Abstract
In this report, we describe a case of homozygous delta-beta (δβ) thalassaemia, a rare genetic disorder characterized by severe deficiency in delta (δ) and beta (β)-globin chain production, leading to ineffective erythropoiesis and chronic haemolytic anaemia. The patient, a 26-year-old female with δβ-thalassaemia, experienced a miscarriage. High-performance liquid chromatography revealed 89.5% foetal haemoglobin (HbF) and 14.4% glycated HbF. Sebia capillary electrophoresis showed haemoglobin peak of 97.2% and 2.8%. Kleihauer Bekte test indicated a pancellular pattern of foetal cells, while morphology analysis demonstrated microcytic, hypochromic red cells and target cells. Gene analysis confirmed compound heterozygosity for two large deletions in the β-globin gene cluster.
Introduction
Homozygous δβ-thalassaemia is a rare yet clinically significant haemoglobinopathy, drawing attention for its unique genetic and physiological manifestations [1]. It is characterized by the complete absence or severe reduction of δ and βglobin chain production, an essential component of haemoglobin (Hb), the oxygen-carrying protein in red blood cells (RBCs) [2]. This deficit leads to ineffective erythropoiesis—the impaired production and maturation of RBCs—culminating in chronic haemolytic anaemia [3]. The genetic origin of this condition lies in mutations of both the delta (δ) and β-globin genes located on chromosome 11, which disrupt the normal production of adult haemoglobin (HbA) [4]. Consequently, this abnormality triggers the gamma genes on the affected chromosomes to compensate by increasing the synthesis of HbF, which is normally produced during foetal development but largely replaced by HbA after birth [5, 6].
Clinically, homozygous δβ-thalassaemia and hereditary persistence of foetal haemoglobin (HPFH) often present with features similar to thalassaemia intermedia, typically characterized by mild anaemia [7]. From a diagnostic perspective, high-performance liquid chromatography (HPLC) and capillary electrophoresis—two commonly used diagnostic tools—typically show a predominance of HbF in affected individuals [8]. However, due to the rarity of this condition and its relatively mild clinical presentation, it is frequently underdiagnosed or misidentified.
Globally, thalassaemia syndromes are more prevalent in regions where malaria was or remains endemic, including parts of Africa, the Mediterranean, the Middle East, and Southeast Asia [9]. However, homozygous δβ-thalassaemia is a much rarer condition, with its prevalence varying significantly across different populations [10]. In fact, the exact epidemiology of homozygous δβ-thalassaemia is not well-established, as it is less commonly encountered and studied compared to more prevalent forms like β-thalassaemia or alpha (α)-thalassaemia [11].
Despite its scarcity, homozygous δβ-thalassaemia remains an important condition for clinicians and researchers to understand due to its unique genetic and haematological features, as well as the impact it can have on affected individuals [1, 12]. Its mild presentation can mask the underlying genetic complexity, making accurate diagnosis crucial for appropriate patient management and family genetic counselling.
Most existing research on the impact of thalassaemia syndromes on pregnancy primarily focuses on beta-thalassaemia major and intermedia, with relatively little attention given to pregnancies in women carrying thalassaemia traits. Although there is a growing awareness of the haemoglobin E syndromes, data on pregnancy outcomes for women affected by these conditions remains sparse. The limited information available suggests a heightened risk of perinatal loss and intrauterine growth restriction (IUGR) among these patients [13]. However, when it comes to obstetric management, the primary determinants of maternal and foetal risk, as well as pregnancy outcomes, are largely influenced by factors such as the severity of anaemia, existing maternal complications, and any organ damage resulting from iron overload. The effects of severe anaemia on pregnancy, combined with pre-existing organ damage, further complicate management [14].
In this case report, we present the haematological findings of a young female patient diagnosed with homozygous δβ-thalassaemia. By examining her case, we aim to shed light on the diagnostic nuances of this rare disorder, offering a deeper understanding of its clinical course, diagnostic markers, and the importance of recognizing it in a broader spectrum of haemoglobinopathies.
Case Description
A 26-year-old black African female referred by her general practitioner (GP) to south-west Birmingham NHS Hospitals Trust, for a full blood count (FBC) and investigation of sickle cell based on the patient’s report of a positive family history. Upon examination the patient was not pregnant, however 2 years ago, she experienced an unexplained miscarriage in the first trimester.
Medical records indicated that the patient had no history of receiving blood transfusions, but iron therapy was administered to manage low mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) levels.
Discussion and Diagnostic Assessment
In our case, this variant of thalassemia was diagnosed at 26 years of age. Mondal et al. reported a wide age range for affected individuals, from 5 months to 72 years [15]. Typically, these cases exhibit mild to moderate anaemia, with RBCs counts varying from slightly elevated to normal or decreased [16]. In this case the clinical assessment showed no evidence of anaemia or fatigue. To investigate potential underlying causes of the miscarriage, the GP ordered blood tests including a FBC, hematinic profile, urea and electrolyte levels, liver function tests, and a hemoglobinopathy screen (Table 1).
TABLE 1
| Parameters | Patient value | Reference range |
|---|---|---|
| WBCs (10*9/L) | 6.1 | 4–11 |
| RBCs (10*12/L) | +7.05 | 3.8–5.2 |
| Hb (g/L) | +170 | 115–160 |
| HCT (L/L) | 0.49 | 0.37–0.45 |
| MCV (fL) | −69.8 | 80–100 |
| MCH (pg) | −24.1 | 27–32 |
| MCHC (g/L) | 346 | 310–350 |
| PLT (10*9/L) | 269 | 150–450 |
| B12 (ng/L) | +981 | 188–883 |
| Folate (ug/L) | 4.0 | 3.1–20 |
| Ferritin (ug/L) | 18 | 10–300 |
| Urea (mmol/L) | 3.6 | 2.5–7.8 |
| Sodium (mmol/L) | 140 | 135–145 |
| Potassium (mmol/L) | 5.0 | 3.5–5.3 |
| Creatinine (µmol/L) | 97 | 45–110 |
| eGFR (mL/min/1.73 m2) | 65 | — |
| Albumin (g/L) | 44 | 35–50 |
| Bilirubin (µmol/L | 12 | <21 |
| Alkaline Phosphatase (IU/L) | 142 | 30–130 |
Patient clinical characteristics.
WBCs, white blood cells; RBC, red blood cells; Hb, haemoglobin; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; PLT, platelets; B12, vitamin B12; eGFR, estimated glomerular filtration rate.
Heterozygous δβ-thalassemia is usually characterized by elevated levels of HbF, ranging from 5% to 20%, along with thalassaemic red blood cell indices, such as high RBC count and low MCV and MCH, while HbA2 levels are normal or reduced [17]. In contrast, the homozygous form presents with similar thalassaemic red cell indices (high RBC count, low MCV and MCH, and elevated reticulocyte count) but is distinguished by very high HbF levels and reduced HbA2. Additionally, it often shows biochemical signs of haemolytic anaemia, such as increased indirect bilirubin and decreased serum haptoglobin. Hepatosplenomegaly may or may not be observed [18].
The patient’s blood analysis revealed a slight elevation in RBCs count, high Hb concentration, reduced MCV and a microcytic, hypochromic appearance, with target cells present, consistent with findings from other studies [17, 19].
The patient had also elevated vitamin B12 levels, with folate and ferritin levels within the normal reference range. Blood urea and electrolyte levels were also normal, but alkaline phosphatase levels were elevated (Table 1).
The patient’s blood sample was further analysed using two different analytical instruments: HPLC (Tosoh-G11) and Sebia Capillary Electrophoresis. The HBLC analysis detected 89.5% HbF and 14.4% glycated HbF (Figure 1A), with no haemoglobin peaks observed in the HbA or haemoglobin A2 (HbA2) windows. Which contrast those of Sameen et al., who showed varied HbF from 7.5% to 17.8% with normal to reduced HbA2 from 2.5% to 2.8% [8].
FIGURE 1
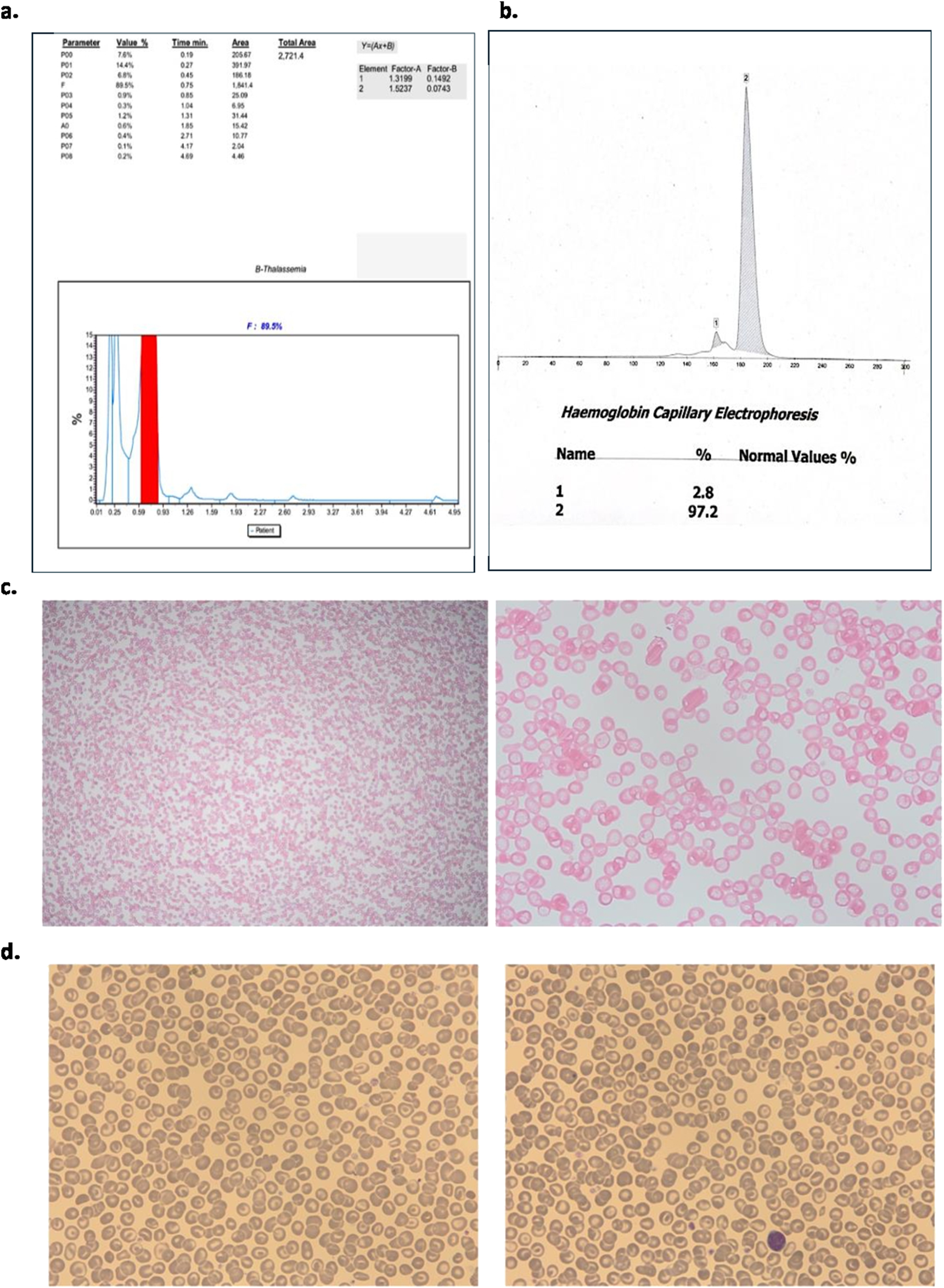
(A) Tosoh-G11 Chromatogram demonstrated foetal haemoglobin as a major peak and minor peak for glycated foetal haemoglobin; (B) Sebia Capillary electrophoresis; (C) Kleihauer Bekte showing pancellular expression; (D) Morphological analysis.
Elevated levels of HbF are typical in newborns but generally decrease to less than 2% by the first year of life [20]. If HbF levels remain high beyond this age, it may be indicative of conditions such as pregnancy, stressed bone marrow, or juvenile myelomonocytic leukaemia, though values typically do not exceed 10%. Notably high levels of HbF, combined with either absent or less than 10% HbA, are characteristic of β-thalassemia major and homozygous hereditary persistence of foetal haemoglobin (HPFH). Despite these high levels of HbF, both conditions are distinguished by their unique clinical and haematological profiles, and HbA2 levels remain normal in both disorders [21].
On the other hand, Sebia capillary electrophoresis showed a haemoglobin peak of 97.2% and a secondary peak of 2.8%, however, these peaks were not zoned, and thus both remained unlabelled (Figure 1B).
The electropherogram revealed a single dominant peak, which was not clearly separated into distinct zones and, as a result, was not labelled. This lack of zoning made it difficult to differentiate between specific haemoglobin variants. A Kleihauer-Bekte test to detect foetal cells in maternal blood was performed, demonstrating an evenly spread of HbF across all cells in the sample (pan-cellular distribution of foetal cells). This pan-cellular staining pattern effectively rule out the diagnosis of δβ thalassemia. δβ thalassemia typically presents with heterogeneous HbF distribution, often showing a patchy or uneven staining pattern across the cells [22], which was not observed in this case (Figure 1C).
Morphological analysis indicated the presence of uniform microcytic, hypochromic red cells along with target cells. No other abnormal cells were observed, and the FBC confirmed reduced MCV and MCH and this was supported by red cell morphology observed (Figure 1D).
Normal ferritin level ruled out iron deficiency and the reduced MCV and MCH suggested the possibility of co-existence of thalassaemia. The absence of HbA and HbA indicated a leaning towards a total deletion of β and δ-globin genes, along with co-existence of α(+)-thalassaemia. The phenotype of homozygotes δβ-thalassaemia with co-existing α(+)-thalassaemia dictated FBC results very similar to that of β (+) thalassaemia carrier, and Hb analysis showed >93.0% HbF with total absence of HbA and HbA2. The microcytosis of RBCs is caused by the co-existence of α-thalassaemia and the erythrocytosis is caused by high levels high oxygen affinity of HbF.
Genomic Report
Gene analysis demonstrated that this patient is compound heterozygous for two large deletions in the β-globin gene cluster, consistent with the black hereditary persistence of foetal hemoglobin-1 (HPFH-1) deletion mutation and the Ghanaian hereditary persistence of foetal hemoglobin-2 (HPFH-2) deletion mutation. In addition, this patient is heterozygous for the 3.7 kb single α-globin gene deletion.
Clinical Implications
Individuals with homozygous for δ and β deletions have reported to have erythrocytosis with a high haemoglobin level which is likely to result from high affinity of HbF. The heterozygous α(+) thalassaemia mutation may be contributing to the microcytosis and hypochromia observed in this individual.
Reproductive Implications
These HPFH deletions do not have any reproductive implications although they cause confusion during neonatal screening if either is inherited in combination with another significant beta globin gene mutation.
The α(+) thalassaemia mutation can result in Hb H disease if inherited alongside an α(0) thalassaemia deletion and Hb H is normally associated with mild to moderate anaemia which may require clinical management in elderly patients.
Conclusion
These findings collectively validate the presence of homozygous δβ-thalassaemia with α-thalassaemia and erythrocytosis. Through the implementation of two distinct laboratory methods, we substantiated the diagnosis, and as far as our understanding extends, this report stands as one of the limited literatures detailing this condition. Consequently, it enhances our understanding of the underlying pathogenesis of this rare disorder and its possible complications.
Summary Sentence
This case represents a significant advance in biomedical science by highlighting a rare genetic combination that expands our understanding of thalassemia syndromes and their complex haematological presentation.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The collected data cannot be disclosed owing to the confidentiality of patient information. Requests to access these datasets should be directed to sukhjinder.marwah@nhs.net.
Ethics statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM, designed the research study, performed the research and wrote the paper; HaS, data analysis, data presentation and wrote the paper; MM, data analysis, data presentation and wrote the paper; HiS performed the research and wrote the paper; CW performed the research. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Ahmad S Zafar S Malik H Ahmed S . Delta-Beta Thalassaemia in a Pathan Family. J Coll Physicians Surgeons--Pakistan (2017) 27:722–4.
2.
Waghela S Sharma S Shah N Uchil H Ghildiyal R . Varied Clinical Presentation of Compound Heterozygous Thalassemia With Delta Beta or Hereditary Persistence of Foetal Hemoglobin. Pediatr Hematol Oncol J (2023) 8(1):31–3. 10.1016/j.phoj.2023.01.002
3.
Kinney TR Friedman S Cifuentes E Kim HC Schwartz E . Variations in Globin Synthesis in Delta-Beta-Thalassaemia. Br J Haematol (1978) 38(1):15–22. 10.1111/j.1365-2141.1978.tb07103.x
4.
Cao A Moi P . Genetic Modifying Factors in β-Thalassemia. 2000;38(2):123–32.
5.
Barrett AN Saminathan R Choolani M . Thalassaemia Screening and Confirmation of Carriers in Parents. Best Pract Res Clin Obstet Gynaecol (2017) 39:27–40. 10.1016/j.bpobgyn.2016.10.015
6.
Sripichai O Fucharoen S . Fetal Hemoglobin Regulation in β-Thalassemia: Heterogeneity, Modifiers and Therapeutic Approaches. Expert Rev Hematol (2016) 9(12):1129–37. 10.1080/17474086.2016.1255142
7.
Nienhuis AW Nathan DG . Pathophysiology and Clinical Manifestations of the β-Thalassemias. Cold Spring Harb Perspect Med (2012) 2(12):a011726. 10.1101/cshperspect.a011726
8.
Sameen S Aden D Jairajpuri ZS Jetley S . Delta-Beta Thalassemia Trait – A Case Series. J Med Surg Public Health (2023) 1:100019. 10.1016/j.glmedi.2023.100019
9.
Kattamis A Kwiatkowski JL Aydinok Y . Thalassaemia. Lancet (2022) 399(10343):2310–24. 10.1016/S0140-6736(22)00536-0
10.
M K Ve M S M P M A A J D et al Prevalence of Delta Beta Thalassemia Minor in Southern Iran. Iranian J Blood Cancer (2012) 4(4):153–5.
11.
Langer AL . Beta-Thalassemia. In: AdamMPFeldmanJMirzaaGMPagonRAWallaceSEBeanLJet al editors. GeneReviews®. Seattle (WA): University of Washington, Seattle (1993). Available from: http://www.ncbi.nlm.nih.gov/books/NBK1426/September 16, 2024).
12.
Quality of Life in Thalassemia - TELFER. Annals of the New York Academy of Sciences. Wiley Online Library (2005). Available from: https://nyaspubs.onlinelibrary.wiley.com/doi/full/10.1196/annals.1345.035?casa_token=-JsfrBMmPxkAAAAA%3A8qEHjCEyyrHQSBaciOYGTP0IPl49Qb7awSTmzEVz1biGbjyjcs__46Jl_mrL5p4IOHcOZ7bg1SZeF_U (Accessed June 14, 2023).
13.
Leung TY Lao TT . Thalassaemia in Pregnancy. Best Pract Res Clin Obstet Gynaecol (2012) 26(1):37–51. 10.1016/j.bpobgyn.2011.10.009
14.
Psihogios V Rodda C Reid E Clark M Clarke C Bowden D . Reproductive Health in Individuals With Homozygous β-Thalassemia: Knowledge, Attitudes, and Behavior. Fertil Sterility (2002) 77(1):119–27. 10.1016/s0015-0282(01)02933-8
15.
Mondal SK Mandal S . Prevalence of Thalassemia and Hemoglobinopathy in Eastern India: A 10-Year High-Performance Liquid Chromatography Study of 119,336 Cases. Asian J Transfus Sci (2016) 10(1):105–10. 10.4103/0973-6247.175424
16.
Jain P Marwah N Dalal N Pawar R Gill M Kumar S . Delta Beta Thalassemia, a Rare Hemoglobin Variant: An Experience From Nodal Centre in North Indian State. J Appl Hematol (2022) 13(1):1–4. 10.4103/joah.joah_198_20
17.
Sharma DC Singhal S Woike P Rai S Yadav M Gaur R . Hereditary Persistence of Fetal Hemoglobin. Asian J Transfus Sci (2020) 14(2):185–6. 10.4103/ajts.AJTS_71_16
18.
Verma S Bhargava M Mittal S Gupta R . Homozygous Delta-Beta Thalassemia in a Child: A Rare Cause of Elevated Fetal Hemoglobin. Iran J Ped Hematol Oncol (2013) 3(1):222–7. 10.4103/0973-6247.175424
19.
Bollekens JA Forget BG . δβ Thalassemia and Hereditary Persistence of Fetal Hemoglobin. Hematol Oncol Clin North Am (1991) 5(3):399–422. 10.1016/s0889-8588(18)30422-2
20.
Kumar BV Choccalingam C Samuel P . Incidental Identification of Possible Delta-Beta Thalassemia Trait in a Family: A Rare Cause of Elevated Hb F. J Clin Diagn Res (2016) 10(3):BD01–2. 10.7860/JCDR/2016/16352.7409
21.
Sharma S Sehgal S Das R Gulati S . Phenotypic Heterogeneity of Delta–Beta Thalassemia. Indian J Pathol Microbiol (2019) 62(1):185–6. 10.4103/IJPM.IJPM_314_17
22.
Bogdanova A Kaestner L Simionato G Wickrema A Makhro A . Heterogeneity of Red Blood Cells: Causes and Consequences. Front Physiol (2020) 11:392. 10.3389/fphys.2020.00392
Summary
Keywords
delta-beta thalassemia, foetal haemoglobin, RBC indices, thalassemia, inherited blood disorders
Citation
Shokr H, Marwah MK, Siddiqi H, Wright C and Marwah S (2024) Homozygous Delta-Beta Thalassaemia With Alpha Thalassaemia and Erythrocytosis- a Rare Case Report. Br J Biomed Sci 81:13663. doi: 10.3389/bjbs.2024.13663
Received
16 August 2024
Accepted
30 October 2024
Published
21 November 2024
Volume
81 - 2024
Updates
Copyright
© 2024 Shokr, Marwah, Siddiqi, Wright and Marwah.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sukhjinder Marwah, sukhjinder.marwah@nhs.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.