Abstract
The alleviating manoeuvres (AMs), classically referred to as “sensory tricks” are voluntary manoeuvres that temporarily improve dystonic postures. Although self-induced application of sensory stimuli is the most common AM, clinical experience suggests that the phenomenon is more diverse, possibly reflecting the complexity of the pathophysiological mechanisms provoking dystonia. We specifically explored five different categories of AMs in patients with cervical dystonia (CD): 1) pure sensory; sensorimotor manoeuvres in which sensory input is associated with a motor output component incorporating 2) active non-oppositional, 3) active oppositional or 4) passive motion; and 5) complex motor manoeuvres. Using an ad hoc structured clinical interview, we collected data on the frequency and efficacy of each subgroup and the possible correlation with some clinical features of CD. One-hundred patients were included in this study. Seventy-five percent of patients reported at least one AM. Half of those reporting AMs acknowledged the use of different phenomenological categories of AMs. Different categories of AMs showed noteworthy differences in prevalence of use amongst CD patients, and in the relationship of frequency of use and efficacy to patient demographic and clinical characteristics. Our observational study supports the existence of different AMs that are phenomenologically different and could be related to different degrees of sensorimotor integration dysfunction. Given that AMs are probably the most efficacious, non-invasive strategy to ameliorate CD and other dystonias, accurate phenotyping and physiological exploration of their diversity may produce relevant insight for new therapeutic strategies or appraisal of existing ones.
Introduction
Dystonia is the third most common movement disorder, characterized by involuntary, repetitive twisting movements, that leads to intermittent or constant abnormal posture (1, 2). Alleviating manoeuvres (AMs), classically referred to as sensory tricks or “gestes antagonistiques,” are part of the motor phenomenology relevant to dystonia. These have been defined in the Consensus Update of Phenomenology and Classification of Dystonia (3) as “voluntary actions that specifically correct the abnormal posture or alleviate the dystonic movements.” AMs are usually simple movements, “gestes” involving, or directed to, the body region affected by dystonia, but not consisting in a forceful opposition to the involuntary dystonic movements or postures. The first report of this peculiar feature of dystonia came in 1894, when Brissaud described a new phenomenon in which a violent muscular contraction could be reversed by a minor reaction (4). Among the different forms of dystonia, cervical dystonia (CD) is the one in which AMs, often characterized merely as the act of touching gently facial or cervical areas, are described most frequently, and possibly in association with the greatest efficacy (5, 6). Over the past 3–4 decades, several studies explored the AM in dystonia, evaluating their frequency (7), duration (8), features and complexity (9–11), direction (5), and relationship to botulinum toxin treatment (7, 12, 13) or to other motor or sensory features of dystonia (14).
Beyond clinical characterization, a few groups have interrogated possible pathophysiological mechanisms underlying or related to AMs (15–17). Indeed, the presence of sensory trick as part of the behavioural phenomenology of dystonia has been acknowledged as a marker of abnormal sensorimotor integration in the pathophysiology of dystonia (18). Dystonia is conceptualized today as a network disorder, in which several structures implicated in sensory processing and sensorimotor integration, including the basal ganglia, cerebellum and the somatosensory cortical regions are dysfunctional (15, 16, 18–22). However, the diversity of the effective manoeuvres reported in the literature, ranging from gestures that apply a sensory input to the body areas expressing dystonia (e.g., gently touching the face or the neck) to complex, articulated motor actions (e.g., walking), likely reflects a greater mechanistic complexity of AMs. Furthermore, the emerging idea that different CD phenotypes -with predominant motor or sensory aspects- may underpin different pathophysiological mechanisms suggests the necessity of identifying and analysing possible individual subtypes of AMs, to forward our comprehension of how different AMs relate to the key pathophysiological mechanisms provoking dystonia. In this context, it would be important to capture systematically the phenomenological diversity of AMs in CD and observe the relationship between frequency of use and efficacy of different AMs and other clinical features of the CD phenotype.
We aimed to specifically explore five different categories of AMs reported by the patients and previously sparsely described in the literature (23). These comprised pure sensory manoeuvres consisting of a sensory input in the absence of associated motor output (S), sensorimotor manoeuvres in which sensory input is associated with a motor output component incorporating either active motor/non-oppositional gestures (AMNO) or active motor/oppositional gestures (AMO), sensorimotor passive manoeuvres implying relaxation (P), and complex motor manoeuvres including those involving muscles anatomically distant from the dystonic body region (CM). Using a structured clinical interview developed for the purpose of this study, we collected data on the frequency of each subgroup, their efficacy on symptoms, and the possible correlation with some clinical features of CD such as dystonia severity, tremor, and pain.
Materials and Methods
Study Participants and Inclusion/Exclusion Criteria
One hundred patients with a diagnosis of adult-onset isolated focal CD (3) were included in this study, 50 of whom recruited from the Movement Disorders Centre of the IRCCS San Martino Hospital of Genova, Italy and 50 recruited from the Movement Disorders Clinic at the University of Calgary, Canada. Eligible patients were recruited consecutively at both sites between April 2018 and February 2021. Exclusion criteria were the following: a Mini-Mental Status Examination score ≤24 or a Montreal Cognitive Assessment score <26; current presence of any other movement disorder apart from dystonia and tremor, or of any other neurological condition; prior neurosurgical intervention, including deep brain stimulation surgery. All patients were undergoing treatment with botulinum neurotoxin (BoNT) injections. To avoid the potential effect of BoNT therapy on clinical measures, data were collected from each patient at least 3 months after the previous BoNT treatment session. Study procedures were approved by the Ethical Committee of the University of Genoa (Protocol number 2021.48) and by the Conjoint Health Research Ethical Board (CHREB) of the University of Calgary (Protocol number REB17-0054) and were conducted in accordance with in agreement with legal requirements and international norms (World Medical Association Declaration of Helsinki: “Ethical Principles for Medical Research Involving Human Subjects” 2013). All participants gave written informed consent before participation.
Demographic and Clinical Variables
Basic demographic/clinical data collected from participants included age at study period, biological sex, and disease duration. The current severity of dystonia was measured using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), sub-scores 1, 2 and 3, and global score (24). Current tremor severity in body areas affected and not affected by dystonia was measured using the Fahn Tolosa Marin Rating Scale (FTMRS); (25).
Clinical Interview on Alleviating Manoeuvres
The structured clinical interview on AMs gathered information to describe phenomenological characteristics and frequency and efficacy of use of the different categories of AM (Supplementary File). The interview was developed in English language and was professionally translated in Italian language for use at the University of Genoa site. After an introductory description of AM, patients were asked whether they had used any of 15 AMs to improve CD symptoms at any time since CD onset. The 15 AMs were selected following a rapid literature review, collating all the AM reported by clinical studies, including both small clinical series and large observational studies or studies exploring pathophysiological mechanisms or physiomarkers. Patients had to select the three (or less than three) AMs that they used most frequently or were most familiar with. If one or more of the three AMs was not included in the list of 15, the patient described it to the examiner for further assessment. For each of the selected AM, two detailed questions followed. The first question concerned frequency: patients were asked to provide a Likert rating from 1 to 5, with 1 indicating “less often than once per week,” 2 “not every day but at least once per week,” 3 “1 to 4 times per day,” 4 “5 to 10 times per day” and 5 “more than 10 times per day.” The second question was related to efficacy: patients were asked to rate the alleviating power of the manoeuvre a 10-cm long visual analogue scale (VAS), with maximum efficacy expressed by the extreme right of the scale.
For analysis, the 15 pre-identified AMs (plus any additional AM reported by patients during the study) were grouped in the following categories of manoeuvre:
(1) Pure sensory (S): wearing a scarf or a neck collar, wear glasses, a hat, or helmet.
(2) Sensorimotor, active non-oppositional (AMNO): patient gently touching with their hand parts of the face (chin and/or nose, and/or cheek), the neck or the back of the head.
(3) Sensorimotor, active oppositional (AMO): patient’s hand pushing against the chin, the forehead, the cheek, the neck, or the nuchal region; pushing the head against a car headrest, or other vertical surfaces.
(4) Sensorimotor, passive (P): resting the back of the head on the hand or laying the head on the car headrest or on a pillow, on the couch, laying on a side. The “passive” attribution of this category relates to the relaxation of muscles supporting the erect position of the cervical spine, the engagement of which is a well-established activation state for CD.
(5) Complex Motor (CM): walking, talking, yawning or chewing, closing the eyes.
Statistical Analysis
A cumulative anonymized dataset was created pooling data from the two sites. A descriptive statistical analysis was performed to assess demographic and clinical data. Descriptive statistics (mean and standard deviation) are reported also for frequency and efficacy of each AM category. We subsequently conducted an exploratory analysis to assess if efficacy and frequency of use of each AM category were associated with basic demographics, natural history features of dystonia (disease duration, degree of dystonia severity, degree of disability), and other phenotypical features (presence and severity of pain and co-existing tremor). Spearman’s rank correlation coefficient (r or ρ, as appropriate) was used to evaluate the strength of the association between mean value of frequency and efficacy of the different AM in each category, and age, sex, and clinical variables. Finally, we explored whether the most prevalent combinations of AM were associated with demographic and clinical variables, using t test (or Mann-Whitney’s U statistic, as appropriate) and Fisher’s exact test. A p value ≤0.05 was considered statistically significant. For all the analyses IBM SPSS version 23 was used.
Results
Demographic and clinical data from the 100 included patients are summarized in Table 1. Seventy-five percent of patients reported at least one AM. The most represented category of AM was the Sensorimotor, passive category, which includes several gestures leading to relaxation of the dystonic muscles, followed in decreasing order by the Sensorimotor, active non-oppositional, the Complex motor, the Sensorimotor, active motor oppositional, and the Pure sensory categories (Figure 1; Table 2).
TABLE 1
| Total number of patients | 100 |
| Number of female patients | 71 |
| Age (mean ± SD) | 61.71 ± 14.21 |
| Disease duration (mean ± SD) | 14.48 ± 11.40 |
| Number of patients with head tremor | 47 |
| Number of patients with tremor in a non-dystonic body area | 27 |
| TWSTRS sub-score I (mean ± SD) | 15.03 ± 5.34 |
| TWSTRS sub-score II (mean ± SD) | 5.26 ± 5.43 |
| TWSTRS sub-score III (mean ± SD) | 4.67 ± 4.40 |
| TWSTRS Total score (mean ± SD) | 24.96 ± 11.82 |
| FTMRS Total score (mean ± SD) | 2.80 ± 4.44 |
| Number of patients reporting at least 1 AM (percentage) | 75 (75%) |
| Number of AMs/patient across patients reporting an AM (mean ± SD) | 1.54 ± 1.22 |
Demographic and clinical data.
N, number; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; FTMRS, Fahn Tolosa Marin Rating Scale; AM, alleviating manoeuvres; SD, standard deviation.
FIGURE 1
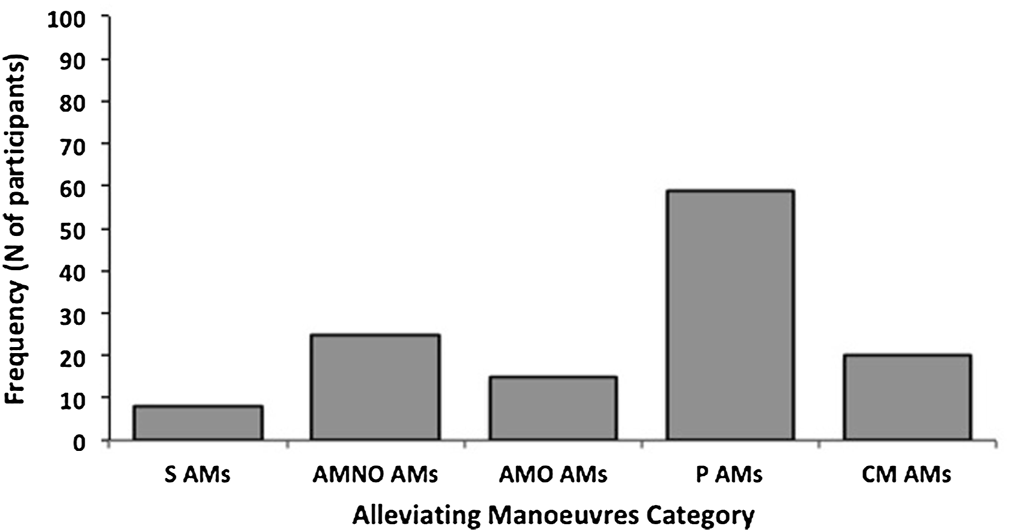
Frequency of Alleviating Manoeuvres (AM) categories in all patients. S, pure sensory; AMNO, sensorimotor active motor non-oppositional, AMO, sensorimotor active motor oppositional, P, Sensorimotor passive, CM, complex motor. In total 75 out of 100 CD patients reported at least 1 AMs. Of the total sample of 100 patients, 8% reported S AMs, 25% reported AMNO AMs, 15% AMO AMs, 58% reported P AMs and 20% reported CM AMs. In half of the patients AMs were reported in combination.
TABLE 2
| Patients reporting only one category of AM | 38 |
| Patients reporting only S category | 2 |
| Patients reporting only AMNO category | 10 |
| Patients reporting only P category | 21 |
| Patients reporting only AMO category | 1 |
| Patients reporting only CM category | 4 |
| Patients reporting more than one category of AM | 37 |
| Patients reporting two categories of AM | 27 |
| Patients reporting two categories of AM including the P category | 25 |
| Patients reporting three categories of AM | 10 |
| Patients reporting three categories of AM including the P category | 9 |
Number of patients reporting one or more Alleviating Manoeuvre (AM).
S, pure sensory; AMNO, sensorimotor, active non-oppositional; AMO, sensorimotor, active oppositional; P, sensorimotor, passive; CM, complex motor.
Half of the patients adopted two AMs or more (Table 2). The vast majority (34/37) of patients using more than one AM at different times reported the use of Sensorimotor, passive AMs.
The mean efficacy of the AM was not significantly different across the different categories, with the only exception for the comparison between the mean efficacy of the Sensorimotor, passive and of the mean efficacy of the Pure sensory categories (Sensorimotor, passive > Pure sensory, p = 0.049; see Table 3). There were several significant differences when comparing the mean frequency of the AM across the different categories (summarized in Table 3), with the Sensorimotor, active non-oppositional exhibiting the highest mean frequency and the Pure sensory one exhibiting the lowest mean frequency.
TABLE 3
| Category | Efficacy mean (SD) | Statistical analysis efficacy | Frequency mean (SD) | Statistical analysis frequency |
|---|---|---|---|---|
| (All AM) | 6.95 (1.96) | 3.67 (0.97) | ||
| S (9) | 6.06 (2.34) | vs. P category: p = 0.049 | 2.39 (1.45) | vs. AMNO category: p = 0.0002 |
| vs. P category: p = 0.0013 | ||||
| vs. AMO category: p = 0.0054 | ||||
| AMNO (25) | 6.66 (1.96) | n.s. | 4.18 (0.95) | vs. S category: p = 0.0002 |
| vs. CM category: p = 0.0014 | ||||
| P (58) | 7.49 (1.94) | vs. S category: p = 0.049 | 3.72 (1.05) | vs. S category: p = 0.0013 |
| vs. CM category: p = 0.0263 | ||||
| AMO (14) | 7 (1.95) | n.s. | 3.89 (0.88) | vs. S category: p = 0.0054 |
| vs. CM category: p = 0.0386 | ||||
| CM (19) | 6.82 (2.23) | n.s. | 3.07 (1.20) | vs. AMNO category: p = 0.0014 |
| vs. P category: p = 0.0263 | ||||
| vs. AMO category: p = 0.0386 |
Mean/SD of (mean) efficacy (range 0–10) and mean/SD of (mean) frequency (range 0–5) for all Alleviating Manoeuvres and for each category (including only those who have at least one AM in that category). Statistical analysis for efficacy and frequency between categories is also reported.
S, pure sensory; AMNO, sensorimotor active non-oppositional; AMO, sensorimotor active oppositional, P, sensorimotor passive, CM, complex motor. Under the column “Category”, in brackets we report the number of CD patients reporting that category of AMs.
Table 4 summarizes the results of our correlation analyses. A few associations between specific categories of AMs and demographic and clinical features of CD patients were observed. The frequency of use of pure sensory AMs was positively correlated with age. The frequency of use of Sensorimotor, passive AMs was positively correlated with severity and disability of CD, whereas their efficacy correlated positively with CD severity. Both frequency of use and efficacy of Sensorimotor, active motor oppositional AMs were negatively correlated with disease duration. We did not find any correlation between measures related to the Sensorimotor, active motor non-oppositional or Complex motor categories of AMs and demographic/clinical variables of CD patients.
TABLE 4
| AMs | Correlation with frequency of use and efficacy |
|---|---|
| Sensory | Frequency: Age, r = 0.21, p = 0.04 |
| Active Motor Oppositional | Frequency: Disease duration, r = −0.22, p = 0.03; Efficacy: Disease duration, r = −0.21, p = 0.04 |
| Sensorimotor Passive | Frequency: TWTRS I, r = 0.2, p = 0.048; TWTRS II, r = 0.29, p = 0.003; TWTRS tot, r = 0.3, p = 0.002; Efficacy: TWTRS tot, r = 0.20, p = 0.049 |
Correlations/associations with demographic and other clinical features.
Alleviating Manoeuvres (AMs).
The most common AM combinations included the Sensorimotor, passive category, which was combined with the Sensorimotor active non-oppositional in 13 patients, with the Sensorimotor active oppositional in 12 patients, and with the Complex motor in 15 patients (Table 5). Any of the above combinations was associated with greater dystonia severity compared to the presence of the Sensorimotor, passive AM in isolation; the combination Sensorimotor, passive + Sensorimotor, active non-oppositional was associated with greater dystonia-related disability and severity, and frequency of CD-related pain, compared to the presence of the Sensorimotor, passive AM in isolation; finally, the combination Sensorimotor, passive + Sensorimotor, active oppositional was associated with younger age and shorter disease duration, compared to the presence of the Sensorimotor, passive AM in isolation (Table 5). Within each patient subgroup exhibiting any of these combinations, we did not detect any difference in frequency between the co-existing manouevres (all p > 0.05).
TABLE 5
| Variable | P In isolation N = 22 mean (SD) | P + AMNO N = 13 mean (SD) | P + AMO N = 12 mean (SD) | P + CM N = 15 mean (SD) |
|---|---|---|---|---|
| Age (years) | 65.87 (11.26) | 62.08 (10.56) | 57.25 (9.93)ap = 0.03 | 62.6 (11.81) |
| Sex (F/M) | 19/3 | 9/4 | 9/3 | 11/4 |
| Disease duration (years) | 18.64 (10.03) | 16.38 (14.98) | 9 (7.76)ap = 0.007 | 16.73 (14.59) |
| TWSTRS part I | 12.32 (5.24) | 17.38 (4.19)ap = 0.006 | 18.25 (4.49)ap = 0.002 | 18.87 (5.03)ap = 0.0006 |
| TWSTRS part II | 5.18 (5.98) | 9.31 (4.23)ap = 0.034 | 7.33 (5.6) | 5.27 (4.4) |
| TWSTRS part III | 4.95 (5.19) | 6.92 (4.15) | 5.73 (3.18) | 3.48 (3.09) |
| Presence of pain (Y/N) | 14/8 | 11/2 | 11/1 | 11/4 |
| FTM total score | 3.54 (4.66) | 5.15 (8.19) | 0.92 (1.73) | 2.2 (2.48) |
| Presence of tremor (Y/N) | 14/8 | 11/2 | 4/8 | 10/5 |
Associations between demographic/clinical variables and combinations of AM.
AMNO, sensorimotor active non-oppositional; AMO, sensorimotor active oppositional; P, sensorimotor passive, CM, complex motor; TWSTRS, toronto western spasmodic torticollis rating scale; FTM, fahn tolosa marin scale.
Significantly different from the “P in isolation” group.
Discussion
Administering an ad hoc structured questionnaire to inquire specifically on the presence of different categories of AMs, we observed that 75% of patients with adult-onset, isolated CD, consecutively recruited from two specialist movement disorders clinics, report at least one type of AM. Almost half of those reporting AMs acknowledged the use of different phenomenological categories of AMs. The different categories of AMs explored showed a few differences in their relationship of frequency of use and efficacy to basic patient demographic and clinical characteristics. Unfortunately, due to a likely recall bias, we were not able to collect reliable information on which AM occurred first during the course of the illness in patients reporting more than AM.
The observed prevalence of AMs falls within the upper range of prevalence estimates reported by other clinical studies of CD (6, 7, 9, 20, 26). There is, however, a substantial variability of estimates across studies, which is likely to depend on the phenomenology of the different behaviors included in the definition of AM adopted by each individual study, as well as by the ascertainment method used. Most studies limited the definition of AM to the description of the classical “sensory trick” phenomenon, i.e., “a touch or other movement that influences the severity of the abnormal movement” (8, 27). This definition focuses the attention primarily on active gestures that correspond to only one of the five categories investigated by our questionnaire (the active motor non-oppositional), thus potentially underestimating or neglecting other types of manoeuvres, such as passive relaxation manoeuvres, complex actions, or pure sensory input.
Assessment of AMs associated with the Dystonia Coalition registry has used video-based rating focused on the TWSTRS sensory trick item (6, 8). However, this may be affected by limited assessment time. Additionally, the video format may limit the range of demonstrable manoeuvres in keeping with different types of AMs used by an individual patient. Similarly, the video format can constrain the degree of appreciable dystonia severity and is also limited by the angle of head rotation. Given the inclusion of different categories of AMs reported in the literature in association with CD beyond the classical definition of “sensory trick,” our structured questionnaire probably provided a more comprehensive assessment compared to most previous studies that explored this phenomenon.
The observation that the Sensorimotor, passive (P) is the most prevalent (59%) and efficacious category of AM in CD is relatively novel, albeit not surprising. The most represented AM in this category is resting the back of the head or neck on a surface (e.g., wall, cushion, headrest). It is likely that the highest efficacy of this AM stems from the abolition of dystonia-inducing postures, while the role of a sensory input applied by the surface on which the head rests could be secondary. One possible mechanism of action of the sensorimotor passive AM is related to the action on proprioceptors, and particularly on muscle spindles. There is evidence in the literature that proprioceptive dysfunction is involved in the pathophysiology of dystonia (28). The sensory afferents innervating muscle spindles convey information about muscle stretch, contributing to the sense of body segment position and movement, and regulate muscle contraction by activating motor neurons via the stretch reflex to resist muscle stretch. The sensitivity of muscle spindles is under the control of a population of motor neurons, namely the γ-motor neurons, that selectively innervate the intrafusal fibers of muscle spindles and control the sensitivity of spindle afferent discharge. Experimental evidence suggests that afferent information coming from muscle spindles from the neck are misinterpreted in patients with CD, likely contributing to dystonic symptoms (29, 30). The relaxation of dystonic muscles induced by P AMs may thus contribute to a modulation of afferent inputs from muscle spindles. This would lead to a modulation in the activity of γ-motor neurons, engaging a brainstem pathway that could ultimately reduce muscle spindle sensitivity. One of the possible mechanisms of action of botulinum neurotoxin, the first-line treatment for CD, is to block γ-motor neurons, causing reduced afferent activity of muscle spindles (31). We can speculate that P AMs produce their effect in a similar fashion.
The underreporting of Sensorimotor, passive AMs may depend on different factors. First, this category of AM does not fulfil the traditional definition of “sensory trick” and may have therefore been consciously excluded in previous studies by both researchers and participants, including those studies that used structured/self-administered questionnaires. Second, the assessment of AM in a clinical setting, or during a videorecording, does not always allow or incorporate the demonstration of how CD changes resting passively the head onto a vertical or horizontal surface. Finally, of all AM, the Sensorimotor, passive are probably the ones that patients are less aware of, given that they involve actions or postures that are automatically adopted to achieve a relaxed or restful position. Given their efficacy, it is not surprising that Sensorimotor, passive AM tend to be used more often, and with greater alleviating outcome, by patients with greater disease severity.
Sensorimotor, active non-oppositional AM category (AMNO) represents the traditional “sensory trick” described and investigated by most studies and includes the patient’s gentle touch of cervical and/or cranial body areas. Despite this being the second most prevalent AM category, the observed 25% prevalence is surprisingly lower than what reported in previous studies (6, 7, 9, 20, 26). This discrepancy may result from a “lumping” approach to AMs detection used by other authors, which may have inflated its prevalence. On the other hand, we acknowledge the possibility that the phrasing of our questionnaire might have been sub-optimal to detect this AM category, leading to specifically lower sensitivity. This notwithstanding, we confirmed Sensorimotor, active non-oppositional AM as the most frequently used AMs per unit of time, likely due to ease of application and immediate effect. Interestingly, in line with several other reports that focused on this type of AM, we did not identify any significant correlation of frequency and efficacy with age, disease duration or severity, suggesting that Sensorimotor, active non-oppositional manouevres may represent a “behavioral” trait of CD, whose underlying mechanism is active throughout different stages of progression of this condition and across different adult age groups.
Sensorimotor, active oppositional manoeuvres (AMO) (present in 15% of our CD patients) correspond to the “forcible tricks” described in the literature (23, 27, 32). These actions were the second most frequently used per unit of time and the second most efficacious in our clinical sample, which reflects the automaticity of their application. Given that this type of AM inevitably incorporates a sensory input, it remains still unclear whether its efficacy depends predominantly on a motor action forcefully opposing to the dystonic movement, or on the applied sensory input, or on a combination of the two. Both its frequency of use and efficacy are higher in patients with shorter disease duration. A possible explanation for this is that dystonic postures can be more easily overcome by force earlier in the disease course and/or that subconscious forceful opposition occurs more naturally in the initial years of living with this condition. Alternatively, it is possible that, earlier in the course of the illness, patients have not yet become aware that light touch may produce a similar effect, leading them to abandon oppositional manoeuvres later. Prospective observational studies are needed to gain accurate information on changes in use of different AMs throughout the natural history of CD.
A surprising finding of our study is the 20% prevalence of use for complex motor AMs (CM). The actions included in this category have traditionally been labelled as “atypical tricks” (23, 33), given that the muscles primarily involved in these manoeuvres are not the primary agonists producing the dystonic postures and movements. This characteristic may have contributed to their underreporting, although their association with CD is increasingly recognized. For instance, one of these actions (raising arms above the head) has been recently associated with an alleviating effect on head tremor in CD (34). Their complexity may justify its relatively low frequency of use per unit of time.
Finally, the application of tactile stimuli (S) to the cervical or cranial body areas through an object like a scarf, hat or goggles may represent another, previously recognized AM which, however, appears to be substantially less prevalent, efficacious, and frequently used than any other category (23, 33). The reduced use and efficacy of these pure sensory AMs is well in line with the already reported, substantial decrease in efficacy of common sensorimotor non-oppositional gestes when these are applied by another person rather than the patient (20, 23).
Accurate phenomenological characterization of AMs may improve our understanding of their underlying mechanisms. The exploration of physiological mechanisms correlated to AMs, or temporally associated with them, has focused on AMs that involved a voluntary movement, hence corresponding to three of the five categories we investigated (sensorimotor active non-oppositional and oppositional movements, and complex movements). Previous research has provided evidence favouring the view of the AM as a behaviour that adjusts sensorimotor integration normalizing abnormal gating mechanisms (16), likely through the modulation of earlier brain potentials related to the motor expectation and preparation phases (35). In line with this, during the execution of active, non-oppositional AMs, a temporary rise in the activity concerned with motor preparation and execution has been observed (36). Other authors have observed a correlation between greater efficacy of this category of AM and multimodal sensory processing (lower visuotactile discrimination threshold (16), different whole-body response to neck vibration (15)) in CD. Future research should clarify the relative contributions of sensory processing and motor preparation to the different types of AMs, particularly noting the varying complexity of motor organization across AMs. Likewise, these elements (sensory processing and motor preparation) can be explored in AMs that are not associated with active movements.
In conclusion, our observational study supports the existence of different AMs that are phenomenologically different and could be related to different degrees of dysfunction of sensorimotor integration. Given that AMs are probably the most efficacious, non-invasive strategy to ameliorate CD and other forms of dystonia, accurate phenotyping and physiological exploration of their diversity may produce relevant insight for new therapeutic strategies or appraisal of existing ones.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the University of Genoa (Protocol number 2021.48) and the Conjoint Health Research Ethical Board (CHREB) of the University of Calgary (Protocol number REB17-0054). The patients/participants provided their written informed consent to participate in this study.
Author contributions
LA, FB, EP, and DM: Data analysis, writing and finalizing manuscript. LA, GB, NC, RM, and DM: Data collection. LA, FB, and DM: Data analysis and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by grants from Italian Ministry of Health assigned to LA (Ricerca Corrente).
Acknowledgments
We would like to thank all CD participants for their time and commitment to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/dyst.2022.10283/full#supplementary-material.
References
1.
Fahn S . Concept and Classification of Dystonia. Adv Neurol (1988) 50:1–8.
2.
Fahn S Bressman SB Marsden CD . Classification of Dystonia. Adv Neurol (1998) 78:1–10.
3.
Albanese A Bhatia K Bressman SB Delong MR Fahn S Fung VSC et al Phenomenology and Classification of Dystonia: A Consensus Update. Mov Disord (2013) 28:863–73. 10.1002/mds.25475
4.
Broussolle E Laurencin C Bernard E Thobois S Danaila T Krack P . Early Illustrations of Geste Antagoniste in Cervical and Generalized Dystonia. Tremor and Other Hyperkinetic Movements (2015) 5:332. 10.7916/D8KD1X7410.5334/tohm.272
5.
Schramm A Reiners K Naumann M . Complex Mechanisms of Sensory Tricks in Cervical Dystonia. Mov Disord (2004) 19:452–8. 10.1002/MDS.10689
6.
Norris SA Jinnah HA Espay AJ Klein C Brüggemann N Barbano RL et al Clinical and Demographic Characteristics Related to Onset Site and Spread of Cervical Dystonia. Mov Disord (2016) 31:1874–82. 10.1002/mds.26817
7.
Filip P Šumec R Baláž M Bareš M . The Clinical Phenomenology and Associations of Trick Maneuvers in Cervical Dystonia. J Neural Transm (2016) 123:269–75. 10.1007/S00702-015-1488-Z
8.
Cisneros E Stebbins GT Chen Q Vu JP Benadof CN Zhang Z et al It's Tricky: Rating Alleviating Maneuvers in Cervical Dystonia. J Neurol Sci (2020) 419:117205. 10.1016/J.JNS.2020.117205
9.
Molho ES Stacy M Gillard P Charles D Adler CH Jankovic J et al Impact of Cervical Dystonia on Work Productivity: An Analysis From a Patient Registry. Mov Disord Clin Pract (2016) 3:130–8. 10.1002/MDC3.12238
10.
Fahn S . The Varied Clinical Expressions of Dystonia. Neurol Clin (1984) 2:541–54. 10.1016/s0733-8619(18)31090-9
11.
Weiner WJ Nora LM . "Trick" Movements in Facial Dystonia. J Clin Psychiatry (1984) 45:519–21.
12.
Ramirez-Castaneda J Jankovic J . Long-term Efficacy and Safety of Botulinum Toxin Injections in Dystonia. Toxins (2013) 5:249–66. 10.3390/TOXINS5020249
13.
Müller J Wissel J Masuhr F Ebersbach G Wenning GK Poewe W . Clinical Characteristics of the Geste Antagoniste in Cervical Dystonia. J Neurol (2001) 248:478–82. 10.1007/S004150170156
14.
Di Biasio F Marchese R Abbruzzese G Baldi O Esposito M Silvestre F et al Motor and Sensory Features of Cervical Dystonia Subtypes: Data From the Italian Dystonia Registry. Front Neurol (2020) 11:906. 10.3389/FNEUR.2020.00906
15.
Brugger F Peters A Georgiev D Kägi G Balint B Bhatia KP et al Sensory Trick Efficacy in Cervical Dystonia Is Linked to Processing of Neck Proprioception. Parkinsonism Relat Disord (2019) 61:50–6. 10.1016/J.PARKRELDIS.2018.11.029
16.
Kägi G Katschnig P Fiorio M Tinazzi M Ruge D Rothwell J et al Sensory Tricks in Primary Cervical Dystonia Depend on Visuotactile Temporal Discrimination. Mov Disord (2013) 28:356–61. 10.1002/MDS.25305
17.
Sarasso E Agosta F Piramide N Bianchi F Butera C Gatti R et al Sensory Trick Phenomenon in Cervical Dystonia: a Functional MRI Study. J Neurol (2020) 267:1103–15. 10.1007/S00415-019-09683-5
18.
Avanzino L Tinazzi M Ionta S Fiorio M . Sensory-motor Integration in Focal Dystonia. Neuropsychologia (2015) 79:288–300. 10.1016/J.NEUROPSYCHOLOGIA.2015.07.008
19.
Antelmi E Erro R Rocchi L Liguori R Tinazzi M Di Stasio F et al Neurophysiological Correlates of Abnormal Somatosensory Temporal Discrimination in Dystonia. Mov Disord (2017) 32:141–8. 10.1002/mds.26804
20.
Patel N Hanfelt J Marsh L Jankovic J . Alleviating Manoeuvres (Sensory Tricks) in Cervical Dystonia. J Neurol Neurosurg Psychiatry (2014) 85:882–4. 10.1136/JNNP-2013-307316
21.
Tinazzi M Fiorio M Fiaschi A Rothwell JC Bhatia KP . Sensory Functions in Dystonia: Insights from Behavioral Studies. Mov Disord (2009) 24:1427–36. 10.1002/MDS.22490
22.
Avanzino L Cherif A Crisafulli O Carbone F Zenzeri J Morasso P et al Tactile and Proprioceptive Dysfunction Differentiates Cervical Dystonia with and without Tremor. Neurology (2020) 94:e639–e650. 10.1212/WNL.0000000000008916
23.
Ramos VFML Karp BI Hallett M . Tricks in Dystonia: Ordering the Complexity. J Neurol Neurosurg Psychiatry (2014) 85:987–93. 10.1136/JNNP-2013-306971
24.
Comella CL Fox SH Bhatia KP Perlmutter JS Jinnah HA Zurowski M et al Development of the Comprehensive Cervical Dystonia Rating Scale: Methodology. Mov Disord Clin Pract (2015) 2:135–41. 10.1002/MDC3.12131
25.
Fahn S Tolosa E Conception M . Clinical Rating Scale for Tremor. In: JankovicJTolosaE, editors. Parkinson’s Disease and Movement Disorders. p. 271–80.
26.
Martino D Liuzzi D Macerollo A Aniello MS Livrea P Defazio G . The Phenomenology of the Geste Antagoniste in Primary Blepharospasm and Cervical Dystonia. Mov Disord (2010) 25:407–12. 10.1002/MDS.23011
27.
Patel N Jankovic J Hallett M . Sensory Aspects of Movement Disorders. Lancet Neurol (2014) 13:100–12. 10.1016/S1474-4422(13)70213-8
28.
Avanzino L Fiorio M . Proprioceptive Dysfunction in Focal Dystonia: from Experimental Evidence to Rehabilitation Strategies. Front Hum Neurosci (2014) 8:1000. 10.3389/FNHUM.2014.01000
29.
Kaji R Rothwell JC Katayama M Ikeda T Kubori T Kohara N et al Tonic Vibration Reflex and Muscle Afferent Block in Writer's Cramp. Ann Neurol (1995) 38:155–62. 10.1002/ana.410380206
30.
Bove M Brichetto G Abbruzzese G Marchese R Schieppati M . Neck Proprioception and Spatial Orientation in Cervical Dystonia. Brain (2004) 127:2764–78. 10.1093/BRAIN/AWH291
31.
Hallett M . Mechanism of Action of Botulinum Neurotoxin: Unexpected Consequences. Toxicon (2018) 147:73–6. 10.1016/J.TOXICON.2017.08.011
32.
Ochudło S Drzyzga K Drzyzga ŁR Opala G . Various Patterns of Gestes Antagonistes in Cervical Dystonia. Parkinsonism Relat Disord (2007) 13:417–20. 10.1016/J.PARKRELDIS.2007.01.004
33.
Mulroy E Ganos C Latorre A Terkelsen AJ Balint B Agarwal PA et al Self-concocted, Curious and Creative Coping Strategies in Movement Disorders. Parkinsonism Relat Disord (2021) 83:140–3. 10.1016/J.PARKRELDIS.2020.10.031
34.
Cisneros E Vu JP Lee HY Chen Q Benadof CN Zhang Z et al Does Raising the Arms Modify Head Tremor Severity in Cervical Dystonia? Tremor and Other Hyperkinetic Movements (2021) 11:21. 10.5334/TOHM.623
35.
Shin H-W Cho HJ Lee SW Shitara H Hallett M . Sensory Tricks in Cervical Dystonia Correlate with Enhanced Brain Activity during Motor Preparation. Parkinsonism Relat Disord (2021) 84:135–8. 10.1016/J.PARKRELDIS.2021.02.005
36.
Lee SW Cho HJ Shin H-W Hallett M . Sensory Tricks Modulate Corticocortical and Corticomuscular Connectivity in Cervical Dystonia. Clin Neurophysiol (2021) 132:3116–24. 10.1016/J.CLINPH.2021.08.019
Summary
Keywords
pain, tremor, cervical dystonia, alleviating manoeuvre, sensory trick
Citation
Avanzino L, Di Biasio F, Bonassi G, Pelosin E, Cothros N, Marchese R and Martino D (2022) Observing the Diversity of Alleviating Manoeuvres in Cervical Dystonia. Dystonia 1:10283. doi: 10.3389/dyst.2022.10283
Received
05 December 2021
Accepted
06 April 2022
Published
17 May 2022
Volume
1 - 2022
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Reviewed by
Aasef Shaikh, Case Western Reserve University, United States
Scott A. Norris, Washington University in St. Louis, United States
Updates
Copyright
© 2022 Avanzino, Di Biasio, Bonassi, Pelosin, Cothros, Marchese and Martino.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Avanzino, lavanzino76@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.