Abstract
Objective: Blepharospasm is a type of dystonia where the diagnosis is often delayed because its varied clinical manifestations are not well recognized. The purpose of this study was to provide a comprehensive picture of its clinical features including presenting features, motor features, and non-motor features.
Methods: This was a two-part study. The first part involved a systematic literature review that summarized clinical features for 10,324 cases taken from 41 prior reports. The second part involved a summary of clinical features for 884 cases enrolled in a large multicenter cohort collected by the Dystonia Coalition investigators, along with an analysis of the factors that contribute to the spread of dystonia beyond the periocular region.
Results: For cases in the literature and the Dystonia Coalition, blepharospasm emerged in the 50s and was more frequent in women. Many presented with non-specific motor symptoms such as increased blinking (51.9%) or non-motor sensory features such as eye soreness or pain (38.7%), photophobia (35.5%), or dry eyes (10.7%). Non-motor psychiatric features were also common including anxiety disorders (34–40%) and depression (21–24%). Among cases presenting with blepharospasm in the Dystonia Coalition cohort, 61% experienced spread of dystonia to other regions, most commonly the oromandibular region and neck. Features associated with spread included severity of blepharospasm, family history of dystonia, depression, and anxiety.
Conclusions: This study provides a comprehensive summary of motor and non-motor features of blepharospasm, along with novel insights into factors that may be responsible for its poor diagnostic recognition and natural history.
Introduction
Dystonia is a disorder characterized by sustained or intermittent muscle contractions causing abnormal repetitive movements and postures [1]. Blepharospasm (BSP) is a subtype characterized by bilateral synchronous spasms of the orbicularis oculi and surrounding muscles [2, 3]. Additional manifestations include exaggerated blinking, eyelid fluttering, and apraxia of eyelid opening. Commonly reported non-motor features include dry or gritty sensations in the eyes, photophobia, depression, and anxiety [4-11]. BSP is particularly disabling because it interferes with vision, sometimes rendering subjects functionally blind. In approximately half of those affected, dystonia spreads from the upper face to the oromandibular area, neck, or other regions [12-17].
Idiopathic BSP has a reported prevalence between 20 and 133 cases per million [18]. However, its varied presentations are often not appreciated, and it is probably under-diagnosed. Several reports describe frequent misdiagnoses [5, 7, 10]. Initial diagnostic accuracy was only 19% in one study [10] and 10% in another [7]. The most common misdiagnoses included eye strain or eye irritation from dryness, conjunctivitis, or keratitis. These diagnostic errors contribute to delays between onset and diagnosis that may span many years [4, 7, 10, 11, 19-22]. Although improved awareness in recent years has shortened these delays, more than half of all affected individuals typically require more than a year to reach a diagnosis [21]. The challenges in diagnosing BSP are important to address, because treatments are available.
Our understanding of BSP is based mostly on case reports and case series from expert centers. Some of these focused on idiopathic cases, while others included cases with known causes. Some focused strictly on motor features, while others focused on non-motor features. Methods of ascertainment and assessment varied considerably. The purpose of the current study was to provide a comprehensive description of the clinical features of isolated BSP. First, we provide a summary of 10,324 cases reported in 41 separate manuscripts from the literature. Next, we describe the clinical features of BSP from the Dystonia Coalition (DC), an international multicenter project involving all types of dystonia, 884 of whom had BSP. Finally, we provide details on clinical features associated with spread of dystonia beyond the upper face. By providing a detailed description for a large number of subjects with BSP, we aim to improve clinical recognition and facilitate more timely diagnosis and treatment.
Methods
Systematic Review of Literature
The Embase (Elsevier), PubMed (National Library of Medicine), and Web of Science: Core Collection (Clarivate Analytics) databases were searched with various combinations of keywords (blepharospasm, eyelid spasm, orbicularis oculi muscle spasm, eyelid contraction, eyelid twitch, cohort studies, longitudinal studies, follow-up studies, prospective studies, retrospective studies) and controlled vocabulary (i.e., MeSH, EMTREE) terms for BSP and cohort studies. The search included cases described as Meige syndrome, a term that usually refers to BSP variably combined with involvement of the lower face, jaw, tongue, and sometimes neck [23]. Because of its varied usage, this eponym is not used here. The searches were limited to English language and human studies, with no publication year limit. The authors also reviewed reference lists for potential additional citations. Studies that provided original results for cohorts ≥100 subjects were selected based on the abstract and full text (55 papers for 100–500 cohort and six papers for >500 cohort). Papers that included at least three demographic or clinical features (age at onset, sex, symptoms, triggering or alleviating factors, disease progression, treatment response) were selected for final review. The selection process was completed by two authors independently (LS and HC) and a third author (MH) adjudicated any discrepancies. Forty-one papers including 10,324 total cases were included in the final analysis (Figure 1) [5, 7, 9-11, 14, 15, 17, 21, 22, 24-35]. No effort was made to address potential duplicate reporting, except for three reports describing the same cohort [36-38].
FIGURE 1
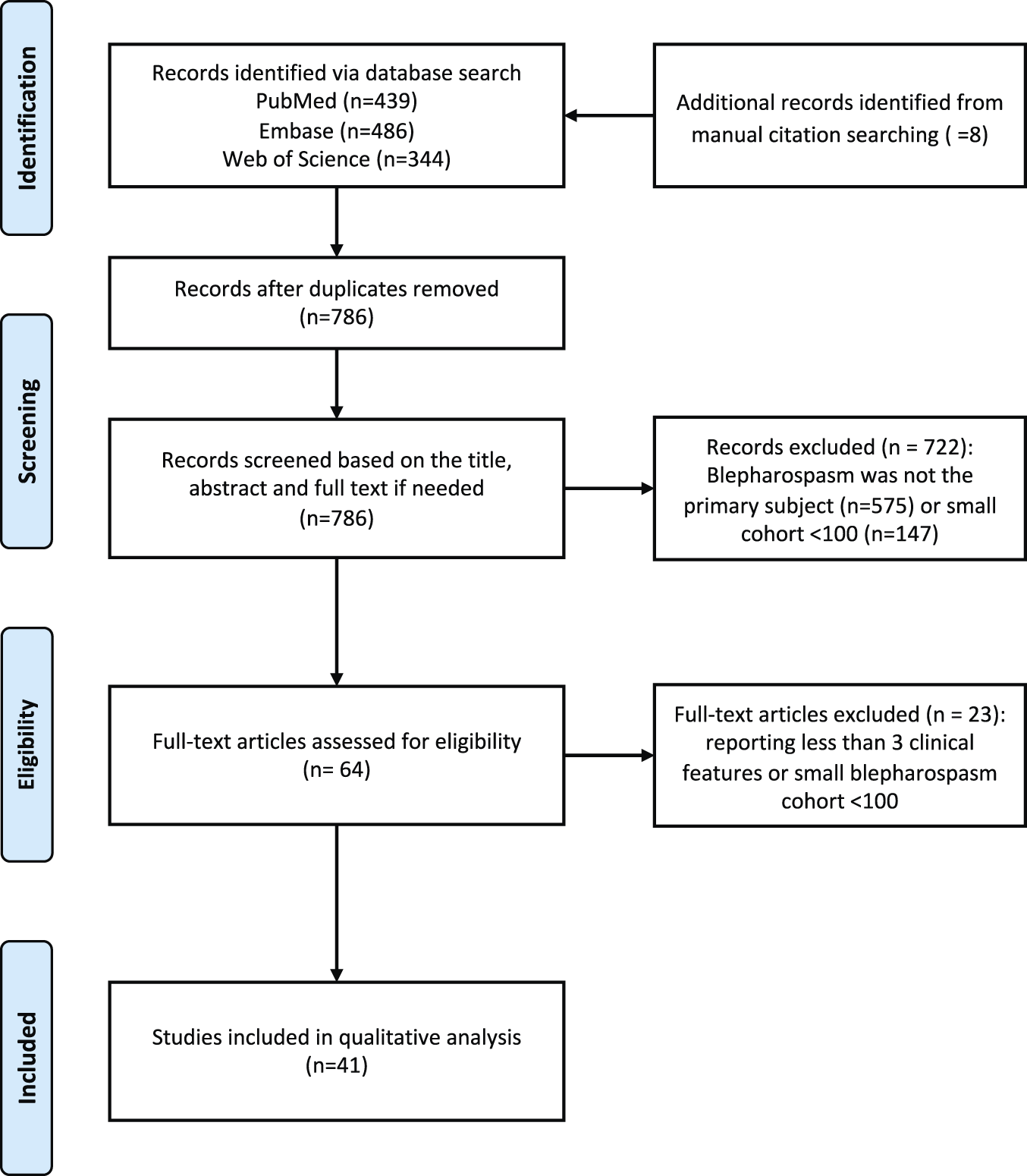
PRISMA flow diagram for literature review.
Dystonia Coalition (DC) Cohorts
Data from the DC were collected and analyzed for subjects enrolled across 45 international sites from 2011–2020 [39]. Subjects recruited for the DC had to be diagnosed with isolated dystonia (focal, segmental, multifocal, generalized). Inclusion in the current study required dystonia to be present in the upper face. Exclusion criteria included any evidence that dystonia that was acquired (e. g., medication-related tardive dystonia), functional (psychogenic), or associated with a known or suspected neurodegenerative disease (e. g., Parkinson’s disease). Also excluded were cases with significant medical or neurologic conditions that might preclude proper evaluation. For this study, focal BSP was defined as dystonia limited to the upper face (orbicularis oculi and nearby muscles). Additional involvement of the oromandibular region was considered segmental craniofacial dystonia.
The DC database includes several sub-studies [39]. Data for subjects with BSP were collected from the Biorepository Project, the Natural History Project, and the Blepharospasm Diagnosis Project. Each of these projects collected a core dataset for a total of 884 subjects that included demographic features along with the distribution and severity of dystonia according to individual body regions assessed using the Global Dystonia Rating Scale (GDRS) and the Burke-Fahn-Marsden dystonia rating scale (BFM) [40]. The GDRS is a Likert-like scale in which dystonia is rated from 0 (absent) to 10 (maximal severity). The BFM is a 120-point rating scale used to assess the severity of dystonia in nine body regions taking into account the severity and frequency of movements with a higher score indicative of greater impairment. A subgroup of 386 subjects in the Natural History Project also completed the Beck Depression Inventory II scale (BDI), and Leibowitz Social Anxiety scale (LSAS). A subgroup of 155 subjects in the Blepharospasm Diagnosis Project completed a survey of subjective symptoms such as light sensitivity, eye discomfort, and the presence of alleviating maneuvers. For the DC, recruitment of the same subject more than once was prevented by assigning all subjects a unique identifier and by conducting DNA fingerprinting on blood samples [39].
The study was approved by the IRBs of all participating clinical sites. All participants gave written consent for participation following the principles of the Declaration of Helsinki. The Emory University IRB and the National Institute of Health IRB also approved all procedures involving human participants.
Statistical Analysis
Because of the different methods and different types of data collected, analyses were completed separately for the literature review and DC cohorts. Results are given as average values ± standard deviations. Descriptive analyses for demographic and clinical characteristics were completed with two sample t-tests for continuous variables and chi-square tests for categorical variables, with p < 0.05 considered statistically significant.
For the DC cohort, we also compared BSP cases with onset of dystonia in the upper face to BSP cases with onset elsewhere. The DC cohort was also used to determine factors associated with spread of BSP beyond the face. Multivariate logistic regression was performed to estimate associations between spread of dystonia from the upper face and specific characteristics. Presence of dystonia elsewhere was determined by GDRS>0 in another body region. Demographic and clinical features of interest in the regression analysis were age at onset, dystonia duration, gender, family history, GDRS and BFM scores for upper face, BDI total score, and LSAS total score. A small amount of missing data was identified, but did not vary by exposure or outcome, and was treated as missing at random. All data analysis was performed with SAS version 9.4.
Results
Literature Review
Demographics and Risk Factors
Among 41 papers considered, 38 reported more females than males, with an overall weighted average of 71% female (Figure 2A). Twenty-one reported median age at onset in 5–6th decade, with an overall weighted average of 56 ± 6 years (Figure 2B). Six reported diagnosis was significantly delayed; two of these revealed that only about half of the patients received the correct diagnosis within one year [4, 10]. The median time from onset to diagnosis was 2 years in one study [22] and the weighted mean from three other studies was 46 ± 68 months [7, 11, 21].
FIGURE 2
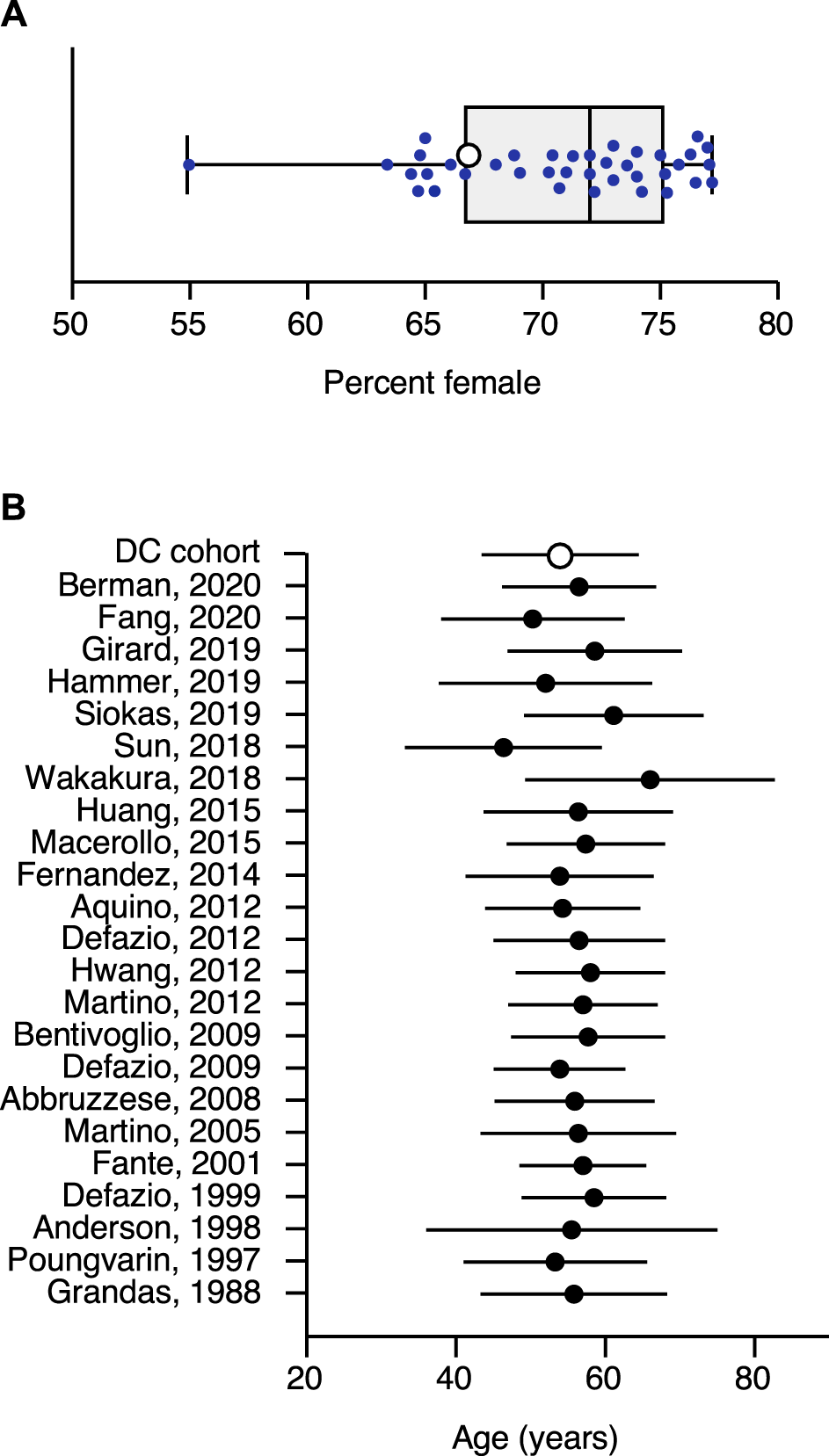
Sex and Age at Onset. (A) shows the percentage of females for all publications reaching criteria for inclusion in the literature review. The sex ratio in each study is represented by a filled circle. The open circle shows the sex ratio in the Dystonia Coalition cohort. The box shows the interquartile range of all studies, with the bar in the middle showing the median. Error bars show the full spread of data across all studies. (B) shows the mean age at onset in blepharospasm. This plot shows the average (filled circles) and standard deviation (error bars) for all publications reaching criteria for inclusion in the literature review. It also shows the average (open circle) and standard deviation for the Dystonia Coalition cohort.
Several studies also addressed potential risk factors for developing BSP including prior eye disease, eye surgery, trauma, stressful event, psychotropic and antiemetic use and white-collar occupation [4, 8-10, 24, 26, 29, 32, 41, 42]. Coffee was reported as potential protective factor in an Italian cohort [41, 43].
Presenting Features
Six reports described presenting symptoms of BSP [4, 5, 7, 10, 24, 26]. Increased blinking was the most common (51.9%), followed by ocular pain or soreness (38.7%), photophobia (35.5%), difficulty with eye opening (23.9%) and dry eyes (10.7%). One paper reported the median time to develop BSP following onset of initial symptoms was 12 months for increased blinking, 12 months for difficulty opening eyes, 11 months for dry eyes, and 6 months for photophobia [7]. Although BSP is defined as bilaterally symmetric disorder, three studies reported unilateral onset in 20%, [24] 26%, [44] or 62% [5] of cases.
Non-Motor Features in Established Cases
Among cases with established diagnoses of BSP, non-motor features were common. A large-scale epidemiological study [9] and one case-control study [33] identified a link between dry eye and BSP. One study performed a Schirmer I-test on 144 BSP patients by placing a filter paper in the inferior conjunctival fornices for 5-min and measuring the length of wetting [11]. In this study, 86.8% had Schirmer I-test values < 15 mm and 76.4% < 10 = mm consistent with dry eye syndrome. Anxiety (34%), depression (21%), insomnia (11%) and panic attacks (10%) were reported in three cohort studies [4, 7, 8]. The presence of non-motor symptoms did not correlate with severity of motor features [10]. Sensory tricks were reported in four cohort studies [4, 5, 8, 24]. The most common trick in one paper involved touching above the eyes (30%), followed by singing (26%) and talking (25%) [4]. Three papers reported improvement of symptoms with sleep and rest [5, 8, 24]. One paper noted sleep benefit and diurnal variations of symptoms in 81% of cases [5].
Progression
BSP is considered a chronic disorder and patients often report symptoms worsen over time. BSP also spreads to other parts of the body more frequently than dystonia that starts in other body regions [14, 15, 17, 27, 44, 45]. Most of the spread occurs in the first 5 years. Previous head trauma with loss of consciousness, age at onset and female sex were associated with an increased risk of spread in an Italian cohort [27]. Other factors such as age at onset and alcohol responsiveness have been associated with higher risk of spread [17]. One study suggested that a genetic variant of TOR1A may be associated with spread [31]. Spontaneous remissions were reported in three studies with frequencies of 1%, [44] 5%, [24] or 11% [46]. However, the majority of cases experienced recurrence after 1 month to 40 years [24, 44].
DC Cohort
Demographics
Among all 884 cases with BSP, the average age was 64 ± 11, the average age at onset was 49 ± 14, and 67% were female. Only 36% of the 884 cases had isolated focal BSP at onset (defined as dystonia limited to the orbicularis oculi and nearby muscles of upper face), and dystonia remained limited to the face in only 18%. The remainder of BSP cases had dystonia onset in other body regions. Table 1 provides a cross-sectional comparison of demographic and clinical features for cases who had onset in the upper face versus onset in other body regions. Those with onset in the upper face were slightly older with shorter durations of illness than those with onset elsewhere, although the magnitude of the difference was small. BFM and GDRS scores showed that those with onset in the upper face had more severe dystonia in the upper face but less severe dystonia overall than those with onset elsewhere.
TABLE 1
| Upper face onset N = 320 | Other site onset N = 564 | p-value | |
|---|---|---|---|
| Age (years) | 64 ± 10 | 63 ± 12 | 0.75 |
| Age of Onset (years) | 51 ± 13 | 48 ± 15 | <0.01 |
| Duration (years) | 12 ± 14 | 15 ± 15 | <0.01 |
| Percent Female | 65% (n = 209) | 68% (n = 382) | 0.46 |
| Areas Affected | |||
| Lower Face, Jaw, Tongue | 58% (n = 186) | 54% (n = 307) | 0.29 |
| Larynx | 13% (n = 43) | 21% (n = 121) | <0.01 |
| Neck | 47% (n = 150) | 68% (n = 383) | <0.01 |
| Limbs | 16% (n = 36) | 17% (n = 29) | 0.79 |
| Trunk | 3% (n = 9) | 8% (n = 42) | <0.01 |
| Sensory Trick | 56% (n = 180) | 54% (n = 305) | 0.53 |
| BFM Severity | |||
| Upper Face | 5 ± 2 | 3 ± 2 | <0.01 |
| Total | 10 ± 7 | 11 ± 11 | <0.01 |
| GDRS Severity | |||
| Upper Face | 5 ± 2 | 3 ± 3 | <0.01 |
| Total | 10 ± 7 | 13 ± 12 | <0.01 |
| BDI-II Total Score | 9 ± 8 | 9 ± 8 | 0.44 |
| LSAS Total Score | 29 ± 28 | 33 ± 29 | 0.20 |
Clinical characteristics of blepharospasm in the Dystonia Coalition cohort.
This table includes data for the entire cohort of 884 subjects with blepharospasm in the Dystonia Coalition cohort, divided according to those who had onset in the upper face, or those who had onset elsewhere with spread to the upper face. Abbreviations: BDI-II, Beck depression inventory version 2; BFM, Burke-Fahn-Marsden dystonia rating scale; GDRS, global dystonia rating scale; LSAS, Liebowitz social anxiety scale.
Specific Motor Features
The precise motor features of BSP were evaluated in more detail in a subset of 131 cases participating in a sub-project aimed at assessing these features. Clinician-rated assessments revealed subjects most commonly had mixed features of excessive blinking, periocular spasm, and apraxia of eyelid opening. Of the 131 cases, excessive blinking was reported for 98%, periocular spasm for 93%, and apraxia of eyelid opening for 29% (Figure 3A). One case had spasm without excessive blinking and eight cases had excessive blinking without spasm. None had isolated apraxia of eyelid opening. Associated lower face dystonia was reported for 51%. Subjects commonly reported mixed motor symptoms including involuntary eye closure (79%), incomplete eye opening (58%) and eyelid fluttering (78%) (Figure 3B).
FIGURE 3
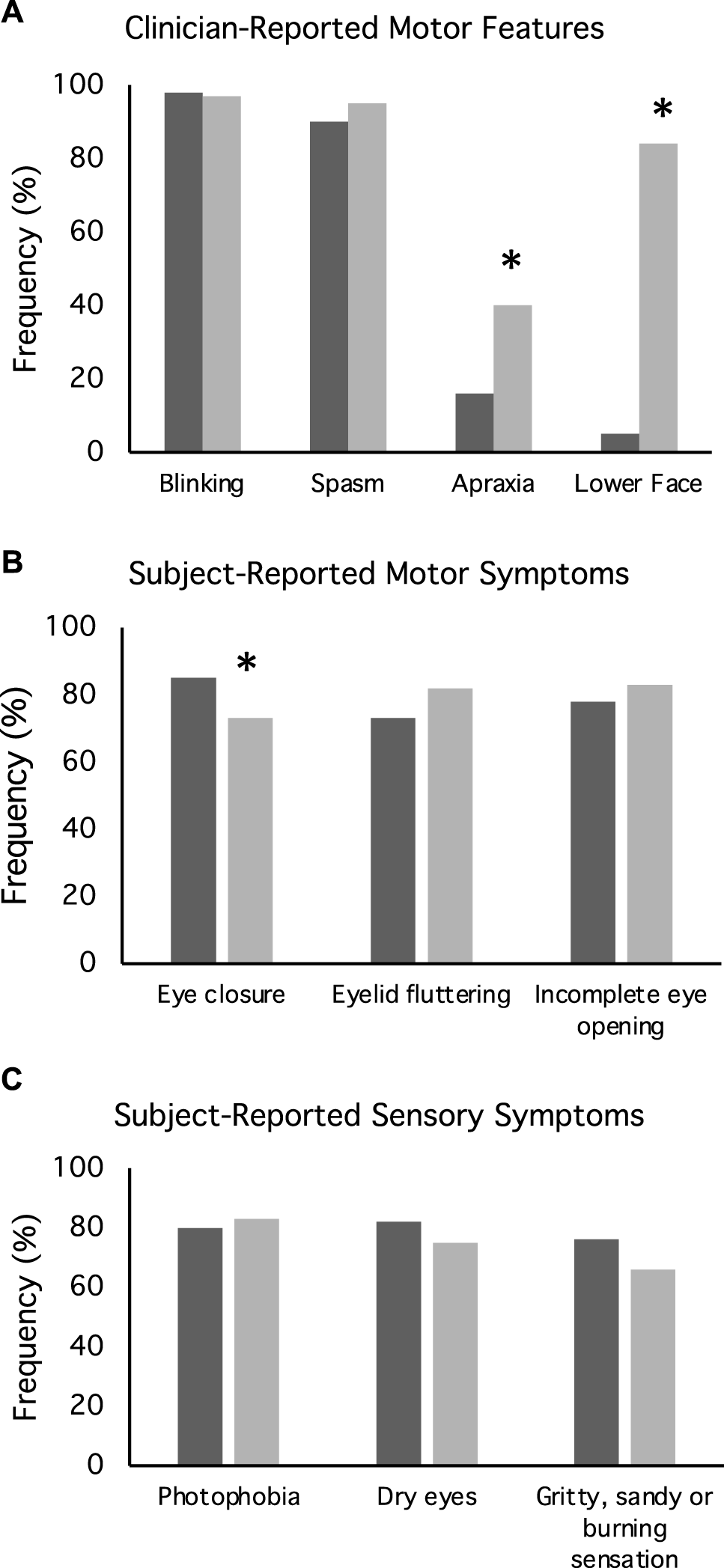
Motor and non-motor features of blepharospasm. These plots illustrate (A) clinician reported motor features, (B) subject reported motor features, and (C) subject reported sensory symptoms for both focal blepharospasm (dark bars) and blepharospasm (light bars) with subsequent spread of dystonia to other body regions. These data were derived from the subgroup of 155 subjects who participated in a blepharospasm rating scales project.
Non-Motor Features
Approximately half of the entire cohort of 884 cases reported a sensory trick lessened the severity of their dystonia. Among the 386 subjects in a sub-study that completed scales assessing psychiatric features, social anxiety was present in 40%, as indicated by LSAS scores greater than 30 points. Depression was present in 24%, as indicated by BDI-II scores greater than 13 points. For the subset of 151 subjects in the BSP scales subproject, patients filled out a questionnaire relating to sensory symptoms (Figure 3C) that revealed a high frequency of photophobia (82%), dry eyes (78%), or a gritty, sandy or burning sensation (70%).
Progression
Detailed information regarding the spread of dystonia beyond the upper face was available for a subgroup of 224 subjects who participated in a sub-study evaluating natural history (Table 2). Among 224 cases presenting with focal BSP, 61% experienced spread of dystonia. The most common region for spread was to the lower face (84%), followed by the neck (63%) (Table 2). Duration of dystonia, age at onset, and gender were not predictive of spread. Subjects reporting a family history of dystonia were 6.8-times more likely to have spread (p < 0.01). Likewise, increased severity of dystonia in the upper face as measured by GDRS and BFM was associated with spread (p < 0.01). Subjects in whom clinicians noted apraxia of eyelid opening (p < 0.01) and those with coincident lower facial dystonia (p < 0.01) were more likely to report subsequent spread (Figure 3A). Subjects reporting involuntary complete eye closure were 4.8 times more likely to develop spread (p = 0.04; Figure 3B). Subjective sensory symptoms were not associated with spread (Figure 3C). Both depression as measured by the BDI (p < 0.01) and social anxiety as measured by the LSAS score (p = 0.03) were associated with spread.
TABLE 2
| Focal blepharospasm N = 87 | Blepharospasm with subsequent spread N = 137 | p-value | |
|---|---|---|---|
| Age | 63 ± 10 | 63 ± 10 | 0.91 |
| Age of Onset (years) | 51 ± 13 | 52 ± 13 | 0.74 |
| Duration (years) | 12 ± 13 | 11 ± 14 | 0.63 |
| Sex | |||
| Female | 70% (n = 61) | 68% (n = 93) | 0.17 |
| Race | 0.17 | ||
| Asian | 7% (n = 6) | 2% (n = 3) | |
| Black | 6% (n = 5) | 4% (n = 6) | |
| Other | 8% (n = 7) | 4% (n = 6) | |
| White | 79% (n = 69) | 89% (n = 122) | |
| Family History of Dystonia | 2% (n = 2) | 14% (n = 19) | <0.01 |
| Areas Affected | |||
| Lower Face, Jaw, Tongue | 84% (n = 115) | ||
| Larynx | 16% (n = 22) | ||
| Neck | 63% (n = 86) | ||
| Limbs | 26% (n = 36) | ||
| Trunk | 3% (n = 4) | ||
| Sensory Trick | 42% (n = 37) | 64% (n = 88) | <0.01 |
| BFM Severity | |||
| Upper Face | 4.2 ± 2.5 | 6.1 ± 1.6 | <0.01 |
| Total | 4.2 ± 2.5 | 12.3 ± 8.0 | <0.01 |
| GDRS Severity | |||
| Upper Face | 4.4 ± 2.1 | 6.4 ± 1.9 | <0.01 |
| Total | 4.4 ± 2.1 | 12.6 ± 6.4 | <0.01 |
| BoNT Treatment | 82% (n = 72) | 79% (n = 108) | 0.46 |
| BDI-II Total Score | 5.6 ± 7.2 | 9.0 ± 8.4 | <0.01 |
| LSAS Total Score | 22.4 ± 26.6 | 32.0 ± 29.3 | 0.03 |
Comparison of blepharospasm cases with and without subsequent spread.
This table includes data for a subset of 224 cases in the Dystonia Coalition database who participated in a sub-study in which information regarding site of origin of dystonia was available. Abbreviations: BDI-II, Beck depression inventory version 2; BFM, Burke-Fahn-Marsden dystonia rating scale; BoNT, Botulinum neurotoxin; GDRS, global dystonia rating scale; LSAS, Liebowitz social anxiety scale.
Discussion
The incorrect and frequently delayed diagnoses reported in the literature highlight the need for better awareness of the many varied clinical features of BSP. To this end, this study provides a comprehensive summary of the clinical features of BSP. Despite different strategies for inclusion and evaluation, results from the literature review are quite similar to those of the DC cohort. Like other focal dystonias [47, 48], BSP tends to emerge in the 50s. Like most other focal dystonias [47, 48], BSP is more common in females. Though spasm of the orbicularis oculi and surrounding muscles may be the most widely recognized feature of BSP, excessive blinking was the most commonly observed sign by clinicians, and eyelid fluttering was frequently reported by subjects. The presence of apraxia of eyelid opening was less common, although its frequency depends on how it is defined. It was observed in approximately a third of DC cases. Finally, non-motor features were common. Sensory features may be a prominent or presenting problem and include irritating eye sensations such as dryness or a gritty feeling, along with photophobia. Depression and anxiety were also common.
The strengths of this study include the use of two independent cohorts, both of which were very large. The literature review included 10,324 cases taken from 41 reports from many parts of the world. The DC cohort is the largest single cohort to be reported, with 884 cases recruited in a multi-center design with 45 centers internationally. The main weakness of this study is inability to directly compare the two cohorts, because of the different methods and designs employed. Another weakness of this study is over-representation of white individuals in the DC cohort, although the results appear to be similar to cohorts of predominantly non-white individuals, such as those from Asia. Another weakness is the lack of information regarding treatment strategies or treatment outcomes, which were not collected for the DC cohort and often not presented for previously published cohorts. The final weakness is the lack of any associated biological measure such as genetics, imaging, or physiology. Unfortunately, the available genes account for <1% of most BSP cases, routine clinical imaging studies rarely reveal any consistent abnormalities, and most centers do not routinely conduct physiological studies. This omission points to the need for additional studies of relevant biomarkers.
Although the clinical features of BSP are quite characteristic, there are several reasons that might account for incorrect or delayed diagnoses. One reason may be that many individuals present first with non-motor symptoms such as irritation of the eyes and/or psychiatric concerns. Eye discomfort with excessive blinking may wrongly suggest allergies or dry eyes, which are very common in this age group. Anxiety and depression may lead to an initial psychiatric diagnosis. The reduction in BSP symptoms when talking while giving the history may wrongly suggest inconsistency or susceptibility to distraction, typical of functional (psychogenic) dystonia. Another reason may be that BSP is relatively uncommon, leading to misdiagnoses of more common disorders. However, common initial misdiagnoses also include myasthenia gravis, which is less common than BSP [10]. One study found myasthenia gravis to be the most common initial misdiagnosis [5], arguing that awareness of BSP may not be as good as awareness of myasthenia gravis.
Regarding the progression of BSP, the results from the DC cohort are consistent with other studies that have revealed a high risk for BSP to spread beyond the upper face [12-17, 27]. Common regions for spread include the lower face, jaw, and neck. Spread to other regions may also occur but appears less common. Factors associated with spread include severity of BSP and family history. Despite the fact that family history is likely to be under-recognized in studies that rely on patient report [49], the association of family history with spread suggests that genetic factors contribute to spread. The current study also revealed that spread was associated with psychiatric features. Whether the psychiatric features are a contributor or consequence of spread cannot be determined from the available data. Similar to prior studies, other sensory features such as dry eyes or photophobia were not associated with spread. Identification of factors related to spread is useful to aid counseling for affected cases.
The importance of early recognition is underscored by the availability of effective treatments. Numerous reviews have been published [50-55]. Several botulinum toxin preparations have proven helpful in the treatment of BSP, with risk/benefit profiles that are similar across brands [50, 51, 53, 54, 56, 57]. Multiple long-term observational studies have demonstrated 68%–89% of individuals show improvement [34, 52, 58-62]. Most studies indicated that mean benefit latency ranged between 4 and 6 days and benefit duration averaged 10 weeks [30, 52, 56, 60], although some describe longer mean response times of 13–16 weeks [22, 25]. Because of their efficacy, botulinum toxins are the treatment of choice for most cases. When botulinum toxins fail, surgical procedures may be offered [63]. Peripheral surgical therapies include orbicularis oculi myectomy, frontalis sling, and differential section of the facial nerve. These procedures were reported to be beneficial in 73–80% of cases [28, 64, 65], although blinded studies with long-term follow-up are not available. Deep brain stimulation also may be offered to patients with BSP. A meta-analysis of 115 cases described in numerous small series suggested deep brain stimulation may be beneficial [66]. However, there is a known bias for publication of positive results, there are no large rigorous blinded studies, and deep brain stimulation has been reported to worsen or trigger BSP in some cases [4, 45]. The optimal surgical approach to BSP remains uncertain. The availability of numerous medical and surgical therapies for BSP emphasizes the need for more rapid accurate diagnosis, which is critical for shortening time from onset of symptoms to treatment in order to alleviate motor symptoms. Treating physicians may also need to address sensory features. For example, artificial tears or other eye lubricants lubrication can be helpful for dry or gritty sensations. Finally, psychiatric features also are common, and they may benefit from direct attention.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data are being shared upon approval of Dystonia Coalition Executive Committee. Requests to access these datasets should be directed to Dr. Gamze Kilic-Berkmen at dystoniacoalition@emory.edu.
Ethics statement
The study was approved by the IRBs of all participating clinical sites. All participants gave written consent for participation following the principles of the Declaration of Helsinki. The Emory University IRB and the National Institute of Health IRB approved all procedures involving human participants.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by grants to The Dystonia Coalition (NS065701, TR001456, NS116025) which is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported by the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Institute of Neurological Diseases and Stroke (NINDS). The Sartain Lanier Family Foundation as well as the Jean and Paul Amos Parkinson’s Disease and Movement Disorders Program Endowment also provided support for this study.
Acknowledgments
Additional DC investigators contributing to the recruitment of subjects for these analyses included Marian Evatt, Michael Johns, Rachel Saunders-Pullman, Alberto Espay, Alex Pantelyat, Sarah Pirio Richardson, William Ondo, Susan Fox, Natividad Stover, Stephen Reich, Claudia Testa, Daniel Truong, Oksana Suchowersky, Samuel Frank, Pinky Agarwal, Julie Leegwater-Kim, Tanya Harlow, Allison Brashear, Stephen Grill, Fatta Nahab, Christopher Groth, Sylvain Chouinard, Andres Deik, Charles Adler, Kailash Bhatia, Tao Xei.
Conflict of interest
RB serves as an associate editor for Neurology: Clinical Practice; performs botulinum toxin injections at the University of Rochester (30% effort); serves/has served on scientific advisory boards for Allergan, Ipsen, Merz and Revance; receives research support from Vaccinex, Fox Foundation, and Revance; NIH (NINDS, ORDR): Dystonia Coalition Projects, Site PI; Consultant for Oscine Corporation and Abvie/Allergan; receives fees as section editor and holds stock options in VisualDx; and has served as an expert witness in legal proceedings including malpractice, not involving commercial entities. SB is currently an investigator for clinical trials sponsored by Takeda Pharmaceutical Company and Bukwang Pharmaceutical Co. Ltd. BB has received research grant support from the Dystonia Coalition (receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke), the Parkinson’s Foundation, and the VCU School of Medicine. He has received honoraria from the MedLink Corporation and the International Parkinson and Movement Disorder Society and serves on the medical advisory board of the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association. SF Honoraria: Lundbeck, Sunovion, Biogen, Impel, Acorda, CereSpir. Grants: Medtronics, Boston Scientific, Sun Pharmaceuticals Advanced Research Company, Biohaven, Impax, Lilly, US World Meds, Sunovion Therapeutics, Neurocrine, Vaccinex, Voyager, Jazz Pharmaceuticals, CHDI Foundation, Michael J. Fox Foundation, NIH (U10 NS077366), Parkinson Foundation Royalties: Demos, Blackwell Futura, Springer for textbooks, Uptodate. Other: Signant Health (Bracket Global LLC), CNS Ratings LLC. VF receives a salary from NSW Health, has received unrestricted research grants from the Michael J Fox Foundation, Abbvie and Merz, is on Advisory Boards and/or has received travel grants from Abbvie, Allergan, Ipsen, Merz, Praxis, Seqirus, Stada, Teva and UCB, and receives royalties from Health Press Ltd. MH is an inventor of patents held by NIH for an immunotoxin for the treatment of focal movement disorders and the H-coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of CALA Health and Brainsway (both unpaid positions). He is on the Editorial Board of approximately 15 journals and receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, Wiley, Wolters Kluwer, and Elsevier. He has research grants from Medtronic, Inc. for a study of DBS for dystonia and CALA Health for studies of a device to suppress tremor. JJ has received research or training grants from AbbVie Inc.; Acadia Pharmaceuticals; Cerevel Therapeutics; CHDI Foundation; Dystonia Coalition; Emalex Biosciences, Inc.; F. Hoffmann-La Roche Ltd.; Huntington Study Group; Medtronic Neuromodulation; Merz Pharmaceuticals; Michael J Fox Foundation for Parkinson Research; National Institutes of Health; Neuraly, Inc.; Neurocrine Biosciences; Parkinson's Foundation; Parkinson Study Group; Prilenia Therapeutics; Revance Therapeutics, Inc.; Teva Pharmaceutical Industries Ltd. JJ has served as a consultant for Aeon BioPharma; Allergan, Inc.; Merck & Co., Inc.; Revance Therapeutics; Teva Pharmaceutical Industries Ltd. JJ has received royalties from Cambridge; Elsevier; Medlink: Neurology; Lippincott Williams and Wilkins; UpToDate; Wiley-Blackwell. Editorial boards: Expert Review of Neurotherapeutics; Medlink; Neurology in Clinical Practice; The Botulinum Journal; PeerJ; Therapeutic Advances in Neurological Disorders; Neurotherapeutics; Toxins; Tremor and Other Hyperkinetic Movements; Journal of Parkinson’s Disease. HJ has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (Cure Dystonia Now), and industry (Revance Therapeutics, Inc.). HJ has also served on advisory boards or as a consultant for Addex, Allergan, CoA Therapeutics, Cavion Therapeutics, EnePharmaceuticals, Ipsen, Retrophin, Revance, and Takaha Pharmaceuticals. He has received honoraria or stipends for lectures or administrative work from the International Parkinson’s Disease and Movement Disorders Society. DHJ serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, the Tourette Association of America, and Tyler’s Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which has received the majority of its support through the NIH (grants NS116025, NS065701 from the National Institutes of Neurological Disorders and Stroke TR 001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences). The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, The Dystonia Medical Research Foundation, and The National Spasmodic Dysphonia Association). CK has active or recent grant support from the DFG, BMBF, and the MJFF. She serves as a medical advisor on genetic testing reports to Centogene and on the Scientific Advisory Board of Retromer Therapeutics, and the Else Kroener Fresenius Foundation. IM has participated in research funded by the Parkinson Foundation, Tourette Association, Dystonia Coalition, AbbVie, Boston Scientific, Eli Lilly, Neuroderm, Prilenia, Revance, Teva but has no owner interest in any pharmaceutical company. She has received travel compensation or honoraria from the Tourette Association of America, Parkinson Foundation, International Association of Parkinsonism and Related Disorders, Medscape, and Cleveland Clinic, and royalties for writing a book with Robert Rose publishers. JM performs compensated consulting services for Biocircuit Technologies. He receives research funding from NIH and the McCamish Foundation. JP has provided medical legal consultation to Wood, Cooper and Peterson, LLC and to Simmons and Simmons LLP. JP serves as Director of Medical and Scientific Advisory Committee of the Dystonia Medical Research Foundation, Chair of the Scientific Advisory Committee of the Parkinson Study Group, Chair of the Standards Committee of the Huntington Study Group, member of the Scientific Advisory Board of the APDA, Chair of the Scientific and Publication Committee for ENROLL-HD (honoraria from this one), and member of the Education Committee of the Huntington Study Group (honoraria from this one). He has received honoraria from CHDI, Huntington Disease Study Group, Parkinson Study Group, Beth Israel Hospital (Harvard group), U Pennsylvania, Stanford U.; U Illinois in Chicago (box of biscotti was my honorarium); Boston University. JP has received research funding from National Institutes of Health NS075321, NS103957, NS107281, NS092865, U10NS077384, NS097437, U54NS116025, U19 NS110456, AG050263, AG-64937, NS097799, NS075527, ES029524, NS109487, R61 AT010753, (NCATS, NINDS, NIA), RO1NS118146, R01AG065214, Department of Defense (DOD W81XWH-217-1-0393), Michael J Fox Foundation, Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson disease research fund), American Parkinson Disease Association (APDA) Advanced Research Center at Washington University, Greater St. Louis Chapter of the APDA, Paula and Rodger Riney Fund, Jo Oertli Fund, Huntington Disease Society of America, Murphy Fund, Fixel Foundation, Cure Huntington’s Disease Initiative, and G. Williams Fund. He is also co-director for the Dystonia Coalition, which has received the majority of its support through the NIH (grants NS116025, NS065701 from the National Institutes of Neurological Disorders and Stroke TR 001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences). ER served on scientific advisory boards for Orkyn, Aguettant, Merz-Pharma, Allergan; received honoraria for speeches from Orkyn, Aguettant, Merz-Pharma, Everpharma, Elivie, International Parkinson and Movement disorders Society; received research support from Merz-Pharma, Orkyn, Aguettant, Elivie, Ipsen, Allergan, Everpharma, Fondation Desmarest, AMADYS, Fonds de Dotation Brou de Lauriè re, ADCY5. org, Agence Nationale de la Recherche, Societé Française de M#233; decine Esthé tique; received travel grant from Vitalaire, PEPS development, Aguettant, Merz-Pharma, Ipsen, Merck, Orkyn, Elivie, Adelia Medical, Dystonia Medical Research Foundation, International Parkinson and Movement disorders Society, European Academy of Neurology, International Association of Parkinsonism and Related Disorders. LS has received grant support from the Dystonia Medical Research Foundation. She also serves as an investigator in clinical trials for Addex, Neurocrine, and Boston Scientific. TW is a consultant for Linden & Associates, Sherrard, Roe, Voigt, and Harbison. AW-S reports grants from the NIH and has received grant support from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders and grant support from NIH (KL2 and K23 NS092957-01A1) as a PI. She receives support from NIH RO1 R01NS121120-01 as a Co-I. AW-S has received consultant fees from Merz and Acadia. She is the current Vice President for the Tremor Research Group. ML owns, and is president of, Veracity Neuroscience LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Albanese A Bhatia K Bressman SB DeLong MR Fahn S Fung VSC et al Phenomenology and Classification of Dystonia: A Consensus Update. Mov Disord (2013) 28:863–73. 10.1002/mds.25475
2.
Defazio G Hallett M Jinnah HA Berardelli A . Development and Validation of a Clinical Guideline for Diagnosing Blepharospasm. Neurology (2013) 81:236–40. 10.1212/wnl.0b013e31829bfdf6
3.
Defazio G Hallett M Jinnah HA Conte A Berardelli A . Blepharospasm 40 Years Later. Mov Disord (2017) 32:498–509. 10.1002/mds.26934
4.
Peckham EL Lopez G Shamim EA Richardson SP Sanku S Malkani R et al Clinical Features of Patients with Blepharospasm: a Report of 240 Patients. Eur J Neurol (2011) 18:382–6. 10.1111/j.1468-1331.2010.03161.x
5.
Hwang WJ . Demographic and Clinical Features of Patients with Blepharospasm in Southern Taiwan: a university Hospital-Based Study. Acta Neurol Taiwan (2012) 21:108–14.
6.
Patel N Jankovic J Hallett M . Sensory Aspects of Movement Disorders. Lancet Neurol (2014) 13:100–12. 10.1016/s1474-4422(13)70213-8
7.
Huang X-F Wang K-Y Liang Z-H Du R-R Zhou L-N . Clinical Analysis of Patients with Primary Blepharospasm: A Report of 100 Cases in China. Eur Neurol (2015) 73:337–41. 10.1159/000381707
8.
Lee JM Baek JS Choi HS Kim SJ Jang JW . Clinical Features of Benign Essential Blepharospasm in Korean Patients. Korean J Ophthalmol (2018) 32:339–43. 10.3341/kjo.2018.0038
9.
Sun Y Tsai P-J Chu C-L Huang W-C Bee Y-S . Epidemiology of Benign Essential Blepharospasm: A Nationwide Population-Based Retrospective Study in Taiwan. PLoS One (2018) 13:e0209558. 10.1371/journal.pone.0209558
10.
Wakakura M Yamagami A Iwasa M . Blepharospasm in Japan: A Clinical Observational Study from a Large Referral Hospital in Tokyo. Neuro-Ophthalmology (2018) 42:275–83. 10.1080/01658107.2017.1409770
11.
Girard BC Lévy P . Dry Eye Syndrome in Benign Essential Blepharospasm. J Français d'Ophtalmologie (2019) 42:1062–7. 10.1016/j.jfo.2019.06.007
12.
Weiss EM Hershey T Karimi M Racette B Tabbal SD Mink JW et al Relative Risk of Spread of Symptoms Among the Focal Onset Primary Dystonias. Mov Disord (2006) 21:1175–81. 10.1002/mds.20919
13.
Svetel M Pekmezović T Jović J Ivanović N Dragašević N Marić J et al Spread of Primary Dystonia in Relation to Initially Affected Region. J Neurol (2007) 254:879–83. 10.1007/s00415-006-0457-8
14.
Abbruzzese G Berardelli A Girlanda P Marchese R Martino D Morgante F et al Long-term Assessment of the Risk of Spread in Primary Late-Onset Focal Dystonia. J Neurol Neurosurg Psychiatry (2008) 79:392–6. 10.1136/jnnp.2007.124594
15.
Martino D Berardelli A Abbruzzese G Bentivoglio AR Esposito M Fabbrini G et al Age at Onset and Symptom Spread in Primary Adult-Onset Blepharospasm and Cervical Dystonia. Mov Disord (2012) 27:1447–50. 10.1002/mds.25088
16.
Svetel M Pekmezovic T Tomic A Kresojevic N Kostic VS . The Spread of Primary Late-Onset Focal Dystonia in a Long-Term Follow up Study. Clin Neurol Neurosurg (2015) 132:41–3. 10.1016/j.clineuro.2015.02.015
17.
Berman BD Groth CL Sillau SH Pirio Richardson S Norris SA Junker J et al Risk of Spread in Adult-Onset Isolated Focal Dystonia: a Prospective International Cohort Study. J Neurol Neurosurg Psychiatry (2020) 91:314–20. 10.1136/jnnp-2019-321794
18.
Steeves TD Day L Dykeman J Jette N Pringsheim T . The Prevalence of Primary Dystonia: a Systematic Review and Meta-Analysis. Mov Disord (2012) 27:1789–96. 10.1002/mds.25244
19.
Powell AT Bidewell JW Walker AC . Diagnosing Idiopathic Dystonia: Must it Take So Long?Aust Health Rev (1995) 18:120–31.
20.
Jog M Chouinard S Hobson D Grimes D Chen R Bhogal M et al Causes for Treatment Delays in Dystonia and Hemifacial Spasm: a Canadian Survey. Can J Neurol Sci (2011) 38:704–11. 10.1017/s0317167100012270
21.
Macerollo A Superbo M Gigante AF Livrea P Defazio G . Diagnostic Delay in Adult-Onset Dystonia: Data from an Italian Movement Disorder center. J Clin Neurosci (2015) 22:608–10. 10.1016/j.jocn.2014.09.014
22.
Fang X-b. Xie M-s. Song Z-b. Zhong Z-g. Wang Y Ou Z-l. et al Long-term Treatment of Blepharospasm with Botulinum Toxin A: a Service-Based Study over a 16-year Follow-Up in Southern China. Neurol Sci (2020) 41:645–52. 10.1007/s10072-019-04123-8
23.
LeDoux MS . Meige Syndrome: What's in a Name?Parkinsonism Relat Disord (2009) 15:483–9. 10.1016/j.parkreldis.2009.04.006
24.
Grandas F Elston J Quinn N Marsden CD . Blepharospasm: a Review of 264 Patients. J Neurol Neurosurg Psychiatry (1988) 51:767–72. 10.1136/jnnp.51.6.767
25.
Poungvarin N Devahastin V Chaisevikul R Prayoonwiwat N Viriyavejakul A . Botulinum A Toxin Treatment for Blepharospasm and Meige Syndrome: Report of 100 Patients. J Med Assoc Thai (1997) 80:1–8.
26.
Anderson RL Patel BCK Holds JB Jordan DR . Blepharospasm. Ophthalmic Plast Reconstr Surg (1998) 14:305–17. 10.1097/00002341-199809000-00002
27.
Defazio G Berardelli A Abbruzzese G Coviello V Carella F De Berardinis MT et al Risk Factors for Spread of Primary Adult Onset Blepharospasm: a Multicentre Investigation of the Italian Movement Disorders Study Group. J Neurol Neurosurg Psychiatry (1999) 67:613–9. 10.1136/jnnp.67.5.613
28.
Fante RG Frueh BR . Differential Section of the Seventh Nerve as a Tertiary Procedure for the Treatment of Benign Essential Blepharospasm. Ophthalmic Plast Reconstr Surg (2001) 17:276–80. 10.1097/00002341-200107000-00007
29.
Martino D Defazio G Alessio G Abbruzzese G Girlanda P Tinazzi M et al Relationship between Eye Symptoms and Blepharospasm: a Multicenter Case-Control Study. Mov Disord (2005) 20:1564–70. 10.1002/mds.20635
30.
Bentivoglio AR Fasano A Ialongo T Soleti F Lo Fermo S Albanese A . Fifteen-year Experience in Treating Blepharospasm with Botox or Dysport: Same Toxin, Two Drugs. Neurotox Res (2009) 15:224–31. 10.1007/s12640-009-9023-3
31.
Defazio G Matarin M Peckham EL Martino D Valente EM Singleton A et al The TOR1A Polymorphism Rs1182 and the Risk of Spread in Primary Blepharospasm. Mov Disord (2009) 24:613–6. 10.1002/mds.22471
32.
Aquino CC Felício AC Castro PCFd. Oliveira RA Silva SMCA Borges V et al Clinical Features and Treatment with Botulinum Toxin in Blepharospasm: a 17-year Experience. Arq Neuro-psiquiatr (2012) 70:662–6. 10.1590/s0004-282x2012000900003
33.
Defazio G Abbruzzese G Stella Aniello M Di Fede R Esposito M Fabbrini G et al Eye Symptoms in Relatives of Patients with Primary Adult-Onset Dystonia. Mov Disord (2012) 27:305–7. 10.1002/mds.24026
34.
Fernandez HH Jankovic J Holds JB Lin D Burns J Verma A et al Observational Study of incobotulinumtoxinA for Cervical Dystonia or Blepharospasm (XCiDaBLE): Interim Results for the First 170 Subjects with Blepharospasm. Tremor Other Hyperkinet Mov (N Y) (2014) 4:238. 10.5334/tohm.181
35.
Hammer M Abravanel A Peckham E Mahloogi A Majounie E Hallett M et al Blepharospasm: A Genetic Screening Study in 132 Patients. Parkinsonism Relat Disord (2019) 64:315–8. 10.1016/j.parkreldis.2019.04.003
36.
Siokas V Kardaras D Aloizou A-M Liampas I Papageorgiou E Drakoulis N et al CYP1A2 Rs762551 and ADORA2A Rs5760423 Polymorphisms in Patients with Blepharospasm. J Mol Neurosci (2020) 70:1370–5. 10.1007/s12031-020-01553-4
37.
Siokas V Kardaras D Aloizou A-M Asproudis I Boboridis KG Papageorgiou E et al BDNF Rs6265 (Val66Met) Polymorphism as a Risk Factor for Blepharospasm. Neuromol Med (2019) 21:68–74. 10.1007/s12017-018-8519-5
38.
Siokas V Kardaras D Aloizou A-M Asproudis I Boboridis KG Papageorgiou E et al Lack of Association of the Rs11655081 ARSG Gene with Blepharospasm. J Mol Neurosci (2019) 67:472–6. 10.1007/s12031-018-1255-3
39.
Kilic-Berkmen G Wright LJ Perlmutter JS Comella C Hallett M Teller J et al The Dystonia Coalition: A Multicenter Network for Clinical and Translational Studies. Front Neurol (2021) 12:660909. 10.3389/fneur.2021.660909
40.
Albanese A Sorbo FD Comella C Jinnah HA Mink JW Post B et al Dystonia Rating Scales: Critique and Recommendations. Mov Disord (2013) 28:874–83. 10.1002/mds.25579
41.
Defazio G Abbruzzese G Aniello MS Bloise M Crisci C Eleopra R et al Environmental Risk Factors and Clinical Phenotype in Familial and Sporadic Primary Blepharospasm. Neurology (2011) 77:631–7. 10.1212/wnl.0b013e3182299e13
42.
Defazio G Abbruzzese G Girlanda P Liguori R Santoro L Tinazzi M et al Phenotypic Overlap in Familial and Sporadic Primary Adult-Onset Extracranial Dystonia. J Neurol (2012) 259:2414. 10.1007/s00415-012-6514-6
43.
Defazio G Martino D Abbruzzese G Girlanda P Tinazzi M Fabbrini G et al Influence of Coffee Drinking and Cigarette Smoking on the Risk of Primary Late Onset Blepharospasm: Evidence from a Multicentre Case Control Study. J Neurol Neurosurg Psychiatry (2007) 78:877–9. 10.1136/jnnp.2007.119891
44.
Jankovic J Orman J . Blepharospasm: Demographic and Clinical Survey of 250 Patients. Ann Ophthalmol (1984) 16:371–6.
45.
Ferrazzano G Conte A Gigante A Defazio G Berardelli A Fabbrini G . Disease Progression in Blepharospasm: a 5-year Longitudinal Study. Eur J Neurol (2019) 26:268–73. 10.1111/ene.13832
46.
Castelbuono A Miller NR . Spontaneous Remission in Patients with Essential Blepharospasm and Meige Syndrome. Am J Ophthalmol (1998) 126:432–5. 10.1016/s0002-9394(98)00099-3
47.
Jinnah HA Berardelli A Comella C DeFazio G DeLong MR Factor S et al The Focal Dystonias: Current Views and Challenges for Future Research. Mov Disord (2013) 28:926–43. 10.1002/mds.25567
48.
Balint B Mencacci NE Valente EM Pisani A Rothwell J Jankovic J et al Dystonia. Nat Rev Dis Primers (2018) 4:25. 10.1038/s41572-018-0023-6
49.
Leube B Kessler KR Goecke T Auburger G Benecke R . Frequency of Familial Inheritance Among 488 index Patients with Idiopathic Focal Dystonia and Clinical Variability in a Large Family. Mov Disord (1997) 12:1000–6. 10.1002/mds.870120625
50.
Colosimo C Tiple D Berardelli A . Efficacy and Safety of Long-Term Botulinum Toxin Treatment in Craniocervical Dystonia: a Systematic Review. Neurotox Res (2012) 22:265–73. 10.1007/s12640-012-9314-y
51.
Hallett M Albanese A Dressler D Segal KR Simpson DM Truong D et al Evidence-based Review and Assessment of Botulinum Neurotoxin for the Treatment of Movement Disorders. Toxicon (2013) 67:94–114. 10.1016/j.toxicon.2012.12.004
52.
Fezza J Burns J Woodward J Truong D Hedges T Verma A . A Cross-Sectional Structured Survey of Patients Receiving Botulinum Toxin Type A Treatment for Blepharospasm. J Neurol Sci (2016) 367:56–62. 10.1016/j.jns.2016.05.033
53.
Simpson DM Hallett M Ashman EJ Comella CL Green MW Gronseth GS et al Practice Guideline Update Summary: Botulinum Neurotoxin for the Treatment of Blepharospasm, Cervical Dystonia, Adult Spasticity, and Headache. Neurology (2016) 86:1818–26. 10.1212/wnl.0000000000002560
54.
Duarte GS Rodrigues FB Marques RE Castelão M Ferreira J Sampaio C et al Botulinum Toxin Type A Therapy for Blepharospasm. Cochrane Database Syst Rev (2020) 11:CD004900. 10.1002/14651858.CD004900.pub3
55.
Anandan C Jankovic J . Botulinum Toxin in Movement Disorders: An Update. Toxins (Basel) (2021) 13(1). 10.3390/toxins13010042
56.
Kollewe K Mohammadi B Köhler S Pickenbrock H Dengler R Dressler D . Blepharospasm: Long-Term Treatment with Either Botox, Xeomin or Dysport. J Neural Transm (2015) 122:427–31. 10.1007/s00702-014-1278-z
57.
Mitsikostas DD Dekundy A Hanschmann A Althaus M Scheschonka A Pagan F et al Duration and Onset of Effect of incobotulinumtoxinA for the Treatment of Blepharospasm in Botulinum Toxin-Naive Subjects. Curr Med Res Opin (2021) 37:1761–8. 10.1080/03007995.2021.1965975
58.
Elston JS . Long-term Results of Treatment of Idiopathic Blepharospasm with Botulinum Toxin Injections. Br J Ophthalmol (1987) 71:664–8. 10.1136/bjo.71.9.664
59.
Jankovic J Kenney C Grafe S Goertelmeyer R Comes G . Relationship between Various Clinical Outcome Assessments in Patients with Blepharospasm. Mov Disord (2009) 24:407–13. 10.1002/mds.22368
60.
Jankovic J Comella C Hanschmann A Grafe S . Efficacy and Safety of incobotulinumtoxinA (NT 201, Xeomin) in the Treatment of Blepharospasm-A Randomized Trial. Mov Disord (2011) 26:1521–8. 10.1002/mds.23658
61.
Truong DD Gollomp SM Gollomp SM Jankovic J LeWitt PA Marx M et al Sustained Efficacy and Safety of Repeated incobotulinumtoxinA (Xeomin) Injections in Blepharospasm. J Neural Transm (2013) 120:1345–53. 10.1007/s00702-013-0998-9
62.
Vivancos-Matellano F Rodríguez-Sanz A Herrero-Infante Y Mascías-Cadavid J . Efficacy and Safety of Long-Term Therapy with Type A Botulinum Toxin in Patients with Blepharospasm. Neuro-Ophthalmology (2019) 43:277–83. 10.1080/01658107.2018.1542009
63.
Jinnah HA . Medical and Surgical Treatments for Dystonia. Neurol Clin (2020) 38:325–48. 10.1016/j.ncl.2020.01.003
64.
Grivet D Robert P-Y Thuret G De Féligonde OP Gain P Maugery J et al Assessment of Blepharospasm Surgery Using an Improved Disability Scale: Study of 138 Patients. Ophthal Plast Reconstr Surg (2005) 21:230–4. 10.1097/01.iop.0000162429.97307.4d
65.
Wabbels B Roggenkämper P . Long-term Follow-Up of Patients with Frontalis Sling Operation in the Treatment of Essential Blepharospasm Unresponsive to Botulinum Toxin Therapy. Graefes Arch Clin Exp Ophthalmol (2007) 245:45–50. 10.1007/s00417-006-0392-5
66.
Wang X Zhang Z Mao Z Yu X . Deep Brain Stimulation for Meige Syndrome: a Meta-Analysis with Individual Patient Data. J Neurol (2019) 266:2646–56. 10.1007/s00415-019-09462-2
Summary
Keywords
Dystonia, Meige syndrome, eyes, jaw, phenotype, Blepharospasm, Oromandibular dystonia
Citation
Scorr LM, Cho HJ, Kilic-Berkmen G, McKay JL, Hallett M, Klein C, Baumer T, Berman BD, Feuerstein JS, Perlmutter JS, Berardelli A, Ferrazzano G, Wagle-Shukla A, Malaty IA, Jankovic J, Bellows ST, Barbano RL, Vidailhet M, Roze E, Bonnet C, Mahajan A, LeDoux MS, Fung VSC, Chang FCF, Defazio G, Ercoli T, Factor S, Wojno T and Jinnah HA (2022) Clinical Features and Evolution of Blepharospasm: A Multicenter International Cohort and Systematic Literature Review. Dystonia 1:10359. doi: 10.3389/dyst.2022.10359
Received
14 January 2022
Accepted
28 February 2022
Published
16 May 2022
Volume
1 - 2022
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Reviewed by
Sanjay Pandey, University of Delhi, India
Camilla Kilbane, Case Western Reserve University, United States
Updates
Copyright
© 2022 Scorr, Cho, Kilic-Berkmen, McKay, Hallett, Klein, Baumer, Berman, Feuerstein, Perlmutter, Berardelli, Ferrazzano, Wagle-Shukla, Malaty, Jankovic, Bellows, Barbano, Vidailhet, Roze, Bonnet, Mahajan, LeDoux, Fung, Chang, Defazio, Ercoli, Factor, Wojno and Jinnah.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. A. Jinnah, hjinnah@emory.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.