Introduction—Drug abuse and health risk
Drug abuse is associated with significant health risk (1, 2). Studies are also showing that there is an important connection between circadian rhythms, metabolism and addiction (3–5). Study analyzing the liver metabolome of mice deficient in the expression of the dopamine D2 receptor (D2R) in striatal medium spiny neurons, found profound changes in the liver circadian metabolome compared to control mice (3). Further drugs that increase dopamine levels like cocaine disrupt circadian metabolic profiles in the liver. It is becoming evident that a strict communication exists between the CNS, and metabolism and this equilibrium can be altered by drug abuse (Figure 1). This loss of equilibrium in drug abusers may increase risk of metabolic dysfunctions which may result in worsening addiction and possible disease states such as those manifested as the metabolic syndrome.
FIGURE 1
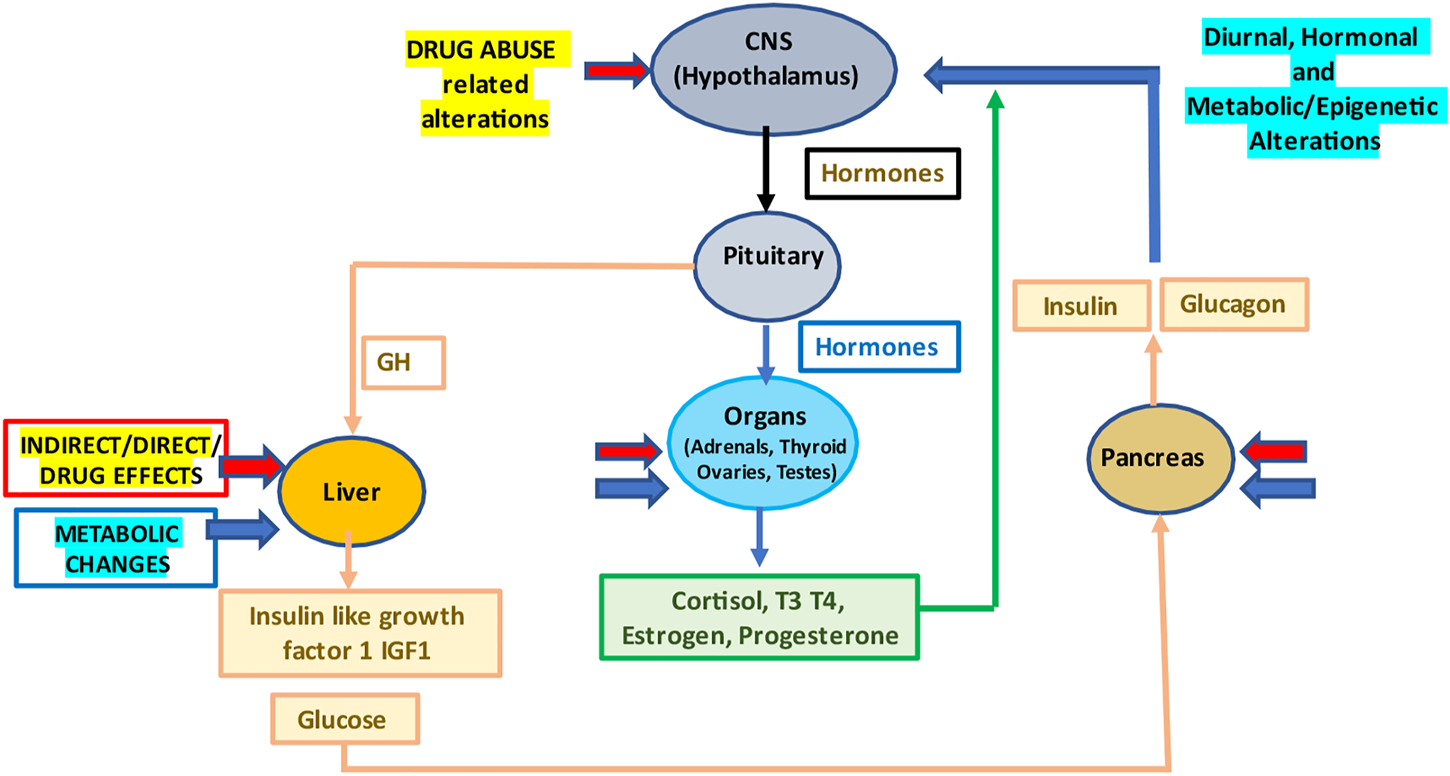
Drug abuse and the resulting diurnal, hormonal and metabolic/epigenetic changes in the body. Abbreviations: CNS, central nervous system; GH, growth hormone; T3, triiodothyronine; and T4, thyroxine.
Drug abuse effects on metabolism and epigenetics
The metabolic syndrome is a collection of metabolic abnormalities, including hyperinsulinemia, hypertension, dyslipidaemia, and abdominal obesity, and may be triggered by initial discrepancies at the cellular level in critical metabolic pathways. These primary, small metabolic pathway disparities probably cascade with time leading in time to significant health problems. Some indications that drug abuse may increase the risk of the metabolic syndrome include the observation that drug-abusing patients have higher rates of diabetes complications. How drug abuse increases the risk to disease is being shown to be linked to changes in metabolism and epigenetics that are associated with the increased disease susceptibility. To counter these drug abuse related deleterious effects various studies, suggest that certain metabolic and antioxidant compounds like L-carnitine, thiamine (B1), co-enzyme Q10 etc., may be useful (6).
Drugs of abuse can be roughly categorised in different groups depending in part on their CNS activities, such as stimulants, amphetamines, hallucinogens, narcotics, inhalants, anaesthetics, anabolic steroids, and antipsychotics/antidepressants etc., (
6). Each category and indeed each type of substance of abuse will affect metabolism differently depending on its unique chemical structure as well as the levels absorbed, processed and metabolised within the body. Furthermore, the effects on the metabolism within the body are manifested at various levels.
• Cell functions such as neurotransmission, release of hormones and inflammatory factors (cytokines, extracellular vesicles).
• Intra-cellular functions such as mitochondrial and endoplasmic reticulum (ER) activity.
• Other body functions such as temperature, intestinal absorption of nutrients, intestinal biota, function of vascular system.
Therefore, each drug of abuse affects overall metabolism in a complex way and the metabolic changes can eventually contribute to damage to cells and organs that manifest as tissue damage and eventually to manifestation of increased ageing rate and diseases (6, 7). Thus, for example, excess alcohol consumption leads mitochondrial dysfunction and inhibited lipid metabolism and reduced insulin sensitivity, eventually causing alcoholic fatty liver disease (8).
Is it possible to protect against/reverse the metabolic and epigenetic changes due to drug abuse?
Every particular type of drug or substance of abuse has its own unique toxicity profile. Thus, amphetamines are known to affect the cardiovascular and neurological systems thereby exacerbating the risk factors for the metabolic syndrome. Methamphetamine users suffer cognitive deficits and abnormal metabolic activity, which affect nutritional status. The abuse of alcohol causes steatosis/liver injury as well as malnutrition leading to deficiencies in vitamin (B1, B2, B6, B12, C, K, A, and D) and minerals (selenium, zinc, magnesium, iron, and phosphorus) that significantly affect cellular metabolism as well as the epigenetic profile. The effects of these metabolic factors and their metabolites can impact gene expression by binding to transcription factors as well as by modifying chromatin structure and function (9–12).
In the common and chronic alcohol use disorder (AUD) the multiple exposure to ethanol produces an overall reduced anxiety which may be linked to opening of chromatin by increased histone acetylation, increased cAMP-response element binding protein (CREB) levels, and histone deacetylase (HDAC) inhibition (11, 12). Acute ethanol exposure was found to increase H3 and H4 histone acetylation as well as increase CREB and CBP in the central nucleus of the amygdala and to decrease HDAC activity in the amygdala (13). Since many organ systems are affected by alcohol, a cross-tissue and cross-phenotypic analysis, showed a differentially methylated region in the proprotein convertase subtilisin/kexin 9 (PCSK9) gene (11). This PCSK9 hypomethylation was conserved across tissues and positively correlated with expression.
Similarly, in the heroin abuse disorder methylation levels of CpG sites HTR1B_07 and HTR1B_26 and the promoter region of the HTR1B gene were hypermethylated in heroin abusers compared to healthy controls (14).
The enzymes involved in epigenetic modifications of the DNA as well as the histone methylation/acetylation are reliant on specific micronutrient cofactors (Figure 3) and their activity can be directly impacted by the levels of these cofactors like nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD) as well as metabolic compounds like L-carnitine, Q10, etc. Chronic alcohol consumption has also been reported to leads to significant reductions in micronutrient especially thiamine and has been reported to contribute to DNA hypomethylation (Figure 2). Thiamine deficiency in causes neurological injury leading to the Wernicke/Korsakoff syndrome (15).
FIGURE 2
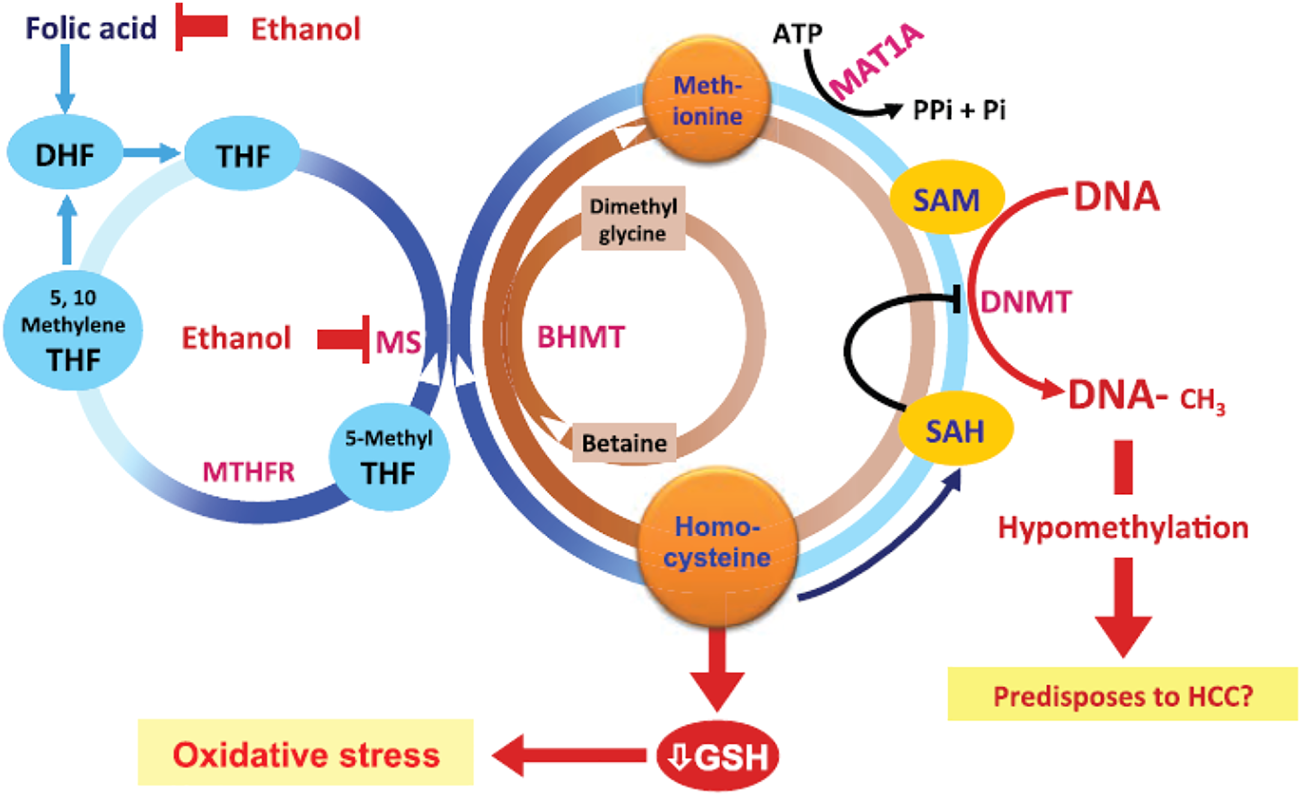
Effects of alcohol on metabolism and gene regulation mechanisms [from Ref (7)]. The effects of ethanol are via effects on homocysteine/methionine metabolism as well as DNA methylation. Abbreviations: ATP, adenosine triphosphate; BHMT, betaine homocysteine methyltransferases; DNMTs, DNA methyltransferases; GSH, glutathione; HCC, hepatocellular carcinoma; MAT, methionine adenosyltransferase; MTHFR, methylene tetrahydrofolate reductase; 5-methyl THF, 5-methyl tetrahydrofolate; MS, methionine synthase; Pi, inorganic phosphate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Future considerations
Studies are showing that drug abuse affects CNS as well as the diurnal rhythms of the whole body. These diurnal rhythm changes are also evident in at the level of cellular and organ metabolism (3). Drug abuse thus causes changes in gene expression and permanent epigenetic pattern changes which are related to the addiction and toxicity associated with long-term drug abuse. The metabolic and genetic/epigenetic dysfunctions over time in drug abuse may be countered or reduced by the use of micronutrients and metabolic cofactors, as well as by strategies utilising caloric restriction and preconditioning strategies. Exercise as well as environmental enrichment may be other strategies that impact on health of drug abusers (16). This may open ways to modulate the protective cellular pathways in a positive manner and reduce the impact of drug abuse.
Drug abuse related changes in metabolism would also have negative effects on various body systems due to increased cell damage, increased excitotoxicity, reduced energy production, and lowered antioxidant potential in cells. Partly this could also be due to mitochondrial dysfunction. This could be limited by adequate nutrient substrates and nutrient cofactors, especially L-carnitine, which play an important role in the mitochondrial function and metabolic flexibility (Figure 3).
FIGURE 3
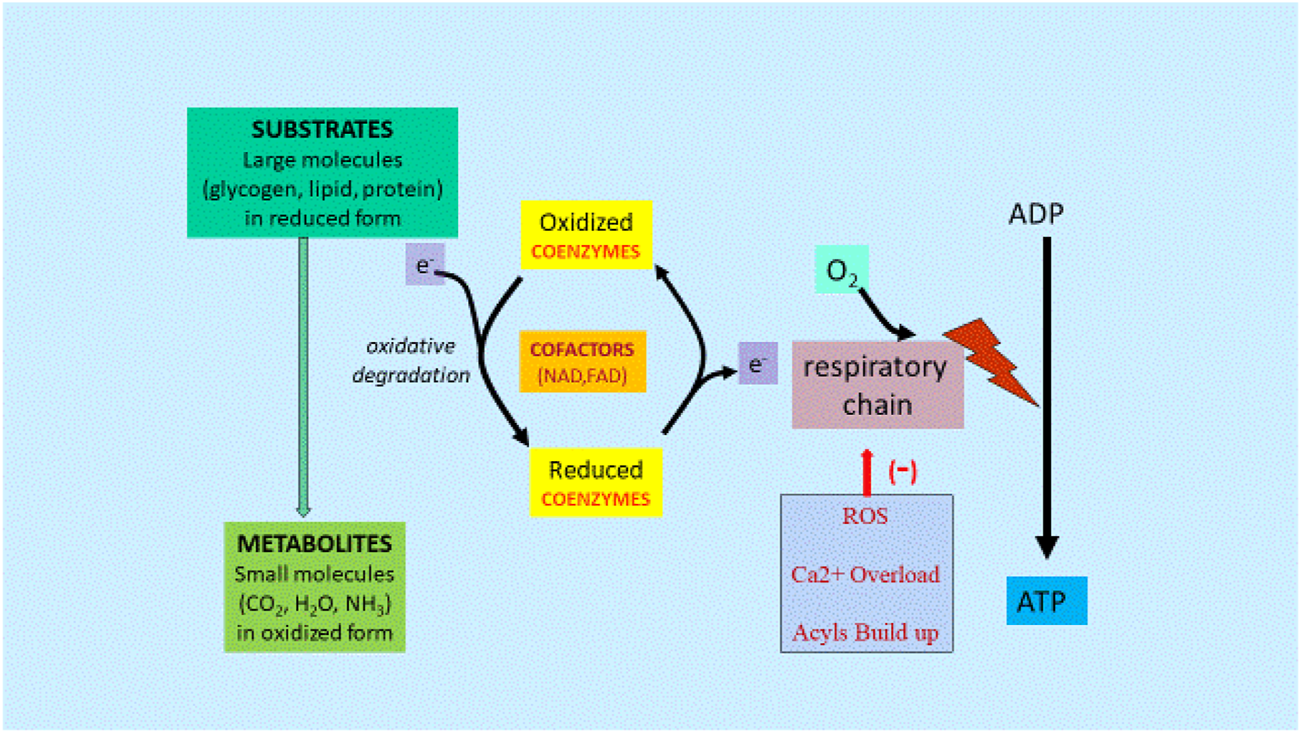
Metabolism and Gene Regulation Mechanisms [From Ref (4)]. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CO2, carbon dioxide; H2O, water; NH3, ammonia; Ca2+, calcium; e, electron; FAD, flavin adenine dinucleotide; NAD, nicotinamide adenine dinucleotide; ROS, reactive oxygen species.
Energy from starting substrates such as glycogen, lipids, and proteins are gradually extracted by the complex series of enzymes in the cellular cytoplasm and mitochondria, resulting in metabolites, mainly CO2 and H2O. Any dysfunction in this chain of events caused by lack of cofactors or mitochondrial dysfunction would lead to reduced ATP formation and increased ROS, Ca2+ and long chain fatty acid acyls and acylcarnitine buildup.
Further studies are needed to examine drug abuse related changes in gene/epigenetic expression and metabolism and to develop effective strategies to limit and reverse these changes. Indeed, certain metabolic compounds positively modulate the gene expression and associated protective cellular pathways and may contribute to limit drug abuse-related alterations and toxicity.
Statements
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
Author AV was employed by the company Alfasigma BV.
References
1.
Warner EA Greene GS Buchsbaum MS Cooper DS Robinson BE . Diabetic ketoacidosis associated with cocaine use. Arch Intern Med (1998) 158(16):1799–802. 10.1001/archinte.158.16.1799
2.
Virmani A Binienda ZK Ali SF Gaetani F . Metabolic syndrome in drug abuse. Ann N Y Acad Sci (2007) 1122:50–68. 10.1196/annals.1403.004
3.
Cervantes M Lewis RG Della-Fazia MA Borrelli E Sassone-Corsi P . Dopamine D2 receptor signaling in the brain modulates circadian liver metabolomic profiles. Proc Natl Acad Sci U S A (2022) 119(11):e2117113119. 10.1073/pnas.2117113119
4.
Virmani MA Cirulli M . The role of L-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int J Mol Sci (2022) 23(5):2717. 10.3390/ijms23052717
5.
Henry JA . Metabolic consequences of drug misuse. Br J Anaesth (2000) 85(1):136–42. 10.1093/bja/85.1.136
6.
Virmani A Ali SF Binienda ZK . Neuroprotective strategies in drug abuse-evoked encephalopathy. Ann N Y Acad Sci (2010) 1199:52–68. 10.1111/j.1749-6632.2009.05171.x
7.
Bachi K Sierra S Volkow ND Goldstein RZ Alia-Klein N . Is biological aging accelerated in drug addiction?Curr Opin Behav Sci (2017) 13:34–9. 10.1016/j.cobeha.2016.09.007
8.
Lamas-Paz A Hao F Nelson LJ Vázquez MT Canals S Gómez Del Moral M et al Alcoholic liver disease: Utility of animal models. World J Gastroenterol (2018) 24(45):5063–75. 10.3748/wjg.v24.i45.5063
9.
Zakhari S . Alcohol metabolism and epigenetics changes. Alcohol Res (2013) 35(1):6–16.
10.
Cadet JL Jayanthi S . Epigenetics of addiction. Neurochem Int (2021) 147:105069. 10.1016/j.neuint.2021.105069
11.
Lohoff FW Sorcher JL Rosen AD Mauro KL Fanelli RR Momenan R et al Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder. Mol Psychiatry (2018) 23(9):1900–10. 10.1038/mp.2017.168
12.
Bohnsack JP Pandey SC . Histone modifications, DNA methylation, and the epigenetic code of alcohol use disorder. Int Rev Neurobiol (2021) 156:1–62. 10.1016/bs.irn.2020.08.005
13.
Pandey SC Ugale R Zhang H Tang L Prakash A . Brain chromatin remodeling: A novel mechanism of alcoholism. J Neurosci (2008) 28:3729–37. 10.1523/JNEUROSCI.5731-07.2008
14.
Li Y Lu Y Xie Q Zeng X Zhang R Dang W et al Methylation and expression quantitative trait locus rs6296 in the HTR1B gene is associated with susceptibility to opioid use disorder. Psychopharmacology (Berl) (2022) 239(8):2515–23. 10.1007/s00213-022-06141-5
15.
Leevy CM Moroianu SA . Nutritional aspects of alcoholic liver disease. Clin Liver Dis (2005) 9:67–81. 10.1016/j.cld.2004.11.003
16.
He Y Madeo G Liang Y Zhang C Hempel B Liu X et al A red nucleus-VTA glutamate pathway underlies exercise reward and the therapeutic effect of exercise on cocaine use. Sci Adv (2022) 8(35):eabo1440. 10.1126/sciadv.abo1440
Summary
Keywords
metabolic, epigenetic, co-factors, L-carnitine, micronutrients, mitochondria, dopamine
Citation
Virmani MA (2023) Drug abuse results in metabolic and epigenetic changes in body which may contribute to disease risk: Role of L-carnitine and nutrients. Adv. Drug. Alco. Res. 3:10901. doi: 10.3389/adar.2023.10901
Received
13 September 2022
Accepted
09 January 2023
Published
18 January 2023
Volume
3 - 2023
Edited by
Emmanuel Onaivi, William Paterson University, United States
Reviewed by
Qing-Rong Liu, National Institute on Aging (NIH), United States
Syed Ali, National Center for Toxicological Research (FDA), United States
Updates
Copyright
© 2023 Virmani.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Ashraf Virmani, ashraf.virmani@alfasigma.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.