- 1Department of Radiodiagnosis and Imaging, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- 2Department of Pulmonary Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Background: Many survivors of severe COVID-19 pneumonia experience lingering respiratory issues. There is limited research on follow-up chest imaging findings in patients with COVID-19 ARDS, particularly in relation to their mMRC dyspnea scores and pulmonary function tests (PFTs). This study addresses this gap by investigating the clinical characteristics, mMRC dyspnea scores, PFTs, and chest CT findings of COVID-19 ARDS patients at the 6 months post-recovery. By analyzing these variables together, we aim to gain a better understanding of the long-term health consequences of COVID-19 ARDS.
Methods: This prospective observational study included 56 subjects with COVID-19 ARDS with dyspnea at the six-month follow-up visits. These patients were evaluated by chest CT, mMRC dyspnea scale, and PFT. The CT severity score was calculated individually for each of the four major imaging findings - ground glass opacities (GGOs), parenchymal/atelectatic bands, reticulations/septal thickening, and consolidation - using a modified CT severity scoring system. Statistics were carried out to find any association between individual CT chest findings and the mMRC dyspnea scale and forced vital capacity (FVC). p values < 0.05 were considered statistically significant.
Results: Our study population had a mean age of 55.86 ± 9.60 years, with 44 (78.6%) being men. Grades 1, 2, 3, and 4 on the mMRC dyspnea scale were seen in 57.1%, 30.4%, 10.7%, and 1.8% of patients respectively. Common CT findings observed were GGOs (94.6%), reticulations/septal thickening (96.4%), parenchymal/atelectatic bands (92.8%), and consolidation (14.3%). The mean modified CT severity scores for GGOs, reticulations/septal thickening, parenchymal/atelectatic bands, and consolidation were 10.32 ± 5.51 (range: 0–21), 7.66 ± 4.33 (range: 0–19), 4.77 ± 3.03 (range: 0–14) and 0.29 ± 0.91 (range 0–5) respectively. Reticulations/septal thickening (p = 0.0129) and parenchymal/atelectatic bands (p = 0.0453) were associated with an increased mMRC dyspnea scale. Parenchymal/atelectatic bands were also associated with abnormal FVC (<80%) (p = 0.0233).
Conclusion: Six-month follow-up chest CTs of COVID-19 ARDS survivors with persistent respiratory problems showed a statistically significant relationship between increased mMRC dyspnea score and imaging patterns of reticulations/septal thickening and parenchymal/atelectatic bands; while parenchymal/atelectatic bands also showed a statistically significant correlation with reduced FVC.
Introduction
The impact of the COVID-19 pandemic has transcended the initial phase of infection, with mounting scientific and clinical evidence of persistent illness in a significant number of patients following recovery from acute illness [1–4]. Frequently observed long-term effects of COVID-19 include fatigue, shortness of breath, cough, chest pain, joint pain, and cognitive difficulties [3–5]. Such a diverse array of long-term health sequelae has been given many names such as “Long COVID,” “Chronic COVID,” “Ongoing Symptomatic COVID-19,” “Post-Acute COVID-19 Syndrome,” “Post-Acute Sequelae of COVID-19” and “Post-COVID-19 condition” [3–7]. While a unified nomenclature and definition to describe this condition remain elusive, the term “Long COVID” has garnered increasing traction within the medical community [3, 4, 6, 7].
Chest computed tomography (CT) has played a critical role in the evaluation of lung changes in COVID-19 survivors [3, 4, 8–10]. The burden of Long COVID is enormous, with persistent lung parenchymal abnormalities seen in up to one-third of COVID-19 hospitalized patients, as reported in recent research and meta-analyses [11, 12]. Ground-glass opacities (GGOs), septal thickening, parenchymal/atelectatic bands, and traction bronchiectasis are the most common radiological abnormalities seen in these patients [1, 2, 10–15].
However, studies specifically investigating the long-term outcomes of patients with COVID-19 acute respiratory distress syndrome (ARDS) are limited. Our present study evaluated clinical features, pulmonary function tests (PFTs), and lung parenchymal abnormalities on chest CT at the six-month follow-up of Long COVID patients who had developed COVID-19 ARDS during the acute phase. Additionally, we investigated potential associations between these CT findings and the modified Medical Research Council (mMRC) dyspnea scale and forced vital capacity (FVC).
Materials and Methods
Study Population
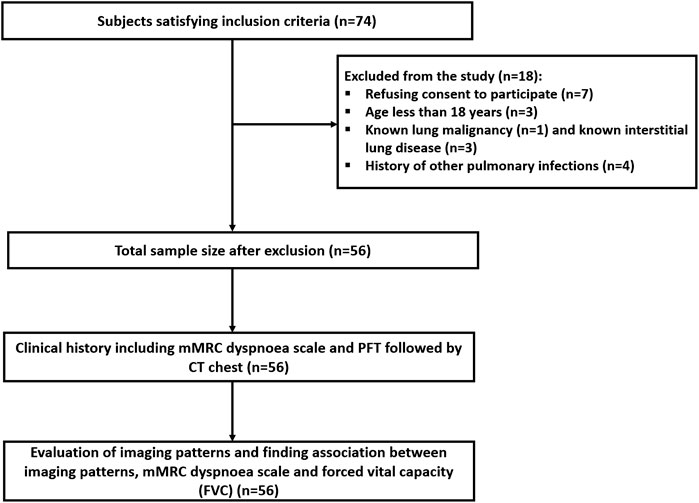
This was a single-center, prospective observational study approved by the institutional ethics committee (IEC number - INT/IEC/2021/SPL-288) and carried out from December 2021 to August 2022. A total of 74 participants who developed acute-phase COVID-19 ARDS, and had persistent respiratory symptoms in the form of dyspnea (shortness of breath) 6 months after discharge, were screened for inclusion. The Berlin criteria for ARDS were taken into consideration for inclusion [16]. Patients with a known history of other pulmonary co-morbidities (pulmonary tuberculosis, pulmonary mucormycosis, pulmonary aspergillosis, any superimposed infection during the acute phase, cystic or interstitial lung disease, and known malignancies), aged less than 18 and who refused to participate in the study were excluded. A total of 56 participants were finally enrolled in the study after exclusion (Figure 1). At the six-month follow-up, participants were evaluated for dyspnea according to the mMRC dyspnea scale [17], PFTs, and abnormalities seen on the chest CT. FVC values obtained from PFT were graded 0 for FVC ≥80% of total lung capacity (TLC) and 1 for FVC <80% of TLC.
The mMRC dyspnea scale [17] is a quick and easy way to measure shortness of breath (dyspnea). As the score increases, breathlessness worsens: grade 0, breathless only with strenuous exertion; grade 1, slightly breathless when hurrying on flat ground or walking uphill; grade 2, walks slower than others due to shortness of breath or needs to stop to catch breath when walking at a normal pace; grade 3, stops to catch breath after about 100 yards (91 m) or a few minutes of walking on flat ground; grade 4, too breathless to leave the house or even to dress or undress. Higher scores on the mMRC scale indicate a greater limitation of daily activities due to shortness of breath.
Chest CT Acquisition Protocol and Interpretation
All the participants underwent a non-contrast chest CT on a 256-slice CT scanner (Philips Brilliance-iCT256; Koninklijke Philips N.V., Netherlands). Scanning was performed under deep inspiration, from the level of the thoracic inlet to the domes of the diaphragm in helical mode with a fixed tube voltage of 120 kV and automatic exposure control (AEC) modulated tube current. The images were then reconstructed from the helical dataset into 1 mm sections in 1 mm increments using the iDose level 6 hybrid iterative reconstruction software.
All scans were randomly assigned and then independently assessed by two chest radiologists (MG and NP) with 25 and 15 years of experience respectively, who were blinded to the patients’ clinical and laboratory data. Disagreements were resolved through discussion and mutual consensus. Each imaging finding was entered in the data sheet.
We implemented a novel, modified CT severity scoring system (Table 1), building upon the original scoring system by Pan et al. [18]. Lung involvement was assessed on a lobar basis, employing a 0–25 point scale; however, an independent score was given to each radiological finding. We scored the four most prevalent CT imaging findings seen in these patients - GGOs, consolidation, parenchymal/atelectatic bands, and reticulations/septal thickening. Additionally, traction bronchiectasis was assigned a binary score of 0 (absent) or 1 (present). These radiological terms were used according to the current recommendations of the Fleischner Society glossary for accurate and precise imaging descriptions of the imaging patterns [19].
Statistical Analysis
Data were entered into a Microsoft Excel spreadsheet. IBM SPSS Statistics Version 22.0 was used for the analysis. Categorical data were presented as counts and percentages. The mean and Standard Deviation were used to depict normally distributed data. The Wilcoxon signed rank test and the Kruskal-Wallis rank test were used to compare various variables. Fischer’s Exact test was used for count data (traction bronchiectasis). Statistical significance was defined as a p-value of less than 0.05.
Results
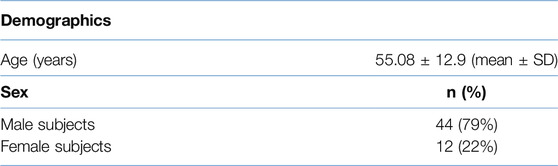
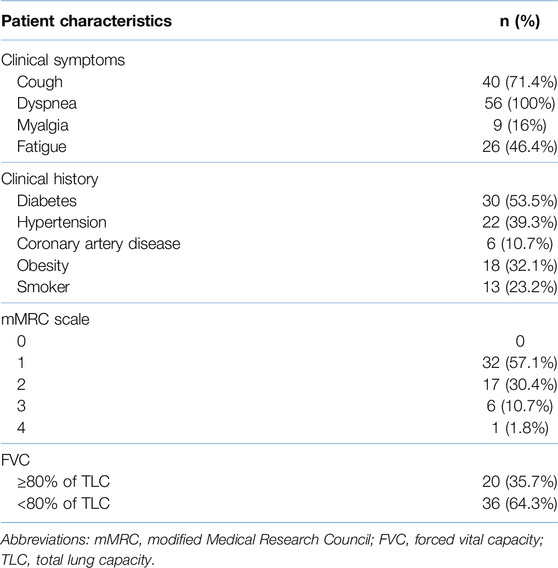
The mean age of our study population was 55.86 ± 9.60 years (mean ± SD) with 44 (78.6%) patients being male. Cough, fatigue, and myalgia were present in 71.4%, 46.4%, and 16% of the participants, respectively, at the six-month follow-up visit. The most frequent comorbidity in the study cohort was diabetes (53.5%). Only one participant (1.8%) had class 4 dyspnea, while 57.1%, 30.4%, and 10.7% of the subjects had grade 1, 2, and 3 dyspnea respectively as per the mMRC dyspnea scale. PFTs revealed an FVC of <80% in 36 (64.3%) subjects. Patient demographics and clinical characteristics are elaborated in Tables 2, 3.
The mean time between the CT scan and patient discharge was 206.5 ± 28.8 days. The most common imaging patterns seen at six-month follow-up were reticulations/septal thickening (96.4%), GGOs (94.6%), parenchymal bands (92.8%), traction bronchiectasis (16%), and consolidation (14.3%). This is shown in the bar diagram in Figure 2. There was considerable overlap in the imaging patterns, with two or more of these patterns seen within a single lobe in many patients. Figures 3, 4 illustrate the lung parenchymal abnormalities identified on chest CTs done at six-month follow-up in our study cohort. The mean modified CT severity score for GGOs was 10.32 ± 5.51 (range 0–21), followed by 7.66 ± 4.33 (range 0–19) for reticulations/septal thickening, 4.77 ± 3.03 (range 0–14) for parenchymal/atelectatic bands and 0.29 ± 0.91 (range 0–5) for consolidation.
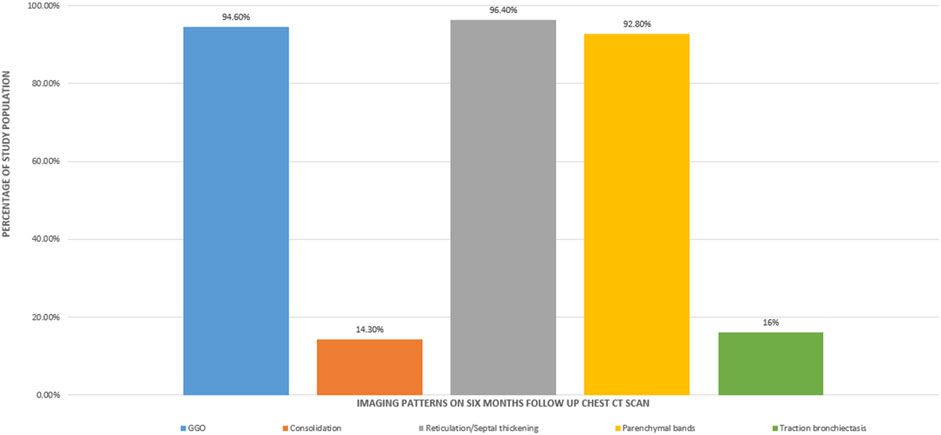
Figure 2. Bar diagram showing the imaging patterns observed at the six-month follow-up CT scan in patients recovering from COVID-19 ARDS.
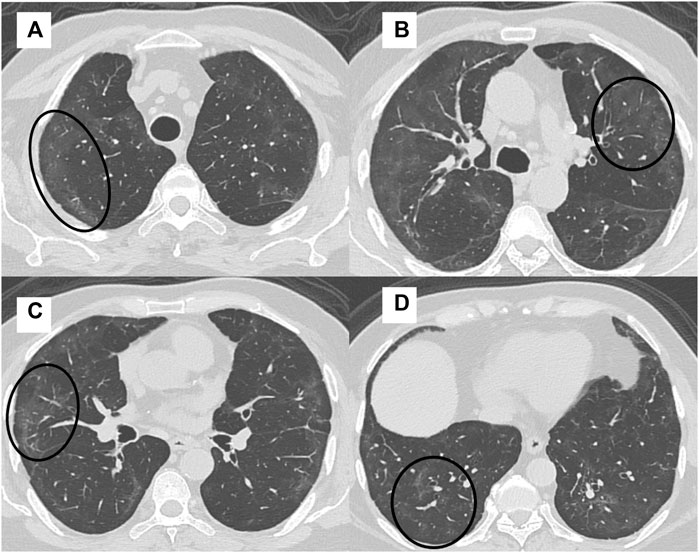
Figure 3. A 41-year-old man with intermittent cough, fatigue, and mMRC class 1 dyspnea had an FVC of 78% at 6 month follow up after COVID-19 ARDS. High-resolution CT (HRCT) chest axial sections (A–D) showed multifocal areas of GGOs in both lungs (black circles).
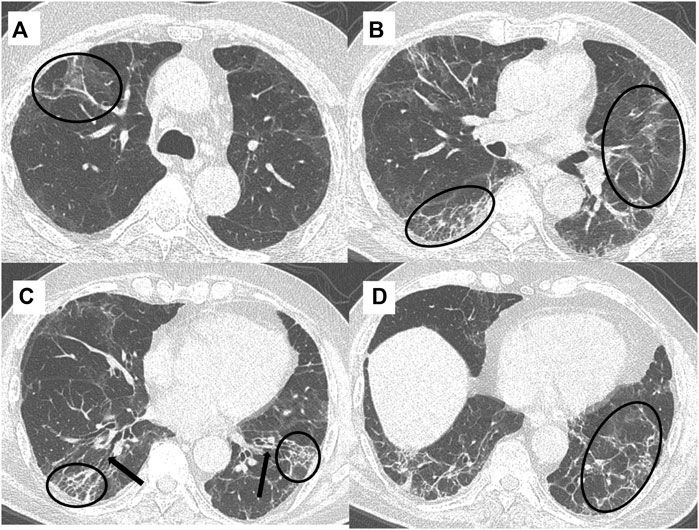
Figure 4. A 49-year-old, COVID-19 ARDS recovered male patient presented with persistent cough and mMRC class 3 dyspnea at six-month follow-up. Spirometry showed an FVC of 60% and high-resolution chest CT (HRCT) axial sections (A–D) showed the presence of reticulations/septal thickening and parenchymal bands (black circles), and traction bronchiectasis (black arrows) interspersed with patchy areas of background GGOs.
Association Between CT Findings and Dyspnea Scores at Six-Month Follow-Up
The association between common CT findings including GGOs, reticulations/septal thickening, parenchymal/atelectatic bands, consolidation, and traction bronchiectasis and the mMRC dyspnea scale was evaluated for statistical significance and is summarized in Table 4. With increasing mean CT severity scores of reticulations/septal thickening and parenchymal/atelectatic bands, there were increasing grades of dyspnea on the mMRC scale (from 1 to 3), and these associations were statistically significant (p-value < 0.05). However, the association between traction bronchiectasis, GGOs, and consolidation with dyspnea scores was not significant.
Association Between CT Findings and PFTs at Six-Month Follow-Up
The association between CT findings and FVC was also evaluated for statistical significance. In patients with abnormal FVC values (<80%), the mean CT severity score of parenchymal/atelectatic bands was found to be increasing and this association was statistically significant (p-value < 0.024). However, there was no significant association seen between FVC and traction bronchiectasis, GGOs, consolidation, or reticulations/septal thickening (Table 5).
Discussion
A significant number of COVID-19 survivors continue to experience long-term health problems such as myalgia, fatigue, dyspnea, and cough with some individuals facing even more debilitating organ injuries [3, 4, 20–22]. Such patients often require close clinical, laboratory, and imaging follow-up to monitor the disease progression and identify potential complications. Several studies have shown that chest CT is a valuable imaging tool for assessing lung damage in patients with persistent respiratory symptoms after COVID-19 [1, 3, 11, 13, 20–22].
According to our prospective study, fatigue followed by dyspnea and cough were the most common symptoms at six-month follow-up in our cohort. This is in agreement with the study conducted by Vijaykumar et al [20] who reported dyspnea and cough as the most common respiratory complaints.
The most common CT findings were GGOs, reticulations/septal thickening, and parenchymal/atelectatic bands; observed in more than 90% of participants in our patient cohort. In contrast in a study by Han and Fan et al [23], fibrotic-like lung changes were observed only in 35% of the patients at he six-month follow-up. This discrepancy may be due to the fact that our study group included only subjects who had developed severe disease in the form of ARDS in the acute phase of the illness. On the other hand, a meta-analysis done by Bocchino et al [11] has shown imaging abnormalities to be present at a pooled frequency of 43.5% at one-year follow-up.
We found that an increase in the mean CT severity score of parenchymal/atelectatic bands was associated with decreased FVC (p-value = 0.023), and an increase in the mean scores of reticulations/septal thickening and parenchymal/atelectatic bands was associated with increased dyspnea severity (p-value 0.012 and 0.045 respectively). This association between FVC and imaging patterns is consistent with the research done by Balbi et al [22] and González et al. [15] who found that the persistence of imaging abnormalities on chest CT was associated with dyspnea and deranged PFTs. Another study by Kumar et al [14] showed that consolidation and honeycombing were associated with dyspnea, while GGOs, traction bronchiectasis, and reticular pattern were associated with abnormal PFTs. However, they found no association between parenchymal/atelectatic bands and dyspnea or PFT.
While previous studies [5, 12, 15, 22, 23] have explored associations between imaging findings and clinical symptoms/PFTs, their correlations were broad, often limited to “versus “abnormal” or “versus “non-fibrotic” lungs (Table 6). Our study meticulously examined how individual imaging findings related to both the mMRC dyspnea scale and PFTs.
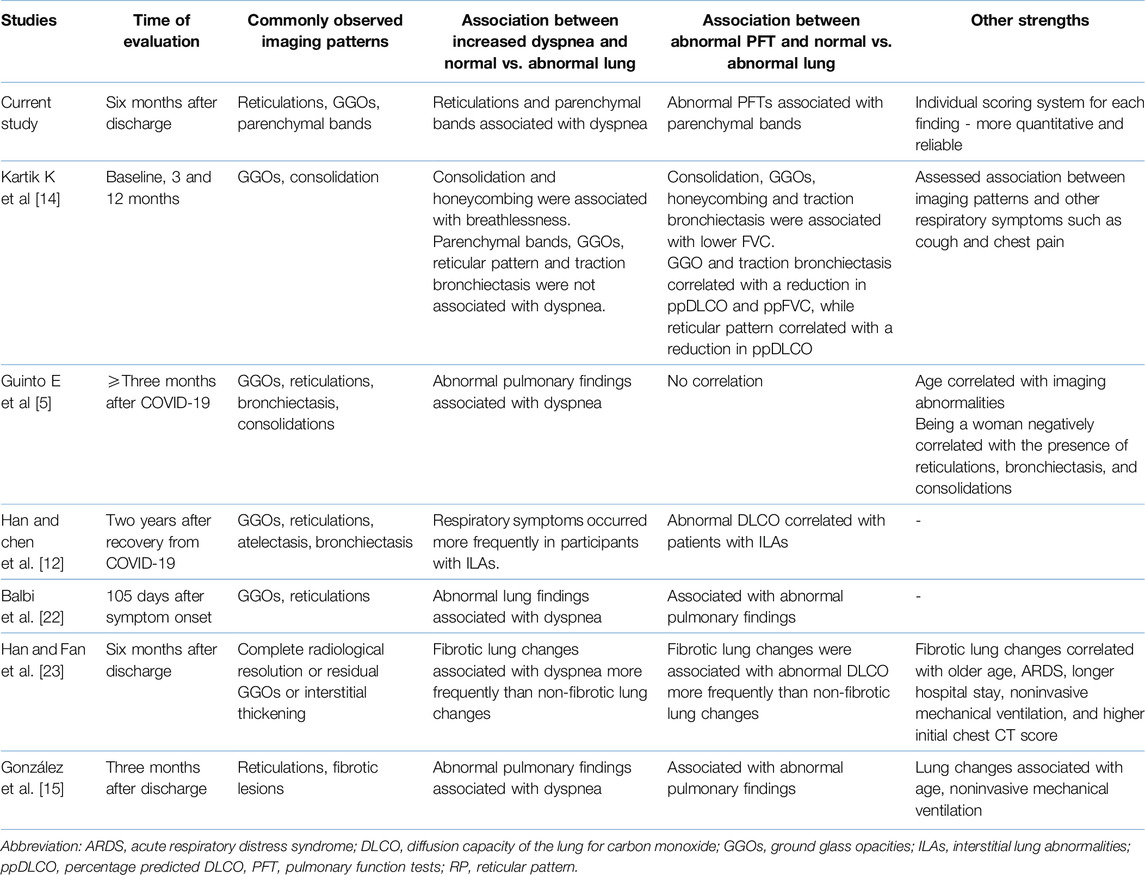
Table 6. Comparison of our current study with other post-COVID-19 studies evaluating imaging patterns in patients recovering from severe acute COVID-19.
Furthermore, our study employed an individualized scoring system for each CT finding. This meticulous approach has been shown to improve both the precision and specificity of our observations, allowing for a better understanding of the intricate relationships between radiologic features and clinical parameters. Our findings reveal a statistically significant correlation between the presence of parenchymal/atelectatic bands and reduced forced vital capacity (FVC), a key measure of lung function. Additionally, both parenchymal/atelectatic bands and reticulation/septal thickening demonstrated a marked association with the mMRC dyspnea scale, which quantifies the severity of breathlessness. These observations suggest a direct link between specific radiographic patterns, pulmonary function impairment and the level of dyspnea. This deeper understanding, facilitated by our individualized scoring system, holds great promise for refining future diagnostic and prognostic strategies for patients recovering from COVID-19 ARDS.
Our study had several limitations. First, it was a single-center study with a small sample size. Second, chest CT scans and PFT results during acute illness were not available for comparison. Third, we only included patients with severe COVID-19 which may have led to statistical bias and overestimation of pulmonary findings.
Conclusion
The most common CT findings observed in COVID-19 ARDS survivors with persistent respiratory problems at six-month follow-up were reticulations/septal thickening, GGOs, parenchymal bands, traction bronchiectasis, and consolidation. Notably, the imaging patterns of reticulations/septal thickening and parenchymal bands/atelectasis were significantly associated with increased scores on the mMRC dyspnea scale; while parenchymal bands/atelectasis also showed a statistically significant correlation with decreased FVC. However, longer follow-up studies are warranted to validate these findings and guide treatment decisions.
Summary Table
What Is Known About This Subject?
• Computed tomography (CT) of the chest is an invaluable tool for assessing lung changes in Long COVID subjects
• Persistent lung parenchymal abnormalities are seen on follow-up CT in one-third of hospitalized COVID-19 survivors
• Ground glass opacities (GGOs), septal thickening, parenchymal bands, consolidation, and traction bronchiectasis are common CT findings
What Does This Paper Add?
• The relationship between clinical features, pulmonary function tests, and lung changes seen on chest CT at 6 months in COVID-19 ARDS survivors
• Reticulations/septal thickening and parenchymal/atelectatic bands showed a significant relationship with an increased mMRC dyspnea score
• Parenchymal/atelectatic bands also showed a statistically significant correlation with decreased forced vital capacity (FVC)
Concluding Statement
This work represents an advance in biomedical science because it shows a statistically significant association between clinical parameters (dyspnea and FVC) and individual CT imaging patterns seen in COVID-19 ARDS survivors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by Postgraduate Institute of Medical Education and Research, Chandigarh, Institutional Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: MG, NP, SDe. Data collection: MG, NP, SDh, UD, AD, TS, HB, MM. Image Analysis: MG, NP. Supervision: MG, MS. Statistical analysis: NP, AD, TS, HB, MM. Manuscript-initial draft: MG, NP, SDe, AD, TS, HB, MM. Critical review: MG, SDh, MS. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cha, MJ, Solomon, JJ, Lee, JE, Choi, H, Chae, KJ, Lee, KS, et al. Chronic Lung Injury After COVID-19 Pneumonia: Clinical, Radiologic, and Histopathologic Perspectives. Radiology (2024) 310(1):e231643. doi:10.1148/radiol.231643
2. Nalbandian, A, Sehgal, K, Gupta, A, Madhavan, MV, McGroder, C, Stevens, JS, et al. Post-Acute COVID-19 Syndrome. Nat Med (2021) 27(4):601–15. Epub 2021 Mar 22. PMID: 33753937; PMCID: PMC8893149. doi:10.1038/s41591-021-01283-z
3. Garg, M, Maralakunte, M, Garg, S, Dhooria, S, Sehgal, I, Bhalla, AS, et al. The Conundrum of 'Long-COVID-19': A Narrative Review. Int J Gen Med (2021) 14:2491–506. PMID: 34163217; PMCID: PMC8214209. doi:10.2147/IJGM.S316708
4. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: Major Findings, Mechanisms and Recommendations. Nat Rev Microbiol (2023) 21(3):133–46. Epub 2023 Jan 13. Erratum in: Nat Rev Microbiol. 2023 Jun;21(6):408. PMID: 36639608; PMCID: PMC9839201. doi:10.1038/s41579-022-00846-2
5. Guinto, E, Gerayeli, FV, Eddy, RL, Lee, H, Milne, S, and Sin, DD. Post-COVID-19 Dyspnoea and Pulmonary Imaging: A Systematic Review and Meta-Analysis. Eur Respir Rev (2023) 32(169):220253. PMID: 37558261; PMCID: PMC10410398. doi:10.1183/16000617.0253-2022
6. NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. London: National Institute for Health and Care Excellence (2020). Available from: http://www.ncbi.nlm.nih.gov/books/NBK567261/ (Accessed January 6, 2024).
7. WHO. Post COVID-19 Condition (Long COVID) (2022). Available from: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (Accessed January 16, 2024).
8. Kwee, TC, and Kwee, RM. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics (2020) 40(7):1848–65. Epub 2020 Oct 23. Erratum in: Radiographics. 2022 Jan-Feb;42(1):E32. PMID: 33095680; PMCID: PMC7587296. doi:10.1148/rg.2020200159
9. Garg, M, Devkota, S, Prabhakar, N, Debi, U, Kaur, M, Sehgal, IS, et al. Ultra-Low Dose CT Chest in Acute COVID-19 Pneumonia: A Pilot Study From India. Diagnostics (Basel) (2023) 13(3):351. PMID: 36766456; PMCID: PMC9914217. doi:10.3390/diagnostics13030351
10. Solomon, JJ, Heyman, B, Ko, JP, Condos, R, and Lynch, DA. CT of Post-Acute Lung Complications of COVID-19. Radiology (2021) 301(2):E383–E395. Epub 2021 Aug 10. PMID: 34374591; PMCID: PMC8369881. doi:10.1148/radiol.2021211396
11. Bocchino, M, Rea, G, Capitelli, L, Lieto, R, and Bruzzese, D. Chest CT Lung Abnormalities 1 Year After COVID-19: A Systematic Review and Meta-Analysis. Radiology (2023) 308(1):e230535. doi:10.1148/radiol.230535
12. Han, X, Chen, L, Fan, Y, Alwalid, O, Jia, X, Zheng, Y, et al. Longitudinal Assessment of Chest CT Findings and Pulmonary Function After COVID-19 Infection. Radiology (2023) 307(2):e222888. Epub 2023 Feb 14. PMID: 36786698; PMCID: PMC9969419. doi:10.1148/radiol.222888
13. Ullal, S, and Goyal, A. Post-COVID-19 Sequelae in Lungs: Retrospective Computed Tomography Analysis in Selected Tertiary Care Hospitals of Mangalore. Pol J Radiol (2023) 88:e98–e102. PMID: 36910884; PMCID: PMC9995240. doi:10.5114/pjr.2023.125210
14. Kumar, K, Ratnakumar, R, Collin, SM, Berrocal-Almanza, LC, Ricci, P, Al-Zubaidy, M, et al. Chest CT Features and Functional Correlates of COVID-19 at 3 Months and 12 Months Follow-Up. Clin Med (Lond) (2023) 23(5):467–77. Epub 2023 Sep 29. PMID: 37775167; PMCID: PMC10541283. doi:10.7861/clinmed.2023-0059
15. González, J, Benítez, ID, Carmona, P, Santisteve, S, Monge, A, Moncusí-Moix, A, et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest (2021) 160(1):187–98. Epub 2021 Mar 4. PMID: 33676998; PMCID: PMC7930807. doi:10.1016/j.chest.2021.02.062
16. The ARDS Definition Task Force, Ranieri, VM, Rubenfeld, GD, Thompson, BT, Ferguson, ND, Caldwell, E, et al. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA (2012) 307(23):2526–33. doi:10.1001/jama.2012.5669
17. Primary Care Respiratory Society. MRC Dyspnoea Scale (2024). Available from: https://www.pcrs-uk.org/mrc-dyspnoea-scale (Accessed January 6, 2024).
18. Pan, F, Ye, T, Sun, P, Gui, S, Liang, B, Li, L, et al. Time Course of Lung Changes at Chest CT During Recovery From Coronavirus Disease 2019 (COVID-19). Radiology (2020) 295(3):715–21. Epub 2020 Feb 13. PMID: 32053470; PMCID: PMC7233367. doi:10.1148/radiol.2020200370
19. Hansell, DM, Bankier, AA, MacMahon, H, McLoud, TC, Müller, NL, and Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology (2008) 246(3):697–722. doi:10.1148/radiol.2462070712
20. Vijayakumar, B, Tonkin, J, Devaraj, A, Philip, KEJ, Orton, CM, Desai, SR, et al. CT Lung Abnormalities After COVID-19 at 3 Months and 1 Year After Hospital Discharge. Radiology (2022) 303(2):444–54. Epub 2021 Oct 5. PMID: 34609195; PMCID: PMC8515207. doi:10.1148/radiol.2021211746
21. Kanne, JP, Little, BP, Schulte, JJ, Haramati, A, and Haramati, LB. Long-Term Lung Abnormalities Associated With COVID-19 Pneumonia. Radiology (2023) 306(2):e221806. Epub 2022 Aug 30. PMID: 36040336; PMCID: PMC9462591. doi:10.1148/radiol.221806
22. Balbi, M, Conti, C, Imeri, G, Caroli, A, Surace, A, Corsi, A, et al. Post-Discharge Chest CT Findings and Pulmonary Function Tests in Severe COVID-19 Patients. Eur J Radiol (2021) 138:109676. Epub 2021 Mar 20. PMID: 33798931; PMCID: PMC7980523. doi:10.1016/j.ejrad.2021.109676
Keywords: COVID-19 ARDS, post-severe COVID-19, long Covid, CT chest-mMRC dyspnoea scale association, CT chest-PFT association
Citation: Garg M, Prabhakar N, Devkota S, Dhooria S, Debi U, Dua A, Singh T, Malarakunte M, Bhatia H and Sandhu MS (2024) Chest CT Findings at Six Months Following COVID-19 ARDS – Correlation With the mMRC Dyspnea Scale and Pulmonary Function Tests. Br J Biomed Sci 81:12871. doi: 10.3389/bjbs.2024.12871
Received: 19 February 2024; Accepted: 12 June 2024;
Published: 10 July 2024.
Copyright © 2024 Garg, Prabhakar, Devkota, Dhooria, Debi, Dua, Singh, Malarakunte, Bhatia and Sandhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mandeep Garg, Z2FyZ21hbmRlZXBAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Mandeep Garg
Mandeep Garg Nidhi Prabhakar
Nidhi Prabhakar Shritik Devkota
Shritik Devkota Sahajal Dhooria2
Sahajal Dhooria2 Ashish Dua
Ashish Dua