- 1Laboratory of Adaptation, Reward and Addiction, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ, United States
- 2Thermo Fisher Scientific Inc., Austin, TX, United States
- 3Department of Cell Biology and Biochemistry, School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, United States
- 4Department of Machine Learning, Mohamed bin Zayed University of Artificial Intelligence, Abu Dhabi, United Arab Emirates
- 5Biologically Inspired Neural & Dynamical Systems Laboratory, The Manning College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, United States
microRNA-9 (miR-9) is one of the most abundant microRNAs in the mammalian brain, essential for its development and normal function. In neurons, it regulates the expression of several key molecules, ranging from ion channels to enzymes, to transcription factors broadly affecting the expression of many genes. The neuronal effects of alcohol, one of the most abused drugs in the world, seem to be at least partially dependent on regulating the expression of miR-9. We previously observed that molecular mechanisms of the development of alcohol tolerance are miR-9 dependent. Since a critical feature of alcohol action is temporal exposure to the drug, we decided to better understand the time dependence of alcohol regulation of miR-9 biogenesis and expression. We measured the effect of intoxicating concentration of alcohol (20 mM ethanol) on the expression of all major elements of miR-9 biogenesis: three pri-precursors (pri-mir-9-1, pri-mir-9-2, pri-mir-9-3), three pre-precursors (pre-mir-9-1, pre-mir-9-2, pre-mir-9-3), and two mature microRNAs: miR-9-5p and miR-9-3p, using digital PCR and RT-qPCR, and murine primary medium spiny neurons (MSN) cultures. We subjected the neurons to alcohol based on an exposure/withdrawal matrix of different exposure times (from 15 min to 24 h) followed by different withdrawal times (from 0 h to 24 h). We observed that a short exposure increased mature miR-9-5p expression, which was followed by a gradual decrease and subsequent increase of the expression, returning to pre-exposure levels within 24 h. Temporal changes of miR-9-3p expression were complementing miR-9-5p changes. Interestingly, an extended, continuous presence of the drug caused a similar pattern. These results suggest the presence of the adaptive mechanisms of miR-9 expression in the presence and absence of alcohol. Measurement of miR-9 pre- and pri-precursors showed further that the primary effect of alcohol on miR-9 is through the mir-9-2 precursor pathway with a smaller contribution of mir-9-1 and mir-9-3 precursors. Our results provide new insight into the adaptive mechanisms of neurons to alcohol exposure. It would be of interest to determine next which microRNA-based mechanisms are involved in a transition from the acute, intoxicating effects of alcohol to the chronic, addictive effects of the drug.
Introduction
Alcohol Use Disorder (AUD) is a chronic, incurable disease affecting people worldwide regardless of their social or economic status. AUD leads to an estimated 132.6 million disability-adjusted life years (DALYs), and an estimated 3 million deaths per year [1]. In the United States AUD is one of the largest drug problems, and alcohol abuse costs the country hundreds of billions of dollars each year in lost revenue, treatments, and mortality [2, 3]. Development of alcohol addiction takes place over time through the complex actions of alcohol on the brain’s reward system. Temporal characteristics of alcohol actions are critical yet poorly understood.
In recent years, many studies have focused on the epigenetic underpinnings of addiction to better understand the development of AUD [4]. MicroRNAs (miRNAs), small (∼21 nt long) endogenous RNA molecules are powerful epigenetic modulators regulating gene expression on a genome-wide scale [5]. It has been estimated that microRNAs modify the expression of approximately 60% of the transcripts in humans [6] and play a fundamental role in the development and maintenance of neurons in the brain [7]. microRNAs are also key elements of the development of drug [8–10] and alcohol addiction [11–13].
One particular microRNA involved in brain development [14], function [15], and malfunction [16] is miR-9 (specifically miR-9-5p). Dysregulation of miR-9-5p by alcohol has a broad impact on the brain, and several downstream targets of miR-9-5p have been well-established ([17], reviewed in depth in [18]). However, effects of upstream changes in miR-9 biogenesis on mature miR-9 are less studied. Understanding alcohol regulation of miR-9 biogenesis could help to uncover new mechanisms of alcohol action, and ultimately may lead to discovery of novel therapeutic options in addiction.
miR-9 is an ancient microRNA found from invertebrates to mammals [19, 20] and has a complex biogenesis. In many species there are three distinct miR-9 genes located on three different chromosomes. In humans, miR-9 genes are located on chromosomes 1, 5, and 15, while their equivalents in mice are on chromosomes 3, 7, and 13, respectively [21]. In both species, each gene gives rise to a separate, long, primary precursor, pri-mir-9-1, pri-mir-9-2, and pri-mir-9-3 (Figure 1). Each pri-precursor is subsequently trimmed to a shorter pre-precursor of a characteristic hairpin loop structure (Figure 1). The next step produces an even shorter, small, double-stranded duplex consisting mostly of two complementarily bound miRNA strands. Ultimate processing of the duplex separates the strands yielding two short, single-stranded, distinct mature microRNAs: miR-9-5p and miR-9-3p. Importantly, in the case of miR-9, all final mature miR-9-5p products of the 3 biogenesis pathways are identical [22]. Similarly, all mature miR-9-3p end products are indistinguishable (Figure 1). Both mature miR-9 strands execute biological action by interacting through complementarity with multiple targets (RNA transcripts), which usually leads to suppression of expression of the targets.
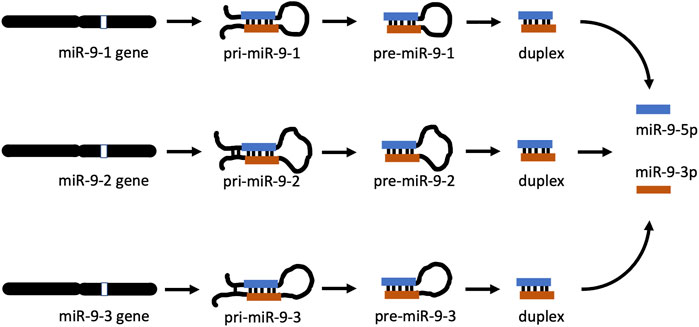
FIGURE 1. miR-9 biogenesis pathways. In apes (including humans) and rodents (including mice), there are 3 mir-9 genes (mir-9-1, mir-9-2, and mir-9-3) located on different chromosomes. Each gene produces its own primary RNA precursor (pri-mir-9-1, pri-mir-9-2, and pri-mir-9-3), which is cleaved to a pre-precursor (pre-mir-9-1, pre-mir-9-2, and pre-mir-9-3). Each precursor is further processed to yield a duplex containing both miR-9-5p and miR-9-3p. Separation of each duplex into single-stranded RNA sequences generates two final forms of miR-9, which are physiologically active: miR-9-5p and miR-9-3p. All miR-9-5p produced via 3 separate biogenesis pathways are identical. Similarly, all final miR-9-3p are indistinguishable.
miR-9-3p has been shown to be biologically active and play an important role in carcinogenesis [23] such as in Burkitt’s lymphoma [24] and breast cancer [23, 25] as well as brain pathologies. Decreased expression of miR-9-3p has been linked to neurological disorders including Alzheimer’s and Huntington’s diseases [26].
Thus, understanding temporal regulation of the expression of various miR-9 precursors as well as both forms of mature miR-9 by alcohol is critical in enhancing our understanding of the mechanisms involved in the development of alcohol addiction and adaptation to alcohol exposure.
Materials and methods
Striatal culture
C57BL6/J mice (Jackson Laboratory, Bar Harbor, ME) were maintained under 12h:12h light:dark cycles at standard temperature and humidity with food and water provided ad libitum at the Bartlett Animal Facility (Rutgers-New Brunswick). Mice were monitored daily, and cages were routinely changed. Mice were bred for litters to use in generating cultures. All animal experiments were approved by the Rutgers Institutional Animal Care and Use Committee (IACUC Protocol # 10-024).
Cultures of Medium Spiny Neurons (MSN) at ∼95% purity [27, 28], were prepared following well-established protocols [29–31]. At day 5 after birth (P5), pups were decapitated, and brains were immediately removed and placed into a 60 mm plate containing ice-cold CMF-HBSS (100 mL of final solution made with 10 mL 10x HBSS (Life Technologies), 0.7 mL 5% NaHCO3 (Sigma), final pH 7.1, brought to final volume with ultrapure water, then filter sterilized and stored at room temperature). The Nucleus Accumbens (NAc) was removed using a mouse brain atlas for visual reference [32]. Equal numbers of male and female pups were used for each preparation to limit the bias of using a single gender. Striatal tissue was diced into smaller fragments, ∼1 mm in diameter in 3.15 mL cold CMF-HBSS, and trypsinized with the addition of 0.35 mL of 2.5% trypsin at 37°C. After 10 min, 8 mL DMEM-FBS medium (178 mL DMEM (high glucose, no sodium pyruvate, no glutamine (Irvine Scientist), with 20 mL FBS, 0.5 mL 10,000 u Pen/Strep and 2 mL 200 mM glutamine (Life Technologies), stored at 4°C in the dark) was added, and the solution was centrifuged at 300 x g for 5 min to pellet the cells. The medium was aspirated from the tube, and 5 mL of Growth Medium (100 mL DMEM/F12 (with Glutamax; Life Technologies), with 2 mL FBS (1.9% v/v), 2 mL B-27 (1.9% v/v; Life Technologies), and 1 mL penicillin/streptomycin (0.95% v/v), stored at 4°C in the dark) was added. Trituration to further break apart aggregates was conducted using a fire-polished Pasteur pipet, and the tube was spun again as above. Excess media was removed, and the cells were resuspended in 10 mL of Growth Medium. Preparations were conducted under a sterile hood to help maintain sterility, except for centrifugation. The concentration of live cells/mL was estimated by hemocytometer counts of live:dead cells using trypan blue, and the cell stock solution was diluted to a final concentration of 0.5 × 106 cells/mL. Plates of striatal cells were prepared by seeding 2 mL of the cell stock solution onto 35 mm cell culture plates that had been coated with ornithine (Sigma-Aldrich, St. Louis, MO) and laminin (Life Technologies, Carlsbad, CA) for cell adherence and enrichment for neurons. Preparations were rapidly conducted as speed was critical for cell viability. Twenty-four hours post-seeding, after allowing cells to adhere, the media was replaced with a 2 mL Neurobasal Medium (NB)/plate (100 mL Neurobasal A Medium supplemented to 2.0 mM glutamine final concentration; Life Technologies), with 2 mL FBS (1.9% v/v), 2 mL B-27 (1.9% v/v; Life Technologies), and 1 mL penicillin/streptomycin (0.95% v/v) stored at 4°C in the dark). Cultures were maintained at 37°C/5% CO2 in a cell culture incubator with saturated humidity for another week before starting exposures, and as a result, the neurons were nearly 2 weeks old since birth (5 days in vivo + 8 days in vitro = 13 days total) at the start of the experiments. Sometimes in microRNA studies alpha-amanitin is added to cultured cells to inhibit RNA polymerases II and III, which process microRNA. Since alpha-amanitin can also cause widespread transcriptional stress and apoptosis [33, 34] we did not add it to our cultures.
Ethanol exposure
We chose 20 mM ethanol for alcohol exposures as it represents a physiologically relevant dose of alcohol while maintaining cell viability. 20 mM ethanol corresponds to a 0.092% Blood Alcohol Content (BAC), which can be achieved in humans by a quick (30–60 min) consumption of 3–4 standard drinks of alcohol by a 150-pound individual [35] causing disinhibition, impaired thinking, and potential DWI/DUI in the US [35]. Previously we have shown that 20 mM ethanol can upregulate the expression of miR-9-5p within 15 min after exposure of the rat brain organotypic cultures containing supraoptic nucleus (SON) neurons leading to alcohol tolerance [17]. Importantly, 20 mM causes minimal neuronal cell death in culture as shown by us [12] and others [36].
Seven days after seeding neurons the cells were subjected to the alcohol exposure and withdrawal with the following collection time points: Control = 0 min exposure +0 h withdrawal, 15 min 20 mM ethanol exposure +0 h withdrawal, 15 min 20 mM ethanol exposure +1 h withdrawal, 15 min 20 mM ethanol exposure +6 h withdrawal, 15 min 20 mM ethanol exposure +12 h withdrawal, 15 min 20 mM ethanol exposure +24 h withdrawal, 6 h 20 mM ethanol exposure +0 h withdrawal, 6 h 20 mM ethanol exposure +6 h withdrawal, 6 h 20 mM ethanol exposure +24 h withdrawal. Collection at each time point was conducted in triplicate. For each control, 5–7 plates were prepared. Cells were treated by aspirating off media and replacing with either a neurobasal medium (“media only” control) or a neurobasal medium with alcohol (NBE with 20 mM final ethanol concentration). Ethanol evaporation was minimized by maintaining NBE plates in a semi-sealed container in the incubator with saturated humidity and additional plates of medium containing the same concentration of ethanol, based upon the methods of Pietrzykowski [12, 17].
After a defined length of exposure, NB or NBE media were removed. For plates without a withdrawal period, cells were collected immediately. For cells with a withdrawal period, the NB medium replaced the NBE medium for a defined length of time after which cells were collected.
Cell collection was carried out by quickly rinsing plates with 2 mL ice-cold PBS followed by scraping cells from the plate with a cell scraper in 200 uL PBS. Cells were immediately flash-frozen in liquid nitrogen and stored at −80°C until processed for total RNA isolation as described previously [16].
Alcohol concentration verification
Media samples were gathered at each collection point in the experimental process (before and after ethanol addition, during exposure and withdrawal) to verify alcohol concentration. Alcohol measurements were conducted using an AMI Analyzer according to the manufacturer’s instructions (Analox Instruments Ltd., Lunenburg, MA). 10, 20, and 50 mM ethanol standards in media were used to calibrate the instrument prior to reads to ensure accuracy. Alcohol measurements confirmed that ethanol loss was minimized using our methodology as previously described in more detail [12, 17].
RNA isolation
Total RNA or Small RNA (for precursor assays) was isolated by miRVana kit according to the manufacturer’s instructions (Life Technologies). Concentration and purity were analyzed by a Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE), and aliquots of each sample were used to prepare 10ng/ul dilutions in nuclease-free water for RT-qPCR. All samples were kept at −80°C.
RT-qPCR
miRNA
We conducted RT and qPCR steps to assess mature miR-9-5p and miR-9-3p based upon the manufacturer’s protocols for TaqMan Small RNA Assays (Applied Biosystems, Inc., Foster City, CA). Using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, Inc., Foster City, CA), mature miRNA was converted into cDNA using a Veriti Thermal Cycler (Applied Biosystems, Inc., Foster City, CA). A working stock of 10 ng/ul of total RNA was prepared and used for RT with each experimental sample in a total volume of 15 μL. RT consisted of 16°C 30 min, 42°C 30 min, 85°C 5 min, and hold at 4°C. The two-step process of RT followed by qPCR permitted finer control/greater accuracy for the final RT-qPCR reaction by allowing us to equalize the quantities of cDNA. cDNAs were amplified with the Taqman Small RNA Assay kit (Applied Biosystems, Inc., Foster City, CA) using an ABI Step One Plus Thermocycler (Applied Biosystems, Inc., Foster City, CA). 1.33 μL of RT sample was used for Taqman qPCR in a total volume of 20 μL. TaqMan reactions were carried out using Universal Master Mix II, no UNG from Applied Biosystems, and 1 µL of TaqMan MicroRNA assay primers. Triplicates of each sample were used in the 96-well plate (except for controls, where n = 5, or n = 7) to ensure greater accuracy. The average was taken as the value for each. For normalization and quality assessment we followed absolute quantification methods which can provide better accuracy without the need of a separate housekeeping gene, as described by Iguchi [37], Arabkari [38], and Wang [39]. We used 7-log dilution range (10 fmol–10−4 fmol) of synthetic miR-9-5p and miR-9-3p oligos (amplification efficiency, R2 = 0.9993). The cycling protocol consisted of 95°C for 10 min, followed by 40 cycles of (95°C for 15 s and 60°C for 1 min), in an ABI Step One Plus Thermocycler (Applied Biosystems, Inc., Foster City, CA). Data collection occurred at the 60°C step.
Pre- and Pri-miRNA precursors
We used the Ambion miRVana kit following the manufacturer’s instructions, to separate small RNA molecules including pre-precursor miRNAs (∼100 nt in length) from the much larger (over 1,000 nt in length) pri-precursors for subsequent studies.
Pre- precursors
RT was conducted using a miScript II RT kit (Qiagen). miR-9 precursors (pre-mir-9-1, pre-mir-9-2, pre-mir-9-3) were pre-amplified using stock primers for RT-qPCR from Qiagen. After this, a 1:20 dilution of the pre-amp product was used for normal qPCR. Standard curves were prepared from 100 fmol using stocks of 9-1, 9-2, and 9-3 oligos. Pre-amplification was carried out using Qiagen miScript Precursor assay kits for pre-mir-9-1, -9-2, and -9-3 respectively, along with a miScript PreAMP PCR Kit (Qiagen).
Pri-precursors
Cell cultures were obtained as indicated above. RT was carried out with SuperScript VILO Master Mix (Invitrogen) using the manufacturer’s recommendations. cDNA samples were sent to Life Technologies for subsequent digital PCR.
Digital PCR
30 ng/μL of each alcohol exposure sample was tested with three TaqMan Pri-miRNA assays (Mm04227702 pri-mmu-mir-9-1, Mm03306269 pri-mmu-mir-9-2, and Mm03307250 pri-mmu-mir-9-3) (Thermo Fisher Scientific Inc.). 1μL of each sample was added to 10 μL QuantStudio 3D Digital PCR Master Mix, 1 μL of TaqMan Assay (20X), and 8 μL of nuclease-free water for 20 μL of the reaction mix. 14.5 μL of reaction mix was loaded on each QuantStudio 3D Digital PCR 20K Chip (Thermo Fisher Scientific Inc.) using QuantStudio 3D Digital PCR Chip Loader (Thermo Fisher Scientific Inc.) according to manufacturer’s instruction. The digital PCR was performed on Proflex 2x Flat PCR System (Thermo Fisher Scientific Inc.) with thermal cycling of 10 min at 96°C, followed by 39 cycles at 60°C for 2 min and 98°C for 30 s, followed by holding at 60°C for 2 min and 10°C for long term. Each chip fluorescence intensity was read using QuantStudio 3D Digital PCR instrument (Thermo Fisher Scientific Inc.) and analyzed copies/μL based on Poisson distribution using QuantStudio 3D Analysis Suite Cloud Software (Thermo Fisher Scientific Inc.).
Statistical analysis
Expression data for statistical analysis were obtained using oligos in a standard curve method for mature miR-9-5p and miR-9-3p, 2−ΔΔCT method for pre-precursors, and Poisson distribution for pri-precursors. The data were analyzed using unpaired, two-tailed t-tests. Data were expressed as fold-change to visualize the relationship between exposure condition and molecule expression. p-value below 0.05 (p < 0.05) was set as statistically significant.
Results
Regulation of miR-9-5p and miR-9-3p expression by short exposure to alcohol
miR-9-5p is a prominent brain microRNA regulated by alcohol. Some reports describe the stimulatory effect of alcohol on miR-9-5p expression [17, 40], while others report the opposite effects [41]. To better understand the intricacies of miR-9-5p regulation by alcohol, we first exposed murine primary neuronal cultures to physiologically relevant 20 mM ethanol for 15 min (the short exposure) and measured its expression at various times after alcohol withdrawal up to 24 h post-exposure (Figure 2A).
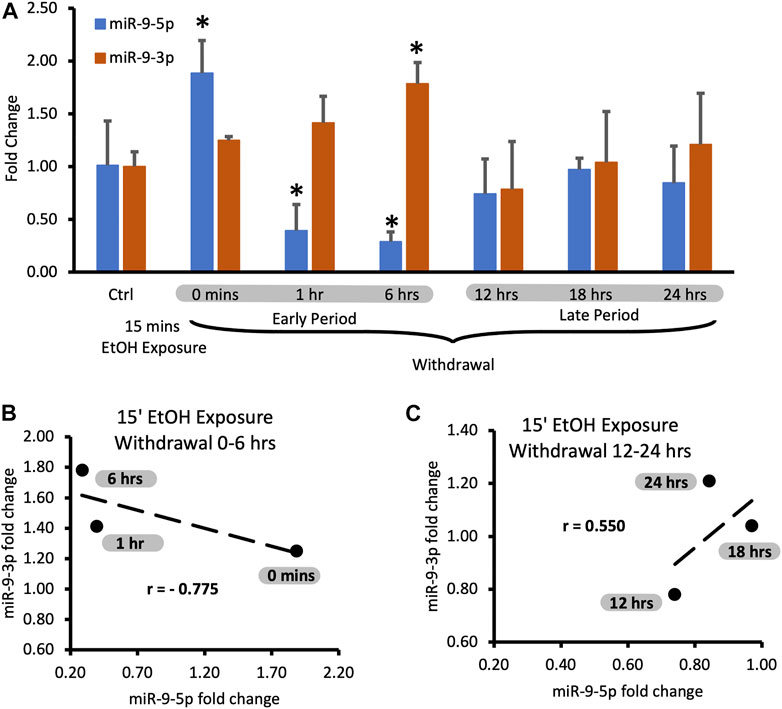
FIGURE 2. Expression levels of miR-9-5p and -3p during 24-hr long withdrawal after 15 min exposure to 20 mM ethanol. (A) Temporal expression profile of miR-9-5p (left bars) and miR-9-3p (right bars) measured by Taqman-based RT-qPCR. Data expressed as mean fold change ± SD. n = 3, except n = 7 in miR-9-5p control group, n = 5 in miR-9-3p control group). Asterisks indicate statistically significant differences comparing to the control, p < 0.05. (B) Strong, negative correlation of miR-9-5p and miR-9-3p expression levels during the first 6 h of alcohol withdrawal. Correlation coefficient r = −0.775. (C) Moderate, positive correlation of miR-9-5p and miR-93p expression levels during 12–24 h of alcohol withdrawal. The correlation coefficient r = 0.550.
We observed that after the short exposure expression levels of miR-9-5p increased almost two-fold (Figure 2A, left bars), in accordance with previously published findings [17]. Alcohol withdrawal caused a fast decrease of the elevated levels of miR-9-5p even below the pre-exposure, normal levels within 1 h after the start of the exposure (Figure 2A, left bars). In the alcohol-free environment, miR-9-5p levels decreased even further with time, reaching the lowest levels of around 40% of the pre-exposure levels at the 6 h post-exposure mark. Somewhere between 6 h and 12 h of the withdrawal miR-9-5p levels started to rebound from their nadir point and went back to the pre-exposure levels (Figure 2A, left bars). They reached the pre-exposure levels 12 h after the exposure and maintained normal levels up to 24 h after the exposure (Figure 2A, left bars).
Although miR-9-5p is the most recognized final product of miR-9 biogenesis, miR-9-3p also plays an important role in neural development [42] and neuronal differentiation [43] with more predicted targets then miR-9-5p (Supplementary Table S1, miR-9-5p: 1242 targets; Supplementary Table S2, miR-9-3p: 4334 targets). Interestingly, there is a quite large overlap of targets between these two microRNAs: over 34% of miR-9-5p targets are also targeted by miR-9-3p (425 targets, Supplementary Table S3).
We observed that short alcohol exposure also regulates the expression of miR-9-3p. The short exposure increased expression of miR-9-3p (Figure 2A, right bars) similar to its effect on the miR-9-5p expression. In contrast to miR-9-5p however, after the removal of alcohol, miR-9-3p levels continue to rise, reaching significantly higher levels 6 h post-exposure (Figure 2A, right bars).
After reaching the peak of expression, miR-9-3p levels return down to pre-exposure levels at the 12 h post-exposure timepoint and maintain that normal level up to the 24 h post-exposure, mimicking temporal dynamics of miR-9-5p expression changes within 12–24 h post-exposure time interval (Figure 2A, right bars).
It seems that, based on changes in the expression of both microRNAs, two withdrawal periods triggered by short alcohol exposure could be distinguished: the early period starting immediately after alcohol withdrawal and lasting around 6 h, and the late period following the early one and lasting up to the 24-hour post-exposure timepoint (Figure 2A).
During both time periods, the expression of miR-9-5p and miR-9-3p seems to be tightly associated with each other as determined by correlation analysis. During the early withdrawal period changes in miR-9-3p and miR-9-5p expression are strongly and negatively correlated (Figure 2B; Table 1, correlation coefficient r = −0.775). During the late withdrawal period, changes in the expression of miR-9-3p and miR-9-5p are moderately and positively correlated (Figure 2C; Table 2, correlation coefficient r = 0.55).

TABLE 1. Correlation between expression fold change of miR-9-5p and miR-9-3p during the early period of alcohol withdrawal.

TABLE 2. Correlation between expression fold change of miR-9-5p and miR-9-3p during the late period of alcohol withdrawal.
Regulation of miR-9-5p and miR-9-3p expression by continuous exposure to alcohol
We compared the short exposure results with the expression of miR-9-5p under the continuous presence of the drug for up to 24 h (continuous exposure).
We assumed that the continuous presence of the drug would maintain the elevated plateau of miR-9-5p since exposure to alcohol increased miR-9-5p levels in the first place. However, it was not the case. We observed that despite alcohol presence, after the initial increase, miR-9-5p levels dropped within 6 h post-exposure (Figure 2B, left bars) and then increased (Figure 2B, left bars) with a similar temporal dynamic seen with the short exposure. Interestingly, in the continuing presence of alcohol beyond 6 h the miR-9-5p expression pattern shifted upwards above the pre-exposure levels presumably trying to set a new, higher equilibrium (Figure 2B, left bars).
During the continuous exposure to alcohol, the miR-9-3p expression did not change sufficiently to achieve standard statistical significance (p < 0.05) except for the last timepoint (Figure 3A, 24 h exposure). However, the changes of the miR-9-3p expression tightly followed the changes of the miR-9-5p expression, showing a strong and positive correlation at each timepoint studied (Figure 3A). We think that two periods with similar time frames can be distinguished here as well based on changes in the expression pattern: the early exposure period starting soon after alcohol addition and lasting about 6 h (Figure 3A) with a correlation coefficient r = 0.720 (Figure 3B; Table 3), and the late exposure period following the first one up to the 24-hour of alcohol exposure (Figure 3C; Table 4) with the correlation coefficient r = 0.853.
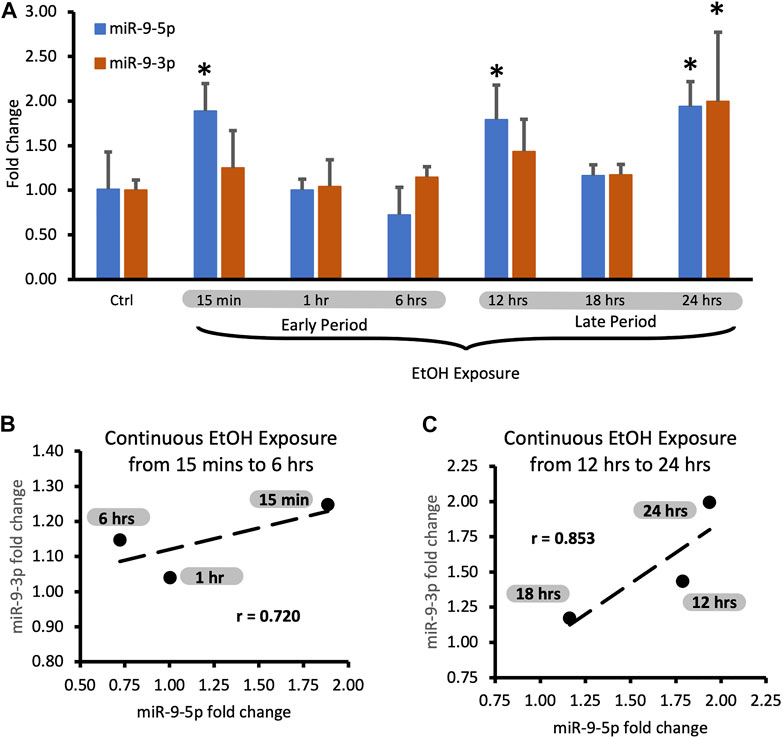
FIGURE 3. Expression levels of miR-9-5p and -3p during continuous, 24-hr long exposure to 20 mM ethanol. (A) Temporal expression profile of miR-9-5p (left bars) and miR-9-3p (right bars) measured by Taqman RT-qPCR. Data expressed as mean fold change ± SD. n = 3, except n = 7 in miR-9-5p control group, n = 5 in miR-9-5p control group). Asterisks indicate statistically significant differences comparing to the control, p < 0.05. (B) Strong, positive correlation of miR-9-5p and miR-9-3p expression levels during the first 6 h of alcohol exposure. The correlation coefficient r = 0.720. (C) Strong, positive correlation of miR-9-5p and miR-93p expression levels during 12–24 h of alcohol exposure. The correlation coefficient r = 0.853.

TABLE 3. Correlation between expression fold change of miR-9-5p and miR-9-3p during the early period of continuous alcohol exposure.

TABLE 4. Correlation between expression fold change of miR-9-5p and miR-9-3p during the late period of continuous alcohol exposure.
Regulation of expression of miR-9 precursors by short alcohol exposure
Both miR-9-5p and miR-9-3p are final products of miR-9 biogenesis (Figure 1, and ref 19). Three separate biogenesis pathways of the miR-9-5p/miR-9-3p pair start with each miR-9 gene generating its own pri-mir-9 precursor, and subsequently pre-mir-9 precursor, which ultimately contributes to the mature miR-9-5p and the mature miR-9-3p pools (Figure 1). We decided to determine the effects of both, the short and the continuous alcohol exposure, on the expression of all of these precursors.
We observed that the short alcohol exposure (15 min) had no effect on the expression of all three pre-mir-9 precursors (Figure 4A). Expression levels of none of the precursors changed immediately after the alcohol exposure. Since they remained consistently at the same, unchanged level for 6 h following the alcohol withdrawal (Figure 4A) we did not explore further time points.
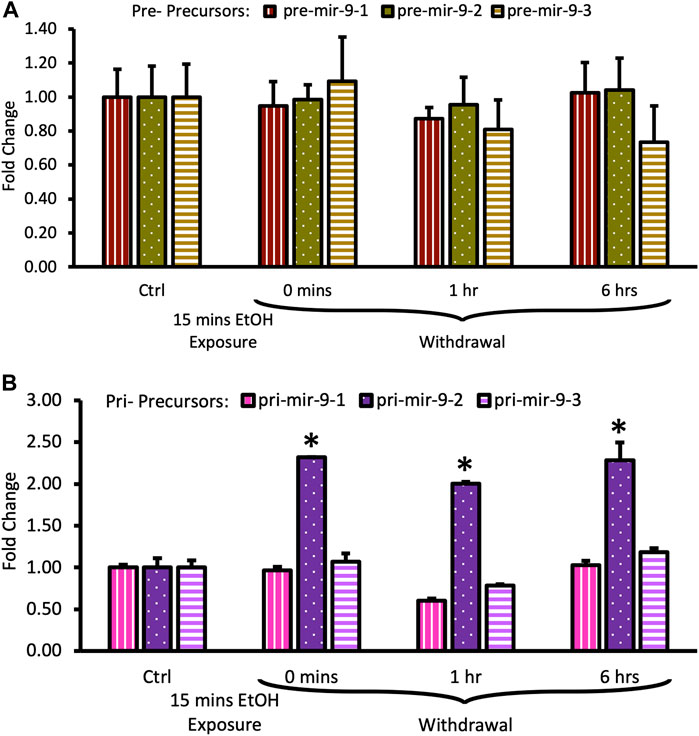
FIGURE 4. Expression levels of pre- and pri-precursors during the first 6 hours of withdrawal after 15 min exposure to 20 mM ethanol. (A) Temporal expression profile of pre-mir-9-1 (left bars), pre-mir-9-2 (middle bars), and pre-mir-9-3 (right bars) measured by miScript RT-PCR. (B) Temporal expression profile of pri-mir-9-1 (left bars), pri-mir-9-2 (middle bars), and pri-mir-9-3 (right bars) measured by QuantStudio 3D Digital RT-PCR. Data expressed as mean fold change ± SD. n = 3 (pre-precursors), n = 2 (pri-precursors). Asterisks indicate statistically significant differences comparing to the control, p < 0.05.
In contrast, within the same timeframe of the early period of withdrawal, we observed a robust, over 2-fold upregulation of pri-mir-9-2 precursor expression by short alcohol exposure immediately following the exposure (Figure 4B). The pri-mir-9-2 precursor expression upregulation was sustained for at least 6 h after the alcohol withdrawal (Figure 4B). This effect was not observable for the other two pri-precursors: pri-mir-9-1 and the pri-mir-9-3 (Figure 4B).
Overall, it seems that a short, 15 min alcohol exposure elicited changes in the expression of pri-miR-9-2 precursor only and that these changes were quick, robust, and unceasing in alcohol absence.
Regulation of expression of miR-9 precursors by long alcohol exposure
The long (6 h) alcohol exposure affected the expression of both, pre- and pri-mir-9 precursors.
Both, pre-mir-9-1, and pre-mir-9-2 were significantly downregulated after 6 h of alcohol exposure, with pre-mir-9-3 following this trend but not reaching a statistical significance at p < 0.05 yet (Figure 5A). Withdrawal of alcohol for 6 h after the 6 hr-long exposure to the drug did not restore expression levels of any of the pre-mir-9 precursors with all of them being decreased. The decreased expressions of all three pre-miR-9 precursors continued in the absence of alcohol for up to 24 h after alcohol withdrawal (Figure 5A).
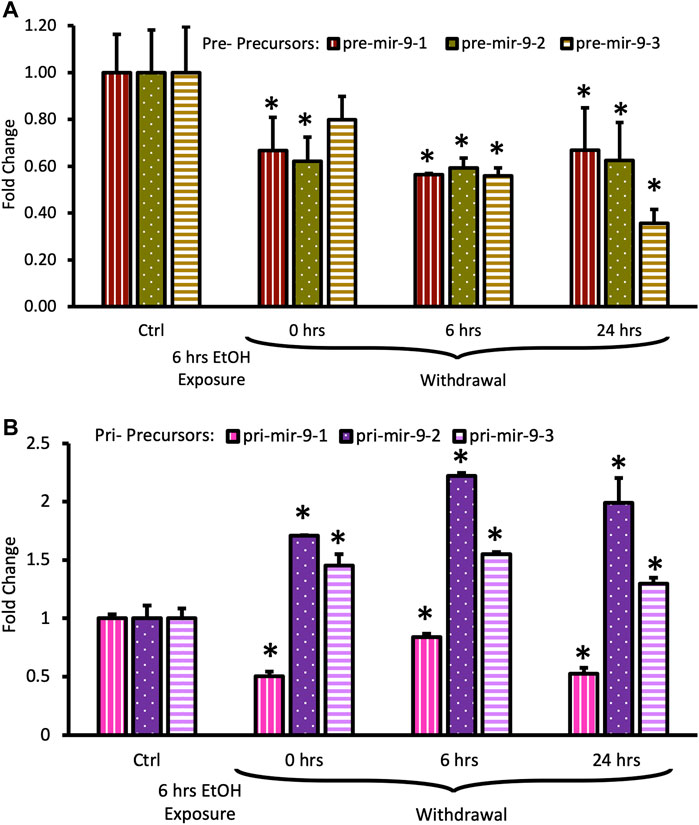
FIGURE 5. Expression levels of pre- and pri-precursors during 24 h withdrawal after 6 h of exposure to 20 mM ethanol. (A) Temporal expression profile of pre-mir-9-1 (left bars), pre-mir-9-2 (middle bars), and pre-mir-9-3 (right bars) measured by miScript RT-PCR. (B) Temporal expression profile of pri-mir-9-1 (left bars), pri-mir-9-2 (middle bars), and pri-mir-9-3 (right bars) measured by QuantStudio 3D Digital RT-PCR. Data expressed as mean fold change ± SD. n = 3 (pre-precursors), n = 2 (pri-precursors). Asterisks indicate statistically significant differences comparing to the control, p < 0.05.
The effects of the long (6 h) alcohol exposure on the expression levels of pri-miR-9 precursors also affected all of these precursors but each in a different way (Figure 5B). The expression of the pri-mir-9-1 precursor was consistently downregulated to about 50% of its pre-exposure levels, and this downregulation persisted in the absence of alcohol for up to 24 h after alcohol withdrawal (Figure 5B). In contrast, the expression of the remaining two pri-precursors (pri-mir-9-2, pri-mir-9-3) was significantly upregulated by the long (6 h) alcohol exposure to about 1.5-fold above their pre-exposure levels. After alcohol withdrawal, the upregulated levels of both pri-precursors were sustained (Figure 5B). The pri-miR-9-3 precursor maintained its 1.5-fold upregulation at both, 6 h and 24 h after alcohol withdrawal (Figure 5B), while the pri-miR-9-2 precursor expression levels 6 h after alcohol withdrawal went even further up, reaching above 2-fold upregulation, and maintaining their higher expression levels 24 h post-exposure (Figure 5B).
Overall, it seems that longer alcohol exposure elicited wider changes in the expression of miR-9 precursors, affecting the expression of all precursors. Nevertheless, it seems that the miR-9-2 biogenesis pathway responded in the most striking way.
Discussion
Alcohol Use Disorder (AUD) is a very complex disease involving an array of biomolecules, multiple biological pathways, and several organismal systems. Time is a fundamental factor of alcohol-triggered changes in the brain’s function as the development of AUD is happening progressively over time. We have attempted to shed some light on the temporal regulation of the biogenesis of miR-9, one of the key master regulators of gene expression in the brain [19], which is affected by alcohol in both, brain development [44, 45] and mature brain function [17, 46] and exists in two biologically active forms: miR-9-5p and miR-9-3p. We measured changes of both mature miR-9 forms in murine, primary cell culture consisting of Medium Spiny Neurons (MSN) derived from the Nucleus Accumbens (NAc), which is a part of the brain reward system integrating information from the cortex and subcortical regions [47–49] and highjacked by alcohol in AUD [50]. Alcohol affects the activity of MSN [51], disrupts information integration, and causes behavioral effects [52].
Although during intoxication, neurons in the brain can be exposed to a wide range of alcohol concentrations from around 10 mM to over 100 mM, 20 mM ethanol concentration has a low apoptotic effect [17, 36] yet significant effects on the CNS neurobiology (e.g., ion channel conductivity, neuronal excitability, neuronal network activity), morphology (e.g., synaptic shape and mitochondrial density [53], and behavior (e.g., sedation, motor incoordination, inability to operate motor vehicles, consistent with intoxication) [54]. We reported previously that exposure of the rat neurohypophysial brain explant to 20 mM alcohol for a short time (15 min) caused an upregulation of miR-9-5p expression and observable changes in expression of some of miR-9-5p targets, including the rearrangement of BK channel splice variants consistent with neuroadaptation [17]. Here, we extended our studies to determine temporal characteristics of miR-9 adaptation to alcohol using murine primary neuronal cultures of medium spiny neurons harvested from the striatum, allowing precise control over alcohol exposure and withdrawal of the pivotal element of the brain reward system.
miR-9-5p homeostatic response to short alcohol exposure and withdrawal
After observing previously the biological effects of a short exposure to 20 mM alcohol [17], we questioned whether the upregulated miR-9-5p levels persist after alcohol withdrawal and for how long. We determined here that the short alcohol exposure triggered changes in miR-9-5p expression observable during withdrawal. These changes could be divided into two, subsequent phases: 1/downregulation below the pre-exposure level, and 2/upregulation to the pre-exposure level. These phases seem to follow a pattern of homeostatic regulation, during which miR-9-5p levels thrown off of the steady state equilibrium by alcohol exposure would undergo changes after alcohol withdrawal to return eventually to the pre-exposure status quo. Based on our collection time points we observed that MSN neurons need roughly around 6–12 h of the drug withdrawal to return miR-9-5p levels to normal (i.e., the pre-exposure steady-state equilibrium). It would be of interest, in the next studies, to further narrow down the time window of this homeostatic adaptation.
miR-9-3p upregulation attenuates the effects of the miR-9-5p downregulation
Recently, miR-9-3p, the passenger strand derived from the same duplex as miR-9-5p, gained recognition as biologically active on its own [43, 55]. Martinez et al. [56] showed that chronic ethanol exposure over the course of 55 days elevated miR-9-3p in the serum of rats. Balaraman proposed that the ratio between these two mature microRNAs is important in the regulation of neuronal differentiation and in the development of cancer [57]. Both microRNAs impact the differentiation of neural stem cells through the co-regulation of a transcription factor, REST (RE1 silencing transcription factor/neuron-restrictive silencer factor). miR-9-5p targets REST directly, while miR-9-3p regulates the expression of coREST, a cofactor of REST [42, 57]. Therefore, miR-9-5p and miR-9-3p working in tandem can create various combinations of REST:coREST, thus influencing neuronal differentiation [58, 59].
Our results postulate an even tighter, joint effect of miR-9-5p and miR-9-3p on gene expression. Simultaneous downregulation of miR-9-5p and upregulation of miR-9-3p observed at some points, and their convergence on a large number of targets (425 transcripts, over 34% of miR-9-5p targets) could be a neuronal attempt to attenuate, at least some acute alcohol effects on miR-9-5p targets, consistent with a homeostatic response and preservation of pre-exposure equilibrium. We also would like to propose that any future studies focused on the regulation of miR-9-5p and its targets by alcohol or other factors should include miR-9-3p and its targets as well.
Coordinated miR-9-5p/-3p allostatic response to continuous alcohol exposure
We expected that in the continuous presence of alcohol, upregulated levels of miR-9-5p and miR-9-3p would be maintained. However, that was not the case. We observed that despite the continuous presence of alcohol, both miR-9-5p and miR-9-3p levels followed a response pattern, similar to one observed in a short exposure/withdrawal experiment, which could be also divided into two phases: 1/initial downregulation, 2/subsequent upregulation, with a demarcation line between these two phases happening after 6–12 h of alcohol exposure. Interestingly, the final outcome after 24 h of alcohol exposure was a significant upregulation of both miR-9-5p and miR-9-3p. One could interpret these results as a neuronal adaptation at the molecular level to the continuous presence of alcohol by attempting to set up a new, overcorrected set-point of miR-9-5p and miR-9-3p expression despite the continued presence of the drug. This is consistent with achieving new stability through change—a tenet of allostasis [60, 61] and the allostatic model of addiction [62].
Regulation of miR-9 precursors and biogenesis pathways by alcohol
Since we observed the presence of the initial phase triggered by a short or continuous exposure lasting about 6 h, we decided to determine whether alcohol differently affects upstream elements of the miR-9 biogenesis pathway (precursors) during that window using two scenarios. First, we used this window as a withdrawal window preceded by the short exposure, second, we used it as an exposure window followed by a 24-hour withdrawal period. As we observed no measurable effect on all three miR-9 pre-precursors’ expression levels during the first scenario, we concluded that the likelihood of alcohol affecting the steps of microRNA biogenesis responsible for the production of pre-precursors from pri-precursors is rather low. However, in scenario 2, alcohol downregulated all three miR-9 pre-precursors suggesting a possibility that alcohol could interfere with one or some of the steps producing pre-precursors from pri-precursors. Production of microRNA pre-precursors starts with pri-precursors cleavage by the Microprocessor machinery, followed by export from the nucleus to the cytoplasm by the exportin5 complex, and capture by Dicer for further processing by the RISC complex [63]. There are many proteins involved in microRNA biogenesis as each microRNA processing complex consists of several proteins. The microprocessor contains Drosha, DGCR8, RIIa and RIIb proteins, and Exportin-5, a mediator of nuclear export that needs a cofactor RanGTP protein [63], while Dicer works with auxiliary proteins TRBP and members of the Argonaute protein family (AGO) to form the RISC complex [63]. It is possible that some of the regulation of precursors by alcohol reported here is due to an alcohol effect on some of these proteins. Indeed, Mulligan [64] showed an association between Drosha and Dicer expression and response to alcohol, while Prins [65] determined that, in the rat hippocampus, alcohol alters Drosha and Dicer expression (also see 18). Moreover, Gedik [66] reported a genetic association of DGCR8, AGO1, and AGO2 alleles with alcohol dependence risk. It would be of great interest to gain a full picture of alcohol regulation of activity of the key elements of the microRNA biogenesis complexes which process precursors.
In order to better understand the temporal regulation of miR-9 expression by alcohol we should also focus our future efforts on the initial steps of the biogenesis, namely, the production of the miR-9 pri-precursors from their respective genes. Our results revealed that even the short alcohol exposure triggered upregulation of pri-mir-9-2, while longer exposure affected the expression of all three miR-9 pri-precursors. At this point we cannot rule out any mechanisms regulating miR-9 gene expression; however, we suspect epigenetic control of the mir-9-2 gene expression by alcohol. Pappalardo-Carter et al. [67] showed that alcohol increases CpG dinucleotide methylation of the mir-9-2 gene promoter. We believe that further, comprehensive studies of the epigenetic regulation of all miR-9 genes by alcohol are fully warranted.
Further studies should also shed some light on the differential regulation of miR-9 expression not only by the temporal aspect of alcohol exposure but also by alcohol concentration. Pappalardo-Carter [67] reported that a high alcohol concentration (130 mM) suppressed miR-9 expression, while Tapocik [68] showed that an alcohol concentration of 70 mM inhibited the expression of miR-9, creating a lower steady-state level in alcohol-dependent rats.
AUD is known to have a genetic component [69, 70]. Because human miR-9-1 and miR-9-3 genes are located near or within the AUD susceptibility loci [8], we believe that exploring the differential effects of alcohol on each miR-9 biogenesis pathway is also of great importance in understanding the genetic predisposition to AUD. We would hypothesize that the first response to alcohol exposure is mostly through the mir-9-2 biogenesis pathway. However, with continuous exposure (longer than 6 h) or possibly multiple exposures (mimicking frequent drinking characteristic of the AUD) the mir-9-2 gene may be eventually substantially turned down, with the remaining contribution shifting to mir-9-1 and mir-9-3 genes. As these two genes combined produce less miR-9 than mir-9-2, this hypothesis would explain lower levels of miR-9 observed in chronic alcohol exposure experiments [67, 68]. This hypothesis would also rationalize the presence of miR-9-1 and miR-9-3 genes in the AUD susceptibility loci.
It is worth mentioning that a deeper understanding of alcohol regulation of miR-9 biogenesis would also benefit research focused on cancer and neurodegenerative diseases. Aberrant levels of miR-9 (either miR-9-5p, miR-9-3p, or both) have been reported in many types of cancer [71]: breast cancer [25, 72, 73], Burkitt’s Lymphoma [24], hepatocarcinoma [74], prostate cancer [75], gastric cancer [76], colorectal cancer [77], as well as Alzheimer’s and Huntington’s diseases [78]. Chronic heavy alcohol consumption increases the risk of all of these cancers and neurodegenerative diseases [79–86].
Summary
AUD is a progressive brain disease. Understanding the temporal effects of alcohol on gene expression in neurons is of great importance. Using murine primary cultures of medium spiny neurons, we attempted to deepen our understanding of temporal regulation by alcohol of expression and biogenesis of miR-9-5p and miR-9-3p, key regulators of gene expression. Based on miR-9-5p and miR-9-3p responses to short alcohol exposure, we concluded that changes in expression of these two microRNAs seem to be consistent with the homeostatic model of addiction, while longer, continuous alcohol exposure evoked possibly allostatic changes. Finally, our results point out that the sensitivity of mir-9 genes to alcohol varies among genes and is also time-dependent. The mir-9-2 gene produces pri-mir-9-2 precursor almost immediately after alcohol exposure, while mir-9-1 and mir-9-3 genes need longer exposure to alcohol. Our studies may help us to understand better mechanisms of addiction, carcinogenesis, and neurodegenerative disorders.
Limitations and future directions
There are several limitations to consider when interpreting the results. We used the primary neuronal culture of the medium spiny neurons harvested from young mice pups’ striatum. One needs to remember that neurons harvested in such a way are taken away from their natural environment of the whole brain “connected” to the whole animal. To preserve more “natural” conditions we could use brain striatal slices, however, their viability over 24 h is poor; we could also consider using whole animals, however, in this model, it is impossible to precisely control alcohol exposure and withdrawal. Thus, with its inherited limitations, this model provides us with precise control over alcohol exposure and withdrawal, as well as direct access to neurons derived from the striatum - a pivotal element of the reward system, which plays a fundamental role in the development of addiction.
Another limiting factor is that neurons harvested from newborn pups are not mature yet and for about 2 weeks correspond to the final in utero period of human development. However, we waited 5 days to harvest the neurons from the striata of P5 pups and then cultivated them on a dish for 8 days before starting alcohol exposure, thus likely passing the period corresponding to the in utero human development.
Another factor to remember is that neurons during a few days after plating undergo proliferation on the culture dish. It has been shown in another model that in the proliferating neurons of the retina miR-9 levels (presumably miR-9-5p) oscillate with a rhythmicity of 3 h [87] meaning the expression of miR-9 follows a sinusoid with the same levels observed every 3 hours. This rhythmicity is transient and stabilizes once the neurons mature. Cultivating neurons on a dish for about a week yields mostly mature neurons. However, it is possible that there are some proliferating neurons still present. Since most of our collection time points were multiplications of three, they were in sync with miR-9 oscillations, thus any miR-9 rhythmicity should have a minimal effect. Therefore, by harvesting MSN from the P5 pups and allowing them for a few days to mature before starting alcohol exposure, we think that we were able to circumnavigate at least some of the shortcomings of this model. Future collections with time intervals shorter than 3 h (or not in sync with 3 h) will require though additional controls.
We measured the expression of miR-9 precursors which are products of mir-9 genes and biogenesis machinery but did not directly study the regulation of gene expression or the machinery activity. Future studies could focus on a systematic approach of determining the alcohol sensitivity of individual elements of microRNA biogenesis (e.g., using antisense oligonucleotides targeting each precursor individually) including temporal characteristics of epigenetic regulation of gene expression by alcohol.
Lastly, we used a single, low-dose alcohol concentration to minimize cellular death. Since higher alcohol concentrations have been shown to also regulate miR-9 expression [67, 68] determination of their effects on miR-9 biogenesis would be of interest.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved the Rutgers Institutional Animal Care and Use Committee (IACUC Protocol # 10-024).
Author contributions
EM, YW, and AZP conceived the idea and planned the experiments; EM, APT, RK, EB-H, VL, and AB performed sample preparations, carried out all experiments except digital PCR experiments, and contributed to the interpretation of the results; EM and YW supervised experiments; SP performed and interpreted digital PCR experiments; EM and AZP interpreted the results and wrote the manuscript; SB and HTS provided critical feedback and contributed to the final version of the manuscript; AZP supervised the project and received grant support. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from NIH-NIAAA grant #AA017920 to AZP.
Acknowledgments
We would like to thank Giles Martin at U. Mass Medical School and Troy Roepke at Rutgers-New Brunswick for assistance with neuron culture protocol development. We would like to thank Jennifer Keefer, Jessica Beck, and Anshul Patel for their assistance with the experiments. We would like to thank Patricia Hegerich for supervising digital PCR experiments during her tenure at Thermo Fisher Scientific Inc. We thank Rutgers Bartlett Hall Animal Care facilities staff for mouse colony maintenance.
Conflict of interest
Author SP was employed by company Thermo Fisher Scientific Inc.
Digital PCR runs (QuantStudio 3D; for research use only, not for use in diagnostic procedures) were conducted by Life Technologies, currently a subsidiary of Thermo Fisher Scientific Inc., as part of an Innovation Grant in the Digital PCR Applications Grant Program awarded to AZP.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/adar.2023.11323/full#supplementary-material
References
1. World Health Organization (WHO). Global status report on alcohol and Health (2018). Available at: https://www.who.int/publications/i/item/9789241565639 (Accessed March 4, 2023).
2. Centers for Disease Control and Prevention (CDC). Alcohol use and Health (2012). Available at: http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm (Accessed March 3, 2023).
3. National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIH-NIAAA). Alcohol facts and statistics (2021). Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-andstatistics (Accessed March 4, 2023).
4. Berkel, TD, and Pandey, SC. Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res (2017) 41(4):666–80. doi:10.1111/acer.13338
5. Farh, KK, Grimson, A, Jan, C, Lewis, BP, Johnston, WK, Lim, LP, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science (2005) 310(5755):1817–21. doi:10.1126/science.1121158
6. Shu, J, Silva, BVRE, Gao, T, Xu, Z, and Cui, J. Dynamic and modularized MicroRNA regulation and its implication in human cancers. Sci Rep (2017) 7(1):13356. doi:10.1038/s41598-017-13470-5
7. Wang, H, Taguchi, YH, and Liu, X. Editorial: miRNAs and neurological diseases. Front Neurol (2021) 12:662373. doi:10.3389/fneur.2021.662373
8. Pietrzykowski, AZ. The role of microRNAs in drug addiction: A big lesson from tiny molecules. Int Rev Neurobiol (2010) 91:1–24. doi:10.1016/S0074-7742(10)91001-5
9. Smith, ACW, and Kenny, PJ. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav (2018) 17(3):e12424. doi:10.1111/gbb.12424
10. Zhao, Y, Qin, F, Han, S, Li, S, Zhao, Y, Wang, H, et al. MicroRNAs in drug addiction: Current status and future perspectives. Pharmacol Ther (2022) 236:108215. doi:10.1016/j.pharmthera.2022.108215
11. Most, D, Workman, E, and Harris, RA. Synaptic adaptations by alcohol and drugs of abuse: Changes in microRNA expression and mRNA regulation. Front Mol Neurosci (2014) 7:85. doi:10.3389/fnmol.2014.00085
12. Pietrzykowski, AZ, Martin, GE, Puig, SI, Knott, TK, Lemos, JR, and Treistman, SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: Decreased ethanol potentiation and decreased channel density. J Neurosci (2004) 24(38):8322–32. doi:10.1523/JNEUROSCI.1536-04.2004
13. Santos-Bezerra, DP, Cavaleiro, AM, Santos, AS, Suemoto, CK, Pasqualucci, CA, Jacob-Filho, W, et al. Alcohol use disorder is associated with upregulation of MicroRNA-34a and MicroRNA-34c in hippocampal postmortem tissue. Alcohol Clin Exp Res (2021) 45(1):64–8. doi:10.1111/acer.14505
14. Tsujimura, K, Shiohama, T, and Takahashi, E. microRNA biology on brain development and neuroimaging approach. Brain Sci (2022) 12(10):1366. doi:10.3390/brainsci12101366
15. Prochnik, SE, Rokhsar, DS, and Aboobaker, AA. Evidence for a microRNA expansion in the bilaterian ancestor. Dev Genes Evol (2007) 217(1):73–7. doi:10.1007/s00427-006-0116-1
16. Mead, EA, Boulghassoul-Pietrzykowska, N, Wang, Y, Anees, O, Kinstlinger, NS, Lee, M, et al. Non-invasive microRNA profiling in saliva can serve as a biomarker of alcohol exposure and its effects in humans. Front Genet (2022) 12:804222. doi:10.3389/fgene.2021.804222
17. Pietrzykowski, AZ, Friesen, RM, Martin, GE, Puig, SI, Nowak, CL, Wynne, PM, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron (2008) 59(2):274–87. doi:10.1016/j.neuron.2008.05.032
18. Miranda, RC. MicroRNAs and ethanol toxicity. Int Rev Neurobiol (2014) 115:245–84. doi:10.1016/B978-0-12-801311-3.00007-X
19. Coolen, M, Katz, S, and Bally-Cuif, L. miR-9: a versatile regulator of neurogenesis. Front Cel Neurosci (2013) 7:220. doi:10.3389/fncel.2013.00220
21. Chen, X, Zhu, L, Ma, Z, Sun, G, Luo, X, Li, M, et al. Oncogenic miR-9 is a target of erlotinib in NSCLCs. Sci Rep (2015) 5:17031. doi:10.1038/srep17031
22. Ramachandran, D, Roy, U, Garg, S, Ghosh, S, Pathak, S, and Kolthur-Seetharam, U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J (2011) 278(7):1167–74. doi:10.1111/j.1742-4658.2011.08042.x
23. Wang, Y, Dong, L, Wan, F, Chen, F, Liu, D, Chen, D, et al. MiR-9-3p regulates the biological functions and drug resistance of gemcitabine-treated breast cancer cells and affects tumor growth through targeting MTDH. Cell Death Dis (2021) 12(10):861. doi:10.1038/s41419-021-04145-1
24. Onnis, A, De Falco, G, Antonicelli, G, Onorati, M, Bellan, C, Sherman, O, et al. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PLoS One (2010) 5(9):e12960. doi:10.1371/journal.pone.0012960
25. Zawistowski, JS, Nakamura, K, Parker, JS, Granger, DA, Golitz, BT, and Johnson, GL. MicroRNA 9-3p targets β1 integrin to sensitize claudin-low breast cancer cells to MEK inhibition. Mol Cel Biol (2013) 33(11):2260–74. doi:10.1128/MCB.00269-13
26. Sim, SE, Lim, CS, Kim, JI, Seo, D, Chun, H, Yu, NK, et al. The brain-enriched MicroRNA miR-9-3p regulates synaptic plasticity and memory. J Neurosci (2016) 36(33):8641–52. doi:10.1523/JNEUROSCI.0630-16.2016
27. Tepper, JM, and Bolam, JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol (2004) 14(6):685–92. doi:10.1016/j.conb.2004.10.003
28. García-González, D, Dumitru, I, Zuccotti, A, Yen, TY, Herranz-Pérez, V, Tan, LL, et al. Neurogenesis of medium spiny neurons in the nucleus accumbens continues into adulthood and is enhanced by pathological pain. Mol Psychiatry (2021) 26(9):4616–32. doi:10.1038/s41380-020-0823-4
29. Ventimiglia, R, and Lindsay, RM. Rat striatal neurons in low-density, serum-free culture. In: Goslin, GBAK, editor. Culturing nerve cells. Cambridge, MA: MIT Press (1998). p. 371–93.
30. Misonou, H, and Trimmer, JS. Determinants of voltage-gated potassium channel surface expression and localization in Mammalian neurons. Crit Rev Biochem Mol Biol (2004) 39(3):125–45. doi:10.1080/10409230490475417
31. Leveque, JC, Macias, W, Rajadhyaksha, A, Carlson, RR, Barczak, A, Kang, S, et al. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci (2000) 20(11):4011–20. doi:10.1523/JNEUROSCI.20-11-04011.2000
32. Franklin, KBJ, and Paxinos, G. The mouse brain in stereotaxic coordinates, 3rd ed. San Diego: Academic Press (2008). p. 351.
33. Ljungman, M. The transcription stress response. Cell Cycle (2007) 6(18):2252–7. doi:10.4161/cc.6.18.4751
34. Mao, Y, Tamura, T, Yuki, Y, Abe, D, Tamada, Y, Imoto, S, et al. The hnRNP-Htt axis regulates necrotic cell death induced by transcriptional repression through impaired RNA splicing. Cel Death Dis (2016) 7(4):e2207. doi:10.1038/cddis.2016.101
35. Calculator.net. Blood alcohol concentration (BAC) calculator (2023). Available at: https://www.calculator.net/bac-calculator.html (Accesed January 15, 2023).
36. Aydin Sinirlioğlu, Z, and Akbaş, F. Differential expression of BDNF, MiR-206, and MiR-9 under the chronic ethanol exposure and its withdrawal. Sigma J Eng Nat Sci (2016) 34(2):191–6.
37. Iguchi, T, Niino, N, Tamai, S, Sakurai, K, and Mori, K. Absolute quantification of plasma microRNA levels in cynomolgus monkeys, using quantitative real-time Reverse transcription PCR. J Vis Exp (2018) 132:56850. doi:10.3791/56850
38. Arabkari, V, Clancy, E, Dwyer, RM, Kerin, MJ, Kalinina, O, Holian, E, et al. Relative and absolute expression analysis of microRNAs associated with luminal A breast cancer – a comparison. Pathol Oncol Res (2020) 26:833–44. doi:10.1007/s12253-019-00627-y
39. Wang, J, Jiang, D, Rao, H, Zhao, J, Wang, Y, and Wei, L. Absolute quantification of serum microRNA-122 and its correlation with liver inflammation grade and serum alanine aminotransferase in chronic hepatitis C patients. Int J Infect Dis (2015) 30:52–6. doi:10.1016/j.ijid.2014.09.020
40. Wang, LL, Zhang, Z, Li, Q, Yang, R, Pei, X, Xu, Y, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod (2009) 24(3):562–79. doi:10.1093/humrep/den439
41. Burrowes, SG, Salem, NA, Tseng, AM, Balaraman, S, Pinson, MR, Garcia, C, et al. The BAF (BRG1/BRM-Associated Factor) chromatin-remodeling complex exhibits ethanol sensitivity in fetal neural progenitor cells and regulates transcription at the miR-9-2 encoding gene locus. Alcohol (2017) 60:149–58. doi:10.1016/j.alcohol.2017.01.003
42. Packer, AN, Xing, Y, Harper, SQ, Jones, L, and Davidson, BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J Neurosci (2008) 28(53):14341–6. doi:10.1523/JNEUROSCI.2390-08.2008
43. Roese-Koerner, B, Stappert, L, Berger, T, Braun, NC, Veltel, M, Jungverdorben, J, et al. Reciprocal regulation between bifunctional miR-9/9(∗) and its transcriptional modulator notch in human neural stem cell self-renewal and differentiation. Stem Cel Rep (2016) 7(2):207–19. doi:10.1016/j.stemcr.2016.06.008
44. Miranda, RC, Pietrzykowski, AZ, Tang, Y, Sathyan, P, Mayfield, D, Keshavarzian, A, et al. MicroRNAs: Master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res (2010) 34(4):575–87. doi:10.1111/j.1530-0277.2009.01126.x
45. Radhakrishnan, B, and Alwin Prem Anand, A. Role of miRNA-9 in brain development. J Exp Neurosci (2016) 10:101–20. doi:10.4137/JEN.S32843
46. Kim, CK, Asimes, A, Zhang, M, Son, BT, Kirk, JA, and Pak, TR. Differential stability of miR-9-5p and miR-9-3p in the brain is determined by their unique cis- and trans-acting elements. eNeuro (2020) 7(3):ENEURO0094-20. doi:10.1523/ENEURO.0094-20.2020
47. Humphries, MD, and Prescott, TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol (2010) 90:385–417. doi:10.1016/j.pneurobio.2009.11.003
48. Wassum, KM, and Izquierdo, A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev (2015) 57:271–83. doi:10.1016/j.neubiorev.2015.08.017
49. Klenowski, PM. Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict Behav (2018) 77:102–6. doi:10.1016/j.addbeh.2017.09.024
50. Fritz, M, Klawonn, AM, and Zahr, NM. Neuroimaging in alcohol use disorder: From mouse to man. J Neuro Res (2022) 100:1140–58. doi:10.1002/jnr.24423
51. Marty, VN, and Spigelman, I. Effects of alcohol on the membrane excitability and synaptic transmission of medium spiny neurons in the nucleus accumbens. Alcohol (2012) 46(4):317–27. doi:10.1016/j.alcohol.2011.12.002
52. Kolpakova, J, van der Vinne, V, Giménez-Gómez, P, Le, T, You, IJ, Zhao-Shea, R, et al. Binge alcohol drinking alters synaptic processing of executive and emotional information in core nucleus accumbens medium spiny neurons. Front Cel Neuro (2021) 15:742207. doi:10.3389/fncel.2021.742207
53. Knabbe, J, Protzmann, J, Schneider, N, Berger, M, Dannehl, D, Wei, S, et al. Single-dose ethanol intoxication causes acute and lasting neuronal changes in the brain. Proc Natl Acad Sci U S A (2022) 119(25):e2122477119. doi:10.1073/pnas.2122477119
54. Harrison, NL, Skelly, MJ, Grosserode, EK, Lowes, DC, Zeric, T, Phister, S, et al. Effects of acute alcohol on excitability in the CNS. Neuropharm (2017) 122:36–45. doi:10.1016/j.neuropharm.2017.04.007
55. Xue, Q, Yu, C, Wang, Y, Liu, L, Zhang, K, Fang, C, et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci Rep (2016) 6:26781. doi:10.1038/srep26781
56. Martinez, M, Rossetto, IMU, Arantes, RMS, Lizarte, FSN, Tirapelli, LF, Tirapelli, DPC, et al. Serum miRNAs are differentially altered by ethanol and caffeine consumption in rats. Toxicol Res (Camb) (2019) 8(6):842–9. doi:10.1039/c9tx00069k
57. Balaraman, S, Tingling, JD, Tsai, PC, and Miranda, RC. Dysregulation of microRNA expression and function contributes to the etiology of fetal alcohol spectrum disorders. Alcohol Res (2013) 35(1):18–24.
58. Abrajano, JJ, Qureshi, IA, Gokhan, S, Zheng, D, Bergman, A, and Mehler, MF. REST and CoREST modulate neuronal subtype specification, maturation and maintenance. PLoS One (2009) 4(12):e7936. doi:10.1371/journal.pone.0007936
59. Abrajano, JJ, Qureshi, IA, Gokhan, S, Molero, AE, Zheng, D, Bergman, A, et al. Corepressor for element-1-silencing transcription factor preferentially mediates gene networks underlying neural stem cell fate decisions. Proc Natl Acad Sci U S A (2010) 107(38):16685–90. doi:10.1073/pnas.0906917107
60. Sterling, P, and Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher, S, and Reason, J, editors. Handbook of life stress, cognition and health. New York: John Wiley & Sons (1988). p. 629–49.
61. Ramsay, DS, and Woods, SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev (2014) 121(2):225–47. doi:10.1037/a0035942
62. Koob, GF, and Moal, ML. Drug addiction and allostasis. In: Schulkin, J, editor. Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge: Cambridge University Press (2004). p. 150–63.
63. Bushati, N, and Cohen, SM. microRNA functions. Annu Rev Cel Dev Biol (2007) 23:175–205. doi:10.1146/annurev.cellbio.23.090506.123406
64. Mulligan, MK, Dubose, C, Yue, J, Miles, MF, Lu, L, and Hamre, KM. Expression, covariation, and genetic regulation of miRNA Biogenesis genes in brain supports their role in addiction, psychiatric disorders, and disease. Front Genet (2013) 4:126. doi:10.3389/fgene.2013.00126
65. Prins, SA, Przybycien-Szymanska, MM, Rao, YS, and Pak, TR. Long-term effects of peripubertal binge EtOH exposure on hippocampal microRNA expression in the rat. PLoS One (2014) 9(1):e83166. doi:10.1371/journal.pone.0083166
66. Gedik, H, Erdal, ME, Yilmaz, SG, Sengul, C, Sengul, CB, and Herken, H. Association of microRNA biogenesis pathway gene variants and alcohol dependence risk. DNA Cel Biol (2015) 34(3):220–6. doi:10.1089/dna.2014.2549
67. Pappalardo-Carter, DL, Balaraman, S, Sathyan, P, Carter, ES, Chen, WJ, and Miranda, RC. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: Evidence from zebrafish and murine fetal neural stem cell models. Alcohol Clin Exp Res (2013) 37(10):1657–67. doi:10.1111/acer.12139
68. Tapocik, JD, Solomon, M, Flanigan, M, Meinhardt, M, Barbier, E, Schank, JR, et al. Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. Pharmacogenomics J (2013) 13(3):286–96. doi:10.1038/tpj.2012.17
69. Chen, J, Hutchison, KE, Bryan, AD, Filbey, FM, Calhoun, VD, Claus, ED, et al. Opposite epigenetic associations with alcohol use and exercise intervention. Front Psychiatry (2018) 9:594. doi:10.3389/fpsyt.2018.00594
70. Luo, A, Jung, J, Longley, M, Rosoff, DB, Charlet, K, Muench, C, et al. Epigenetic aging is accelerated in alcohol use disorder and regulated by genetic variation in APOL2. Neuropsychopharm (2020) 45(2):327–36. doi:10.1038/s41386-019-0500-y
71. Khafaei, M, Rezaie, E, Mohammadi, A, Shahnazi Gerdehsang, P, Ghavidel, S, Kadkhoda, S, et al. miR-9: From function to therapeutic potential in cancer. J Cel Physiol (2019) 234(9):14651–65. doi:10.1002/jcp.28210
72. Ma, L, Young, J, Prabhala, H, Pan, E, Mestdagh, P, Muth, D, et al. miR-9, a MYC/MYCN activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cel Biol (2010) 12(3):247–56. doi:10.1038/ncb2024
73. Khew-Goodall, Y, and Goodall, GJ. Myc-modulated miR-9 makes more metastases. Nat Cel Biol (2010) 12(3):209–11. doi:10.1038/ncb0310-209
74. Zhang, Y, Wei, C, Guo, CC, Bi, RX, Xie, J, Guan, DH, et al. Prognostic value of microRNAs in hepatocellular carcinoma: A meta-analysis. Oncotarget (2017) 8(63):107237–57. doi:10.18632/oncotarget.20883
75. Sang, Z, Jiang, X, Guo, L, and Yin, G. MicroRNA-9 suppresses human prostate cancer cell viability, invasion and migration via modulation of mitogen-activated protein kinase kinase kinase 3 expression. Mol Med Rep (2019) 19(5):4407–18. doi:10.3892/mmr.2019.10065
76. Hang, C, Yan, HS, Gong, C, Gao, H, Mao, QH, and Zhu, JX. MicroRNA-9 inhibits gastric cancer cell proliferation and migration by targeting neuropilin-1. Exp Ther Med (2019) 18(4):2524–30. doi:10.3892/etm.2019.7841
77. Bahrami, A, Jafari, A, and Ferns, GA. The dual role of microRNA-9 in gastrointestinal cancers: oncomiR or tumor suppressor? Biomed Pharmacother (2022) 145:112394. doi:10.1016/j.biopha.2021.112394
78. Wang, S, Jiang, G, and Wang, S. Neuroprotective role of MiRNA-9 in neurological diseases: A mini review. Curr Mol Med (2022) 23. doi:10.2174/1566524023666221025123132
79. Wong, AW, Paulson, QX, Hong, J, Stubbins, RE, Poh, K, Schrader, E, et al. Alcohol promotes breast cancer cell invasion by regulating the Nm23-ITGA5 pathway. J Exp Clin Cancer Res (2011) 30(1):75. doi:10.1186/1756-9966-30-75
80. Morch, LS, Johansen, D, Thygesen, LC, Tjonneland, A, Lokkegaard, E, Stahlberg, C, et al. Alcohol drinking, consumption patterns and breast cancer among Danish nurses: A cohort study. Eur J Pub Health (2007) 17(6):624–9. doi:10.1093/eurpub/ckm036
81. Testino, G, Leone, S, and Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J Gastroenterol (2014) 20(43):15943–54. doi:10.3748/wjg.v20.i43.15943
82. Macke, AJ, and Petrosyan, A. Alcohol and prostate cancer: Time to draw conclusions. Biomolecules (2022) 12(3):375. doi:10.3390/biom12030375
83. Li, BY, Li, HY, Zhou, DD, Huang, SY, Luo, M, Gan, RY, et al. Effects of different green tea extracts on chronic alcohol induced-fatty liver disease by ameliorating oxidative stress and inflammation in mice. Oxid Med Cel Longev (2021) 2021:5188205. doi:10.1155/2021/5188205
84. Dashti, SG, Win, AK, Hardikar, SS, Glombicki, SE, Mallenahalli, S, Thirumurthi, S, et al. Physical activity and the risk of colorectal cancer in Lynch syndrome. Int J Cancer (2018) 143(9):2250–60. doi:10.1002/ijc.31611
85. Peng, B, Yang, Q, B Joshi, R, Liu, Y, Akbar, M, Song, BJ, et al. Role of alcohol drinking in alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Int J Mol Sci (2020) 21(7):2316. doi:10.3390/ijms21072316
86. Byars, JA, Beglinger, LJ, Moser, DJ, Gonzalez-Alegre, P, and Nopoulos, P. Substance abuse may be a risk factor for earlier onset of Huntington disease. J Neurol (2012) 259(9):1824–31. doi:10.1007/s00415-012-6415-8
Keywords: addiction, digital PCR, alcohol adaptation, microRNA miR-9, medium spiny neurons
Citation: Mead EA, Wang Y, Patel S, Thekkumthala AP, Kepich R, Benn-Hirsch E, Lee V, Basaly A, Bergeson S, Siegelmann HT and Pietrzykowski AZ (2023) miR-9 utilizes precursor pathways in adaptation to alcohol in mouse striatal neurons. Adv. Drug Alcohol Res. 3:11323. doi: 10.3389/adar.2023.11323
Received: 01 March 2023; Accepted: 16 May 2023;
Published: 06 June 2023.
Edited by:
Emmanuel Onaivi, William Paterson University, United StatesReviewed by:
Jim Tiao, Pathwest Laboratory Medicine, AustraliaDavid Turner, University of Michigan, United States
Copyright © 2023 Mead, Wang, Patel, Thekkumthala, Kepich, Benn-Hirsch, Lee, Basaly, Bergeson, Siegelmann and Pietrzykowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrzej Zbigniew Pietrzykowski, YW5kcmUuei5waWV0QGdtYWlsLmNvbQ==
†Present address: Edward Andrew Mead, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, United States;
Yongping Wang, Holmdel Township School, Holmdel, NJ, United States;
Austin P. Thekkumthala, Bristol-Myers Squibb, New Brunswick, NJ, United States;
Rebecca Kepich, Syneos Health, Morrisville, NC, United States;
Elizabeth Benn-Hirsch, Ross University School of Medicine, Portsmith, Dominica;
Victoria Lee, JP Morgan Chase, Jersey City, NJ, United States;
Azra Basaly, Fresenius Medical Care, Waltham, MA, United States;
Hava T. Siegelmann, Biologically Inspired Neural & Dynamical Systems Laboratory, The Manning College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, United States;
Andrzej Zbigniew Pietrzykowski, Weight & Life MD, LLC, Hamilton, NJ, United States;
Biologically Inspired Neural & Dynamical Systems Laboratory, The Manning College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, United States
 Edward Andrew Mead1†
Edward Andrew Mead1† Andrzej Zbigniew Pietrzykowski
Andrzej Zbigniew Pietrzykowski