- 1Computational Neurology Center, Institute for Neural Computation, University of California, San Diego, San Diego, CA, United States
- 2Parkinson’s Disease Center and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine, Houston, TX, United States
- 3Department of Neurology, Emory University School of Medicine, Atlanta, GA, United States
- 4Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, United States
- 5Department of Neurology, University of Rochester, Rochester, NY, United States
- 6Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States
- 7Departments of Radiology, Neuroscience, Physical Therapy, and Occupational Therapy, Washington University School of Medicine, St. Louis, MO, United States
- 8Departments of Human Genetics and Pediatrics, Emory University School of Medicine, Atlanta, GA, United States
- 9Department of Neurology, University of New Mexico Health Sciences Center, Albuquerque, NM, United States
- 10Neurology Service, New Mexico Veterans Affairs Health Care System, Albuquerque, NM, United States
- 11Department of Neurology, Southern Illinois University School of Medicine, Springfield, IL, United States
- 12Computational Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, CA, United States
Introduction: A common view is that head tremor (HT) in cervical dystonia (CD) decreases when the head assumes an unopposed dystonic posture and increases when the head is held at midline. However, this has not been examined with objective measures in a large, multicenter cohort.
Methods: For 80 participants with CD and HT, we analyzed videos from examination segments in which participants were instructed to 1) let their head drift to its most comfortable position (null point) and then 2) hold their head straight at midline. We used our previously developed Computational Motor Objective Rater (CMOR) to quantify changes in severity, amplitude, and frequency between the two postures.
Results: Although up to 9% of participants had exacerbated HT in midline, across the whole cohort, paired t-tests reveal no significant changes in overall severity (t = −0.23, p = 0.81), amplitude (t = −0.80, p = 0.43), and frequency (t = 1.48, p = 0.14) between the two postures.
Conclusion: When instructed to first let their head drift to its null point and then to hold their head straight at midline, most patient’s changes in HT were below the thresholds one would expect from the sensitivity of clinical rating scales. Counter to common clinical impression, CMOR objectively showed that HT does not consistently increase at midline posture in comparison to the null posture.
Introduction
Head tremor (HT) commonly accompanies cervical dystonia (CD). Clinical impression suggests that HT is minimized when the head is at its “null” posture (1) and exacerbated when against the null posture. Because HT may be posture-dependent and quantified outcome measures are required for clinical trials for new HT treatments, it is important to quantify how HT differs between these postures. However, the dystonia rating scales that assess HT severity (2, 3) do not specify how the patient should hold their head while HT is assessed. The revised Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-2) implies at least two head postures for dystonia assessment: 1) the head is allowed to assume its unopposed null posture, and 2) the head is held straight within 10 degrees of the midline. Kinematic measures suggest that HT changes when patients hold their head in different orientations (4). However, this has not been examined in a large, multicenter cohort.
Computer vision methods have shown to be consistent with clinical scores and comparable to other objective methods in quantifying tremor (5,6). In this study, we used our computer vision system, Computational Motor Objective Rater (CMOR), to quantify how HT severity, power, and frequency change when participants are instructed to hold their head at midline in contrast to the unopposed posture.
Methods
We analyzed clinical data and examination videos from 206 participants enrolled across 10 sites by the Dystonia Coalition (7). All participants were assessed three or more months after their last botulinum toxin injections and provided informed consent before participation, in accordance with the Declaration of Helsinki. Protocols for this study were approved by the Human Research Protection Offices at Washington University School of Medicine, Rush University Medical Center, and University of California, San Diego (protocol 111255X).
One movement disorders neurologist at each site evaluated HT in each of their participants according to the TWSTRS-2 HT item (3). Because of the TWSTRS’ 0–4 scaling and inherent inter-rater variability, we also had a single movement disorders neurologist (CLC) assess all videos for HT severity using ordinal head tremor scores (HTS) from 0–10 (8). Participants were retained if both raters agreed on presence of HT, which may include jerky head movements, sinusoidal head movements, and any combination of jerky and sinusoidal head movements.
HT was assessed in two head postures. First, participants were instructed to close their eyes and let their head drift to its most comfortable posture for 10 s. This posture is often a null point for dystonia and is hereafter referred to as “null posture.” Second, participants were instructed to hold their head straight at midline for up to 1 min.
Video annotation, quality control review, participant exclusions, and spectral processing were performed as described in our previous study (6). We used our computer vision system, Computational Motor Objective Rater (CMOR) to estimate head pose from each frame in each of the three conventional axes (antero/retrocollis, laterocollis, and torticollis) (6). We used Matlab’s pwelch function to compute log10 peak spectral power (“amplitude,” deg2/Hz) and peak frequency (Hz).
To determine whether there were changes between the null and midline postures, we utilized paired t-tests to compare them in terms of CMOR severity, amplitude, and frequency. To contextualize these changes with the sensitivity of clinical severity assessments, we compared each participant’s CMOR measure of change to the level of change that corresponds to a one-point change (OPC) in HTS and TWSTRS-2 HT. The OPC was computed from linear regression equations from our previous study (Eqs 1, 2) (6). OPCs were simply the inverse of the respective variable’s coefficient. The OPC in HTS is 0.61 deg2/Hz for amplitude and 3.23 Hz for peak frequency. The OPC in TWSTRS-2 is 1.52 deg2/Hz for amplitude and 8.07 Hz for peak frequency.
SH = CMOR’s severity estimate of HTS
ST = CMOR’s severity estimate of TWSTRS-2 HT
A = Amplitude F = Peak frequency
To determine whether there was a significant proportion of participants with an absolute magnitude of change greater than OPC thresholds, we used a Chi-square test. Of those with absolute changes greater than the OPC, we evaluated whether there was a tendency across participants to increase or decrease with two-sided Chi-square tests.
We additionally calculated a conservatively high estimate of the minimal detectable change (MDC) by multiplying 1.96 by the standard deviation of the differences between the two postures for CMOR severity scores, amplitude, and frequency (Eq. 3).
SDd = standard deviation of the differences between two postures
Results
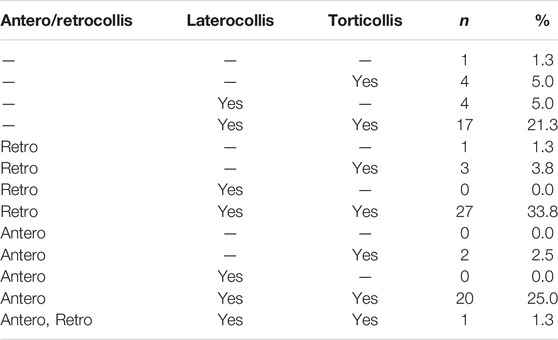
Of 206 participants, 93 had HT according to both raters. Twelve were excluded because video recording standards were not met, and one because their segment duration was less than 5 s. The 80 participants included in this study had a median age of 62 (range 34–80) years, and 64 (80%) were women. The total TWSTRS-2 total motor severity score averaged 17.5 (range 4–29). The body distribution of dystonia is shown in Table 1, and details regarding subtypes are shown in Table 2.
The distributions of the changes in overall HT severity, amplitude, and frequency are provided in Figure 1. One participant had identical peak frequency for both null and midline postures. Paired t-tests revealed no difference in severity (t = −0.23, p = 0.81), amplitude (t = −0.80, p = 0.43), and frequency (t = 1.48, p = 0.14) between the two postures.
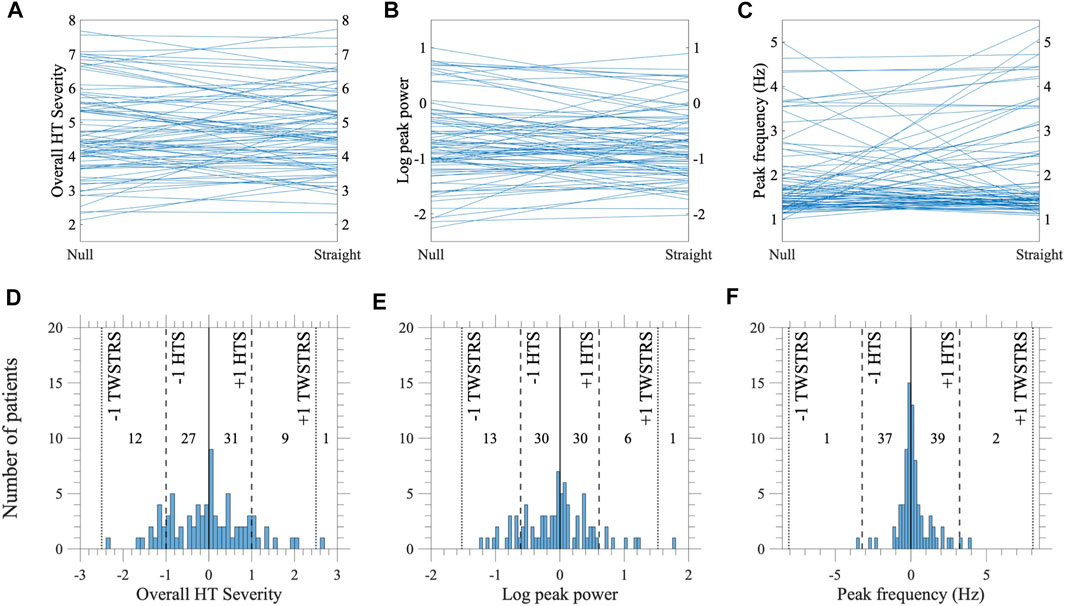
FIGURE 1. Changes in HT as patients go from the null posture to holding their head straight at midline (N = 80). Rows correspond to: [upper, (A–C)] parallel plots showing magnitudes in null and midline postures for every participant, and [lower, (D–F)] distributions of changes in magnitude [vertical lines represent no change (solid), and thresholds for one-point change in HTS (dashed) and TWSTRS-2 HT (dotted)]. Columns correspond to CMOR measures of: (A,D) overall HT severity, (B,E) log peak power (“amplitude”) and (C,F) frequency. (F) One participant had identical peak frequency between the null and midline postures and was excluded from the plot.
Of the 80 participants, the number exhibiting absolute changes greater than the OPC in TWSTRS-2 was: 1 for severity, 1 for amplitude, and 0 for frequency. Chi-square tests revealed no changes in severity (χ2 = 2.37, p = 0.12), and amplitude (χ2 = 2.37, p = 0.12). The number exhibiting absolute changes greater than the OPC in HTS were: 22 for severity, 20 for amplitude, and 3 for frequency. Chi-square tests showed that across the cohort, a significant proportion of participants had absolute changes greater than the OPC in HTS for severity (χ2 = 85.26, p < 0.0001) and amplitude (χ2 = 67.37, p < 0.0001) but not for frequency (χ2 = 0.26, p = 0.6080). Of the participants with absolute changes greater than the OPC, 10 participants increased and 12 decreased in severity, 7 increased and 13 decreased in amplitude, and 2 increased and 1 decreased in frequency. For all but one participant (bottom right quadrant, Figure 2), either amplitude or frequency changed but not both (Figure 2). Two-sided Chi-square tests showed that participants with absolute changes greater than the OPC had no tendency to increase or decrease in severity (χ2 = 1.82, p = 0.67), amplitude (χ2 = 1.80, p = 0.18), and frequency (χ2 = 0.33, p = 0.56).
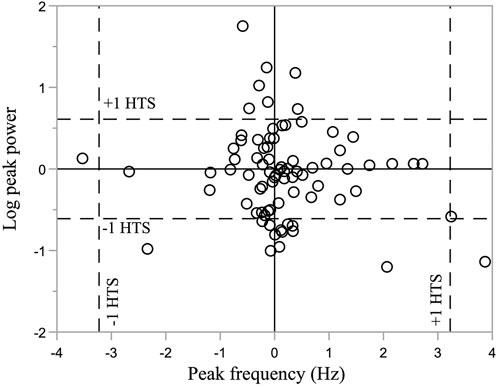
FIGURE 2. Log peak power and frequency changes from Drift to Straight (N = 80). Horizontal and vertical dashed lines represent changes in CMOR metric that corresponds to a one-point change in HTS severity rating. Horizontal and vertical solid lines represent no change in log peak power and frequency, respectively.
The MDC was estimated to be 1.12 for overall HT severity, 0.72 for amplitude, and 1.71 Hz for frequency. An overwhelming majority had absolute changes less than the MDC. For amplitude, 16 (20%) participants displayed changes greater than the MDC; 7 increased and 9 decreased at midline. For frequency, 10 (12.5%) participants displayed changes greater than the MDC; 7 increased (from 1–3 Hz to 3–6 Hz) and 3 decreased (from 3–6 Hz to 1–3 Hz) at midline.
Discussion
We used our computer vision system, CMOR, to quantify HT changes in severity, amplitude, and frequency from videos recorded when participants are instructed to let their head drift to its null posture and then hold their head straight at midline. For most participants, this change in posture did not predictably affect HT in CD. This contrasts with the common clinical impression that the null posture minimizes HT and a posture against the null exacerbates HT. There are multiple potential reasons for this discrepancy. First, the impression may have arisen because human observers are biased to recall patients (9) whose HT is alleviated in the null position. Second, the common impression may be the result of preferential ascertainment of severe cases (10). Relatedly, it could be biased by a slightly different examination protocol in which participants go beyond midline to a posture fully opposite their null posture. This would be expected to induce greater change in HT than the midline posture. The midline posture is in the same direction, but not of the same magnitude, as the posture fully opposite the null. Third, in our procedure, we use the power at the peak frequency, commonly used for quantifying tremor (11,12). Human perception may integrate information across a broader range of frequencies.
We related our objective CMOR measures of change to: 1) one-point changes (OPC) anchored by the TWSTRS-2 and HTS clinical assessments, and 2) minimal detectable changes (MDC). Relative to an OPC in TWSTRS-2, no participants exhibited changes in either severity, amplitude, or frequency. Relative to an OPC in HT severity score (HTS), approximately 25% of participants exhibited changes greater than the OPC in severity and amplitude, but not frequency. This is consistent with studies that found an effect of head posture on amplitude (4,13), but not in frequency (4). Of those who displayed changes greater than the OPC in HTS, some participants’ severity, amplitude, or frequency increased while other participants decreased. This suggests there may be three possible subtypes of CD HT: 1) worse in null, 2) worse in midline, and 3) similar in both. Although one study found no changes in peak frequency using the more sensitive search coil method, there was a focus on sinusoidal HT (4). Meanwhile, frequency changes were found for non-sinusoidal HT (13). Thus, based on our results, non-sinusoidal HT may exhibit posture-dependent changes in peak frequency that could be detected in both video- and search coil-based measures.
It could also be argued that a one-point change in the HTS scale (0–10) is below the resolution of human perception. In previous tremor studies, the minimum change perceptible was estimated to be 1 point on a 5-point scale for HT (14) and 2 points on a 10-point scale for hand tremor (15,16). If those thresholds were applied to participants in our study, the changes in HT that we measured between the two postures would not be clinically perceptible.
Relative to estimated MDCs, most participants had no changes in amplitude and frequency when going from null to midline. Of the 20% with HT amplitude changes greater than the MDC, more participants exhibited decreases than increases. Conversely, of the 12.5% of participants with HT frequency changes greater than our MDC, more participants exhibited increases than decreases. These instances of large frequency change may result from qualitative changes in head movement from HT to low-frequency dystonic movement and vice versa. Our results based on MDC and OPC are consistent, and support the conclusion that most participants in our cohort did not exhibit changes in HT.
This study has a few limitations. First, the video segments utilized may not fully capture the variability of HT. The video segment when participants were instructed to hold their head straight at midline is against, though not fully opposite to, the dystonic posture. In our study, we do not have video data to determine the effect that a posture fully opposite to the null posture would have on HT. Additional sampling across more video segments may better capture the full variability of HT. Second, peak frequencies close to 1 Hz may reflect an artifact of the lower cutoff frequency and large amplitude, irregular movements that may or may not be labelled as HT by clinical observation. Although by prior convention (17) we used a lower cutoff frequency of 1 Hz, future studies should consider using a lower cutoff frequency to detect slower components of head movements that some may label as tremor. Third, although the HTS is more granular, in contrast to the TWSTRS-2, it has not been validated.
Although clinicians recognize that HT may vary on a moment-to-moment basis depending on the posture of the head, objective measures across a large cohort suggest HT is not exacerbated at midline.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Dystonia Coalition (https://www1.rarediseasesnetwork.org/cms/dystonia).
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Research Protection Offices at Washington University School of Medicine, Rush University Medical Center, and University of California, San Diego (protocol 111255X). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JV: software, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, and visualization. EC: data curation, writing—review and editing. JZ: writing—original draft, writing—review and editing. HL and SP: writing—review and editing. JJ, SF, and RB: resources. CG: resources, writing—review and editing. JP and HJ: resources, writing—review and editing, and funding acquisition. GS: methodology, formal analysis, investigation, writing—review and editing. RE: conceptualization, methodology, investigation, writing—review and editing. CC: conceptualization, methodology, investigation, writing—original draft, writing—review and editing, and funding acquisition. DP: conceptualization, methodology, software, formal analysis, investigation, writing—original draft, writing—review and editing, supervision, project administration, and funding acquisition.
Funding
This research was conducted under the auspices of the Dystonia Coalition, which is part of the Rare Diseases Clinical Research Network, an initiative funded by the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences (U54 TR001456) in collaboration with the National Institute of Neurological Disorders and Stroke (U54 NS065701 and U54 NS116025) at the National Institute of Health (NIH). This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer-Reviewed Medical Research Program under Awards W81XWH-17-1-0393 and W81XWH-19-1-0146.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Disclaimer
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Acknowledgments
We gratefully acknowledge assistance from the WUSM Biorepository team, including Laura Wright for managing video recording and clinical data intake, and Matt Hicks for technical support.
References
1. Lenka, A, and Jankovic, J. Tremor Syndromes: An Updated Review. Front Neurol (2021) 12:684835. doi:10.3389/fneur.2021.684835
2. Tsui, JKC, Jon Stoessl, A, Eisen, A, Calne, S, and Calne, DB. Double-blind Study of Botulinum Toxin in Spasmodic Torticollis. Lancet (1986) 2(8501):245–7. doi:10.1016/s0140-6736(86)92070-2
3. Consky, E, Basinski, A, Belle, L, Ranawaya, R, and Lang, A. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): Assessment of Validity and Inter-rater Reliability. Neurology (1990) 40(1):445.
4. Shaikh, AG, Zee, DS, and Jinnah, HA. Oscillatory Head Movements in Cervical Dystonia: Dystonia, Tremor, or Both? Mov Disord (2015) 30(6):834–42. doi:10.1002/mds.26231
5. Uhríková, Z, Šprdlík, O, Hoskovcová, M, Komárek, A, Ulmanová, O, Hlaváč, V, et al. Validation of a New Tool for Automatic Assessment of Tremor Frequency from Video Recordings. J Neurosci Methods (2011) 198(1):110–3. doi:10.1016/j.jneumeth.2011.02.033
6. Vu, JP, Cisneros, E, Lee, HY, Le, L, Chen, Q, Guo, XA, et al. Head Tremor in Cervical Dystonia: Quantifying Severity with Computer Vision. J Neurol Sci (2022) 434:120154. doi:10.1016/j.jns.2022.120154
7. Kilic-Berkmen, G, Wright, LJ, Perlmutter, JS, Comella, C, Hallett, M, Teller, J, et al. The Dystonia Coalition: A Multicenter Network for Clinical and Translational Studies. Front Neurol (2021) 12:660909. doi:10.3389/fneur.2021.660909
8. Comella, C, Leurgans, S, Wuu, J, Stebbins, G, and Chmura, T. Rating Scales for Dystonia: a Multicenter Assessment. Mov Disord (2003) 18(3):303–12. doi:10.1002/mds.10377
9. Roche, N, Reddel, H, Martin, R, Brusselle, G, Papi, A, Thomas, M, et al. Quality Standards for Real-World Research. Focus on Observational Database Studies of Comparative Effectiveness. Ann Am Thorac Soc (2014) 11(2):S99–104. doi:10.1513/AnnalsATS.201309-300RM
10. Lipsitch, M, Donnelly, CA, Fraser, C, Blake, IM, Cori, A, Dorigatti, I, et al. Potential Biases in Estimating Absolute and Relative Case-Fatality Risks during Outbreaks. Plos Negl Trop Dis (2015) 9(7):e0003846–16. doi:10.1371/journal.pntd.0003846
11. Lee, HJ, Lee, WW, Kim, SK, Park, H, Jeon, HS, Kim, HB, et al. Tremor Frequency Characteristics in Parkinson’s Disease under Resting-State and Stress-State Conditions. J Neurol Sci (2016) 362:272–7. doi:10.1016/j.jns.2016.01.058
12. Masuhr, F, Wissel, J, Müller, J, Scholz, U, and Poewe, W. Quantification of Sensory Trick Impact on Tremor Amplitude and Frequency in 60 Patients with Head Tremor. Mov Disord (2000) 15(5):960–4. doi:10.1002/1531-8257(200009)15:5<960:aid-mds1029>3.0.co;2-g
13. Shaikh, AG, Wong, AL, Zee, DS, and Jinnah, HA. Keeping Your Head on Target. J Neurosci (2013) 33(27):11281–95. doi:10.1523/JNEUROSCI.3415-12.2013
14. Elble, RJ, Hellriegel, H, Raethjen, J, and Deuschl, G. Assessment of Head Tremor with Accelerometers versus Gyroscopic Transducers. Mov Disord Clin Pract (2017) 4(2):205–11. doi:10.1002/mdc3.12379
15. Elble, RJ, and Ellenbogen, A. Digitizing Tablet and Fahn–Tolosa–Marín Ratings of Archimedes Spirals Have Comparable Minimum Detectable Change in Essential Tremor. Tremor Other Hyperkinet Mov (2017) 7:481. doi:10.7916/D89S20H7
16. Hopfner, F, Erhart, T, Knudsen, K, Lorenz, D, Schneider, SA, Zeuner, KE, et al. Testing for Alcohol Sensitivity of Tremor Amplitude in a Large Cohort with Essential Tremor. Parkinsonism Relat Disord (2015) 21(8):848–51. doi:10.1016/j.parkreldis.2015.05.005
Keywords: head tremor, video, computer vision, TWSTRS, task dependency
Citation: Vu JP, Cisneros E, Zhao J, Lee HY, Jankovic J, Factor SA, Goetz CG, Barbano RL, Perlmutter JS, Jinnah HA, Pirio Richardson S, Stebbins GT, Elble RJ, Comella CL and Peterson DA (2022) From Null to Midline: Changes in Head Posture do Not Predictably Change Head Tremor in Cervical Dystonia. Dystonia 1:10684. doi: 10.3389/dyst.2022.10684
Received: 31 May 2022; Accepted: 19 August 2022;
Published: 01 September 2022.
Edited by:
Aasef Shaikh, Case Western Reserve University, United StatesReviewed by:
Jean-Francois Daneault, Rutgers, The State University of New Jersey, United StatesSanjay Pandey, University of Delhi, India
Copyright © 2022 Vu, Cisneros, Zhao, Lee, Jankovic, Factor, Goetz, Barbano, Perlmutter, Jinnah, Pirio Richardson, Stebbins, Elble, Comella and Peterson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David A. Peterson, ZGFwQHNhbGsuZWR1
 Jeanne P. Vu
Jeanne P. Vu Elizabeth Cisneros1
Elizabeth Cisneros1 Jerry Zhao
Jerry Zhao Joseph Jankovic
Joseph Jankovic Hyder A. Jinnah
Hyder A. Jinnah Rodger J. Elble
Rodger J. Elble David A. Peterson
David A. Peterson