover, the compromised adaptive immunity, characterized by the lack of functional DCs, loss of CD4 and CD8 T cells likely due to a deregulated DC/T synapse, an increase in the expression level of cell death receptors (e.g., CD95), and the presence of T Cell exhaustion markers [e.g., programmed death protein 1 (PD1) and cytotoxic T lymphocyte antigen-4 (CTLA-4)] on lymphocytes, could be associated with fatal outcomes (Ruibal et al., 2016). While both CD4 T cells and CD8 T cells are affected by the process of cell death through various pathways, resulting in the loss of T cell-derived cytokines, there is no consensus regarding the impact of EBOV infection on B cells in studied models. Thus, hyperinflammatory response together with the abnormal cellular immune response contributes to the severity of EVD. In general, a properly regulated immune response is desirable for efficiently combating highly infectious bat-borne zoonotic viral disease (NiV or Ebola) with minimal or no collateral tissue damage.
Discussion
Emerging and re-emerging viral diseases remain as the major cause of morbidity and mortality. Immunopathological changes brought about by these viruses are sometimes so intense that the patient experience severe complications that can be fatal. The most important part of an initial immune response is inflammation, which is required to directly act against the pathogen as well as in mediating the adaptive immune cell recruitment and activation. However, unfamiliar pathogenic threats such as emerging or re-emerging virus causes excessive immune response leading to immunopathology, including tissue injury, systemic inflammation, multi-organ damage and even death. In this review, we have provided evidence that exaggerated inflammatory response and dysregulated T cell effector functions occurring during pathogenic emerging or re-emerging respiratory, arbovirus or bat-borne viral diseases cause severe complications and mortality in the patients. Currently available therapeutic interventions in dampening the inflammatory response come with undesired side-effects and hampers in reducing the viral load. Future studies addressing the extent and details of the mechanisms involved in emerging or re-emerging virus-induced excessive inflammatory response would support in designing therapeutic strategies to fine tune the immune response in efficiently combating the virus without aggravating immunopathology in the host.
Statements
Author contributions
DG analyzed concerned literature and wrote the manuscript. PB and KN revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This paper was supported by PM-ABHIM program funded by Ministry of Health and Family Welfare, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aleyas A. G. George J. A. Han Y. W. Rahman M. M. Kim S. J. Han S. B. et al (2009). Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J. Immunol.183, 2462–2474. 10.4049/jimmunol.0801952
2
Alhetheel A. F. Albarrag A. M. Shakoor Z. A. Somily A. M. Barry M. A. Altalhi H. et al (2023). Assessment of proinflammatory cytokines among patients with Middle East respiratory syndrome coronavirus infection. Viral Immunol.36, 282–289. 10.1089/vim.2022.0154
3
Baseler L. Chertow D. S. Johnson K. M. Feldmann H. Morens D. M. (2017). The pathogenesis of Ebola virus disease. Annu. Rev. Pathol.12, 387–418. 10.1146/annurev-pathol-052016-100506
4
Baskin C. R. Bielefeldt-Ohmann H. Tumpey T. M. Sabourin P. J. Long J. P. Garcia-Sastre A. et al (2009). Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. U. S. A.106, 3455–3460. 10.1073/pnas.0813234106
5
Bermejo-Martin J. F. Ortiz de Lejarazu R. Pumarola T. Rello J. Almansa R. Ramirez P. et al (2009). Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care13, R201. 10.1186/cc8208
6
Burke D. S. Morill J. C. (1987). Levels of interferon in the plasma and cerebrospinal fluid of patients with acute Japanese encephalitis. J. Infect. Dis.155, 797–799. 10.1093/infdis/155.4.797
7
Chafekar A. Fielding B. C. (2018). MERS-CoV: understanding the latest human coronavirus threat. Viruses10, 93. 10.3390/v10020093
8
Chen H. R. Chuang Y. C. Lin Y. S. Liu H. S. Liu C. C. Perng G. C. et al (2016). Dengue virus nonstructural protein 1 induces vascular leakage through macrophage migration inhibitory factor and autophagy. PLoS Negl. Trop. Dis.10, e0004828. 10.1371/journal.pntd.0004828
9
Chen Y. Maguire T. Marks R. M. (1996). Demonstration of binding of dengue virus envelope protein to target cells. J. Virol.70, 8765–8772. 10.1128/jvi.70.12.8765-8772.1996
10
Chu H. Zhou J. Wong B. H. Li C. Chan J. F. Cheng Z. S. et al (2016). Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis.213, 904–914. 10.1093/infdis/jiv380
11
Dawood F. S. Iuliano A. D. Reed C. Meltzer M. I. Shay D. K. Cheng P. Y. et al (2012). Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis.12, 687–695. 10.1016/s1473-3099(12)70121-4
12
de Jong M. D. Simmons C. P. Thanh T. T. Hien V. M. Smith G. J. Chau T. N. et al (2006). Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med.12, 1203–1207. 10.1038/nm1477
13
Duangchinda T. Dejnirattisai W. Vasanawathana S. Limpitikul W. Tangthawornchaikul N. Malasit P. et al (2010). Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. U. S. A.107, 16922–16927. 10.1073/pnas.1010867107
14
Fauci A. S. (2005). Emerging and reemerging infectious diseases: The perpetual challenge. Acad. Med.80, 1079–1085. 10.1097/00001888-200512000-00002
15
Feldmann H. Bugany H. Mahner F. Klenk H. D. Drenckhahn D. Schnittler H. J. (1996). Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol.70, 2208–2214. 10.1128/jvi.70.4.2208-2214.1996
16
Foo C. X. Bartlett S. Chew K. Y. Ngo M. D. Bielefeldt-Ohmann H. Arachchige B. J. et al (2023). GPR183 antagonism reduces macrophage infiltration in influenza and SARS-CoV-2 infection. Eur. Respir. J.61, 2201306. 10.1183/13993003.01306-2022
17
Gogoi D. Biswas D. Borkakoty B. Dutta M. (2021). T cell responses in symptomatic moderate patients with pandemic 2009 H1N1 influenza A virus infection. Acta Virol.65, 245–253. 10.4149/av_2021_301
18
Gomes de Azevedo-Quintanilha I. Campos M. M. Teixeira Monteiro A. P. Dantas do Nascimento A. Calheiros A. S. Oliveira D. M. et al (2022). Increased platelet activation and platelet-inflammasome engagement during chikungunya infection. Front. Immunol.13, 958820. 10.3389/fimmu.2022.958820
19
Goodman R. B. Pugin J. Lee J. S. Matthay M. A. (2003). Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev.14, 523–535. 10.1016/s1359-6101(03)00059-5
20
Guo L. Wang Y. C. Mei J. J. Ning R. T. Wang J. J. Li J. Q. et al (2017). Pulmonary immune cells and inflammatory cytokine dysregulation are associated with mortality of IL-1R1-/-mice infected with influenza virus (H1N1). Zool. Res.38, 146–154. 10.24272/j.issn.2095-8137.2017.035
21
Huang C. Wang Y. Li X. Ren L. Zhao J. Hu Y. et al (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet395, 497–506. 10.1016/s0140-6736(20)30183-5
22
Jefferson T. Dooley L. Ferroni E. Al-Ansary L. A. van Driel M. L. Bawazeer G. A. et al (2023). Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev.1, CD006207. 10.1002/14651858.CD006207.pub5
23
Kalayanarooj S. Vaughn D. W. Nimmannitya S. Green S. Suntayakorn S. Kunentrasai N. et al (1997). Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis.176, 313–321. 10.1086/514047
24
Kalita J. Srivastava R. Mishra M. K. Basu A. Misra U. K. (2010). Cytokines and chemokines in viral encephalitis: A clinicoradiological correlation. Neurosci. Lett.473, 48–51. 10.1016/j.neulet.2010.02.017
25
Kenmoe S. Demanou M. Bigna J. J. Nde Kengne C. Fatawou Modiyinji A. Simo F. B. N. et al (2019). Case fatality rate and risk factors for Nipah virus encephalitis: a systematic review and meta-analysis. J. Clin. Virol.117, 19–26. 10.1016/j.jcv.2019.05.009
26
Kunkel S. L. Standiford T. Kasahara K. Strieter R. M. (1991). Interleukin-8 (IL-8): The major neutrophil chemotactic factor in the lung. Exp. Lung Res.17, 17–23. 10.3109/01902149109063278
27
Lannes N. Summerfield A. Filgueira L. (2017). Regulation of inflammation in Japanese encephalitis. J. Neuroinflammation14, 158. 10.1186/s12974-017-0931-5
28
Lefebvre A. Fiet C. Belpois-Duchamp C. Tiv M. Astruc K. Aho Glele L. S. (2014). Case fatality rates of Ebola virus diseases: A meta-analysis of world health organization data. Med. Mal. Infect.44, 412–416. 10.1016/j.medmal.2014.08.005
29
Liu J. X. Zhang Y. An M. Wu Q. G. Zhao Y. Li X. et al (2019). Diversity of Th1/Th2 immunity in mice with acute lung injury induced by the H1N1 influenza virus and lipopolysaccharides. J. Infect. Dev. Ctries.13, 536–544. 10.3855/jidc.10338
30
Lo M. K. Miller D. Aljofan M. Mungall B. A. Rollin P. E. Bellini W. J. et al (2010). Characterization of the antiviral and inflammatory responses against Nipah virus in endothelial cells and neurons. Virology404, 78–88. 10.1016/j.virol.2010.05.005
31
Mackay I. M. Arden K. E. (2015). MERS coronavirus: Diagnostics, epidemiology and transmission. Virol. J.12, 222. 10.1186/s12985-015-0439-5
32
Mathieu C. Pohl C. Szecsi J. Trajkovic-Bodennec S. Devergnas S. Raoul H. et al (2011). Nipah virus uses leukocytes for efficient dissemination within a host. J. Virol.85, 7863–7871. 10.1128/jvi.00549-11
33
Min C. K. Cheon S. Ha N. Y. Sohn K. M. Kim Y. Aigerim A. et al (2016). Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep.6, 25359. 10.1038/srep25359
34
Miner J. J. Aw-Yeang H. X. Fox J. M. Taffner S. Malkova O. N. Oh S. T. et al (2015). Brief report: chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol.67, 1214–1220. 10.1002/art.39027
35
Morrison T. E. (2014). Reemergence of chikungunya virus. J. Virol.88, 11644–11647. 10.1128/jvi.01432-14
36
Mukherjee S. (2017). Emerging infectious diseases: epidemiological perspective. Indian J. Dermatol62, 459–467. 10.4103/ijd.IJD_379_17
37
Neumann G. Chen H. Gao G. F. Shu Y. Kawaoka Y. (2010). H5N1 influenza viruses: Outbreaks and biological properties. Cell Res.20, 51–61. 10.1038/cr.2009.124
38
Peiris J. S. Cheung C. Y. Leung C. Y. Nicholls J. M. (2009). Innate immune responses to influenza A H5N1: Friend or foe?Trends Immunol.30, 574–584. 10.1016/j.it.2009.09.004
39
Peiris J. S. Yu W. C. Leung C. W. Cheung C. Y. Ng W. F. Nicholls J. M. et al (2004). Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet363, 617–619. 10.1016/s0140-6736(04)15595-5
40
Petitdemange C. Becquart P. Wauquier N. Beziat V. Debre P. Leroy E. M. et al (2011). Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog.7, e1002268. 10.1371/journal.ppat.1002268
41
Prescott J. de Wit E. Feldmann H. Munster V. J. (2012). The immune response to Nipah virus infection. Arch. Virol.157, 1635–1641. 10.1007/s00705-012-1352-5
42
Ray N. B. Durairaj L. Chen B. B. McVerry B. J. Ryan A. J. Donahoe M. et al (2010). Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat. Med.16, 1120–1127. 10.1038/nm.2213
43
Robinson M. L. Glass D. R. Duran V. Agudelo Rojas O. L. Sanz A. M. Consuegra M. et al (2023). Magnitude and kinetics of the human immune cell response associated with severe dengue progression by single-cell proteomics. Sci. Adv.9, eade7702. 10.1126/sciadv.ade7702
44
Ruibal P. Oestereich L. Ludtke A. Becker-Ziaja B. Wozniak D. M. Kerber R. et al (2016). Unique human immune signature of Ebola virus disease in Guinea. Nature533, 100–104. 10.1038/nature17949
45
Runowska M. Majewski D. Niklas K. Puszczewicz M. (2018). Chikungunya virus: A rheumatologist's perspective. Clin. Exp. Rheumatol.36, 494–501.
46
Solomon T. Kneen R. Dung N. M. Khanh V. C. Thuy T. T. Ha D. Q. et al (1998). Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet351, 1094–1097. 10.1016/s0140-6736(97)07509-0
47
Suhrbier A. Jaffar-Bandjee M. C. Gasque P. (2012). Arthritogenic alphaviruses--an overview. Nat. Rev. Rheumatol.8, 420–429. 10.1038/nrrheum.2012.64
48
Suhrbier A. Mahalingam S. (2009). The immunobiology of viral arthritides. Pharmacol. Ther.124, 301–308. 10.1016/j.pharmthera.2009.09.005
49
Sung J. M. Lee C. K. Wu-Hsieh B. A. (2012). Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS One7, e46292. 10.1371/journal.pone.0046292
50
Teo T. H. Her Z. Tan J. J. Lum F. M. Lee W. W. Chan Y. H. et al (2015). Caribbean and La reunion chikungunya virus isolates differ in their capacity to induce proinflammatory Th1 and NK cell responses and acute joint pathology. J. Virol.89, 7955–7969. 10.1128/jvi.00909-15
51
Teo T. H. Lum F. M. Claser C. Lulla V. Lulla A. Merits A. et al (2013). A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J. Immunol.190, 259–269. 10.4049/jimmunol.1202177
52
Thepparit C. Smith D. R. (2004). Serotype-specific entry of dengue virus into liver cells: Identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol.78, 12647–12656. 10.1128/jvi.78.22.12647-12656.2004
53
Thiagarajan K. (2023). Nipah virus: India's Kerala state moves quickly to control fresh outbreak. BMJ382, 2117. 10.1136/bmj.p2117
54
Titus R. G. Bishop J. V. Mejia J. S. (2006). The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol.28, 131–141. 10.1111/j.1365-3024.2006.00807.x
55
Villinger F. Rollin P. E. Brar S. S. Chikkala N. F. Winter J. Sundstrom J. B. et al (1999). Markedly elevated levels of interferon (IFN)-σ, IFN-α, interleukin (IL)-2, IL-10, and tumor necrosis factor‐α associated with fatal Ebola virus infection. J. Infect. Dis.179 (Suppl. 1), S188–S191. 10.1086/514283
56
Wang X. Wang G. Yang H. Fu S. He Y. Li F. et al (2022). A mouse model of peripheral nerve injury induced by Japanese encephalitis virus. PLoS Negl. Trop. Dis.16, e0010961. 10.1371/journal.pntd.0010961
57
Winter P. M. Dung N. M. Loan H. T. Kneen R. Wills B. Thu le T. et al (2004). Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J. Infect. Dis.190, 1618–1626. 10.1086/423328
58
Wong K. T. Shieh W. J. Kumar S. Norain K. Abdullah W. Guarner J. et al (2002). Nipah virus infection: Pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am. J. Pathol.161, 2153–2167. 10.1016/s0002-9440(10)64493-8
59
Wu-Hsieh B. A. Yen Y. T. Chen H. C. (2009). Dengue hemorrhage in a mouse model. Ann. N. Y. Acad. Sci.1171 (Suppl. 1), E42–E47. 10.1111/j.1749-6632.2009.05053.x
60
Xu Z. Shi L. Wang Y. Zhang J. Huang L. Zhang C. et al (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med.8, 420–422. 10.1016/s2213-2600(20)30076-x
61
Yang B. Yang K. D. (2021). Immunopathogenesis of different emerging viral infections: evasion, fatal mechanism, and prevention. Front. Immunol.12, 690976. 10.3389/fimmu.2021.690976
62
Yang X. Yu Y. Xu J. Shu H. Xia J. Liu H. et al (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med.8, 475–481. 10.1016/s2213-2600(20)30079-5
63
Zhang Q. Itagaki K. Hauser C. J. (2010a). Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock34, 55–59. 10.1097/shk.0b013e3181cd8c08
64
Zhang Q. Raoof M. Chen Y. Sumi Y. Sursal T. Junger W. et al (2010b). Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature464, 104–107. 10.1038/nature08780
65
Zhang R. Kim A. S. Fox J. M. Nair S. Basore K. Klimstra W. B. et al (2018). Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature557, 570–574. 10.1038/s41586-018-0121-3
Summary
Keywords
immunopathology, respiratory viruses, arboviruses, bat-borne viruses, emerging
Citation
Gogoi D, Baruah PJ and Narain K (2024) Immunopathology of emerging and re-emerging viral infections: an updated overview. Acta Virol. 68:12108. doi: 10.3389/av.2024.12108
Received
24 September 2023
Accepted
27 March 2024
Published
08 April 2024
Volume
68 - 2024
Edited by
Natasa Knap, University of Ljubljana, Slovenia
Reviewed by
Katarina Resman Rus, University of Ljubljana, Slovenia
Arash Letafati, Tehran University of Medical Sciences, Iran
Updates
Copyright
© 2024 Gogoi, Baruah and Narain.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimpu Gogoi, dimpu.gogoi@icmr.gov.in
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.