Abstract
Task-specific tremor (TST) is a specific type of tremor that occurs when performing or attempting to perform a specific task, such as writing or playing a musical instrument. The clinical entity of TST remains heterogeneous. Some TSTs can only be induced by conducting a specific task, while others can be elicited when adopting a particular position simulating a task. The pathophysiology of TST is controversial. Whether TST is an isolated tremor syndrome, a spectrum of dystonic tremor syndrome (DTS), or essential tremor (ET) is not yet clear. Evidence from electrophysiological studies suggests that TST patients have normal reciprocal inhibition responses but abnormal motor cortical excitability, especially relating to the maladaptive long-interval intracortical inhibitory circuitry. The blink recovery study and eyeblink classical conditioning studies demonstrated possible hyperexcitability of the brainstem circuits and cerebellar dysfunction in patients with TST. Functional MRI studies have further shown that patients with TST have reduced functional connectivity in the cerebellum, similar to patients with DTS and ET. Due to variable methodologies and the sparsity of functional MRI studies in TST, it remains uncertain if patients with TST share the connectivity abnormalities between the cortical or subcortical areas that have been demonstrated in patients with DTS. Comprehensive electrophysiological and functional neuroimaging studies may help to elucidate the pathophysiology of TST.
Introduction
Task-specific tremor (TST) is a specific type of action tremor occurs only or predominantly when an affected individual is performing or attempting to perform a specific task. The clinical entity remains heterogeneous. TST can be induced by active movements or by adopting a specific position simulating the task, and is usually non-progressive [1]. TSTs mostly involve the upper limbs, especially dominant limbs, during specific skilled tasks though sometimes the orolingual area (e.g., lip, chin) is affected [2]. TSTs have a mean frequency of 5–7 Hz (range 3–8 Hz), which may be accompanied by a jerky component in some cases [3]. To date, there are no known patients with TSTs involving the lower limbs.
The involved tasks are variable. For example, most commonly, TSTs are elicited while writing and referred to as “primary writing tremor” (PWT) [4]. TSTs in musicians (TSTM) occur mainly while playing an instrument, with cases reported in string instrumentalists [5–7] or flutists [8]. TSTs can also occur during other daily activities. For instance, finger tremors when playing carroms [9], lip tremors while drinking [10–13], chin tremors only while brushing teeth [14], finger tremors with the use of scissors [15], and wrist tremors during weightlifting [16–18]. Given its various clinical subtypes, limited case numbers, and diagnosis uncertainty, there are no accurate numbers for the prevalence and incidence of TST among general populations.
Despite limited reported cases, PWT and TSTM are the two most prevalent TST subtypes. Two case series with 21 and 56 patients with PWT, respectively [4, 19], reported the mean age of onset to be around 50 years of age (broad range: 16–72 years), with a male predominance (70%–95.2%), and up to 33%–44% of the patients reported a positive family history of PWT. These findings suggest that there may be a possible genetic susceptibility to PWT, in addition to environmental factors. However, no causative gene or mutation has been identified so far. In contrast to PWT cohorts, a case series of 23 musicians with TSTM reported the age of onset to be 44.6 ± 13.6 years, with equal gender distribution, and without a positive family history. Besides, TSTM was associated with a relatively long average duration of playing an instrument (35 years) prior to tremor onset [6]. The variable clinical features implied that different types of TST may not share an identical pathophysiology.
Some recent studies have alluded to the possibility that TST may be an early symptom before the onset of other parkinsonism features in patients with Parkinson’s disease (PD) [14, 20, 21]. A case series reported that three of the fve patients with PWT (onset age between 46 and 76 years), later developed PD (within 1–5 years of PWT onset). All three patients had reduced uptake in DaTscan contralateral to the tremor-affected side, and were refractory to propranolol/primidone, but responded to carbidopa-levodopa treatment [20]. However, the interval between TST onset and a diagnosis of PD has been reported to be even longer (average: 13.66 years) in another case series [21]. Currently, the relationship between TST and PD is unclear.
The pathophysiology of TST has been debated in the past decade. The clinical presentation of being focally distributed and task-specific, sometimes with abnormal posturing [4, 22], the presence of coactivation and overflow of muscular activity to adjacent muscles in electromyography [23], a better response to botulinum toxin therapy, suggests a possible correlation between TST and dystonic tremor syndromes (DTS), which included both dystonic tremor and tremor associated with dystonia [24]. The alleviation of symptoms by gestes antagonistes has been described as a clinical hallmark characteristic of dystonia but was only reported in one patient with TST in the previous literature [25]. On the other hand, many studies have reported considerable symptomatic relief of TST by ethanol or propranolol [26–28], and identified a genetic susceptibility, with one case reported with bilateral involvements [26], which points to a possible relationship between TST with ET. Therefore, whether TST is an isolated tremor syndrome, a tremor associated with task-specific dystonia, or a variant of ET, remains uncertain [29, 30]. In this review, we explored the current electrophysiological and functional neuroimaging studies of TST and discussed the possible pathophysiology of TST.
Electrophysiological characteristics of TST
Electromyography (EMG) recording
Surface EMG is an important tool for recording muscular activity, especially in various movement disorders including tremor syndromes [31]. The EMG recording site depends on the subtype of TSTs. For example, in patients with PWT and TSTM, the commonly sampled muscles include the distal muscles of the upper limbs, such as the abductor pollicis brevis, abductor digiti minimi, wrist extensors, wrist flexors, and the more proximal muscles, such as the biceps, triceps, deltoid and pectoralis-major muscles [4, 6]. There are no specific hand muscles that are consistently involved in different kinds of TST patients.
Most of the EMG studies of TST were PWT patients. Alternating EMG bursts, with burst activity between the forearm agonist/antagonist muscles and phasic activity in the intrinsic hand muscles is a typical finding [4, 23, 28, 32]. However, a co-contraction pattern of the agonist/antagonist muscles, or solely extensor muscle activity, has also been documented [4, 28]. Usually, the tremor frequency ranges from 3 to 8 Hz, with a mean frequency of 6 Hz [4]. As a comparison, the usual frequency of the action tremor of the upper extremities in ET is 4–12 Hz, while the frequency being more variable with irregular amplitudes in dystonic tremor (mainly less than 7 Hz) [2]. While earlier studies in PWT did not provide definite evidence of excessive overflow of EMG activity into the proximal musculature [4], a recent study on TSTM demonstrated co-activation of the flexor and extensor muscles and excessive EMG activity in the adjacent muscles [33], implying a possible relationship with dystonia such as writer’s cramp [34, 35].
Of note, EMG findings in TST are sometimes difficult to classify, as the muscle groups involved during a specific task, such as holding a pen or playing an instrument may be subtly different for each patient. Moreover, some patients may use excessive force to control their movements, resulting in diverse and sometimes dystonic features when recording the EMG.
Reciprocal inhibition of Hoffmann’s reflex
Hoffmann’s reflex (H-reflex) refers to the reflex response of muscles after low-intensity electrical stimulation of Ia sensory afferents. Reciprocal inhibition of the H-reflex refers to the phenomenon in which the H-reflex response is reduced on a contraction of the antagonist muscle elicited by peripheral nerve stimulation at a certain period before the H-reflex. In forearm reciprocal inhibition, the H-reflex response arises from the flexor carpi radialis muscle when the median nerve is stimulated, while the radial nerve stimulation represents the conditioning stimulation [36, 37]. In healthy subjects, the time course of the forearm reciprocal inhibition has three distinct inhibitory phases, depending on the inter-stimulation interval (ISI) between the two stimulations. The first inhibitory phase is the ISI within 1 ms, which indicates Ia afferent disynaptic inhibition from the radial nerve to the flexor alpha motor neurons. The second phase is the ISI at 5–50 ms, which reflects presynaptic inhibition at the terminals of the flexor Ia afferent fibers. The third phase is the ISI at 50–100 ms, with an undetermined mechanism. There were no significant differences in the first (disynaptic) and second (presynaptic) phases of the forearm reciprocal inhibition between patients with PWT and healthy subjects [4, 38]. The third phase has not been comprehensively explored, but the inhibition has been shown to be normal at 75 ms as well [4]. For patients with ET, a significantly attenuated second phase of reciprocal inhibition (ISI at 10–30 ms) has been demonstrated in some studies [39, 40]. In contrast, Munchau et al. reported normal reciprocal inhibition in patients with ET [41]. For patients with writer’s cramp, most studies have demonstrated attenuation of all three phases of the forearm reciprocal inhibition [42–44]. However, the 2nd phase of the RI was abnormal in patients who presented arm tremor in the beginning and later presented cervical dystonia [41]. These patients can be classified into ET plus syndrome or dystonia with tremor according to the new tremor classification. These findings suggest that patients with PWT may preserve their spinal inhibitory circuits, which distinguishes them from patients with dystonia or patients with ET.
Blink reflex
The R2 blink reflex recovery cycle (R2BRrc) is an electrophysiological measurement of brainstem excitability that measures the orbicularis oculi muscle responses during paired-pulse electrical stimulation of the supraorbital nerve. It is known to be abnormally enhanced in blepharospasm, PD, craniocervical dystonia, and dystonic tremor (DTS), indicating an alteration in brainstem interneuron excitability [44–47]. In contrast, R2BRrc tends to be normal in patients with ET [48].
The conditioning of the eyeblink reflex is a well-established paradigm in motor learning assessment. This is referred to as eyeblink classical conditioning (EBCC), with the neural circuitry involving the cerebellum, hippocampus, and prefrontal cortex [49]. The blink reflex is recorded as the responses of the orbicularis oculi muscle, with auditory condition stimulus (CS) from the ipsilateral ear, at a set frequency and amplitude (1,000 Hz, 70 dB, duration 540 ms) [50]. EBCC tends to be abnormal in patients with ET and DTS [51, 52], indicating underlying cerebellar dysfunction. This is consistent with the concept that a functional disturbance of the olivo-cerebellar circuit contributes to the expression of many types of tremors.
A recent study demonstrated a reduced R2BRrc in patients with PWT, which was similar to the patients with DTS, while those with ET experienced a normal R2BRrc [53]. Overall, in this study, a reduced conditioned response in EBCC was also found in all PWT, ET, and DTS patient groups, but normal in healthy subjects. According to these findings, though with limited large-scale studies, patients with TST tend to have increased brainstem excitability and impaired olivo-cerebellar circuitry, sharing a more common pathophysiology with DTS rather than ET.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a useful modality for exploring the electrophysiology of the brain. By generating induced currents, TMS can activate neurons and interneurons in the cortex. When paired stimulation is delivered, TMS can further assess the function of the intracortical facilitatory/inhibitory circuits at different ISI. Short-interval intracortical inhibition (SICI), a GABAA-mediated inhibitory circuit, is the most frequently used paired-pulse TMS paradigm for evaluating motor cortex excitability. SICI is conducted via motor cortex stimulations with a sub-threshold conditioning stimulus, followed by a supra-threshold test stimulus, at the ISI of 1–6 ms. Likewise, long-interval intracortical inhibition (LICI), a GABAB-mediated inhibitory circuit, is conducted via two supra-threshold stimuli at the ISI of 50–200 ms. Both SICI and LICI reduce the MEP amplitude compared to the MEP generated by a test stimulus alone. Another common TMS parameter used to evaluate the cortical inhibitory circuit is the cortical silent period (CSP). The CSP refers to a period of 50–300 ms of electrical silence in the active background EMG following a supra-threshold TMS pulse to the motor cortex. The duration of the CSP increases with stimulus intensity, but not with the size of the preceding MEP [54] or the contraction strength of the target muscle [55, 56].
A previous study demonstrated normal intracortical excitability at short and long ISIs in patients with PWT [38]. In contrast, some studies have shown a reduction of the SICI in patients with PWT [53, 57] and posterior displacement of the position of the cortical motor maps [57], suggesting possible dysfunction in the cortical inhibitory circuitry and disorganization of the corticomotor representation, similar to the studies in patients with writer’s cramp [58–60]. The suppression ratio of SICI was approximately 40%–50% in patients with PWT and patients with DTS, but >60% in normal subjects [53]. The LICI was reduced by paired associative stimulation (PAS) in normal subjects but paradoxically enhanced in those patients with PWT or DTS, indicating maladaptive plasticity in the motor cortex [53, 61].
In individuals with and without PWT, the CSP duration is the same during writing or performing a voluntary contraction action of the hand of similar intensity on the affected side or between the sides [62]. Interestingly, a significantly shortened duration of the CSP during near-maximum voluntary contraction on both sides has been noted in patients with PWT. These findings indicate that patients with PWT may have impaired cortical inhibitory processes that are only apparent during strong voluntary activations, which are probably not directly linked to unilateral tremulous activity. In contrast, a shortened duration of the CSP was observed in patients with writer’s cramp during dystonic contraction or voluntary contraction of a similar strength, but only on the affected side [63]. Meanwhile, most studies have demonstrated that the baseline cortical excitability including RMT, SICI, or CSP is not significantly different between patients with ET and healthy subjects [64–67].
In brief, TMS studies of patients with TST, or specifically primary PWT patients, suggest impairments in the central GABAergic pathways, and the impairments may be different from the patients with dystonia.
Neuroimaging insights of TST
Functional magnetic resonance image (fMRI) techniques provide a non-invasive assessment of the structural, functional, and metabolic alterations of neurological disorders. Numerous imaging studies have been performed in patients with ET and DTS, but the studies on TST are sparse.
In an early fMRI study involving three patients with PWT, PWT was shown to be associated with increased activity of the cerebellum bilaterally, with a more pronounced area of activation on the side ipsilateral to the affected hand, along with bilateral activation of the parietal lobule with a more pronounced activation on the side contralateral to the affected hand [68]. Conversely, recent studies have shown opposite findings in the cerebellum. For example, Hirdesh Sahni et al. showed overactivations of the primary and supplementary motor areas and reduced activity in the cingulate motor area and the cerebellum in six patients with PWT [69]. Another recent study using voxel-based morphometry and diffusion tensor imaging (DTI) found that there was predominantly gray matter atrophy in the frontal lobe and the cerebellum, along with white matter changes in the frontal lobe and the cingulum in patients with PWT when compared with healthy subjects [70]. Lenka et al. further applied graph theory-based neural network analysis to fMRI to explore connectivity during the resting state of the functional brain [71]. In this study, the brain was modeled as a complex functional network with two measurements including “clustering coefficient”, which quantified the local connectivity as an index of network segregation; and “path length,” which quantified the global connectivity as an index of network integration. The results of this analysis demonstrated that patients with PWT had a significantly lower clustering coefficient and a higher path length in the bilateral medial cerebellum, right dorsolateral prefrontal cortex, and left posterior parietal cortex, suggesting significant disruptions of the small-world brain architecture in these regions.
To our knowledge, to date, there are no studies that directly compared patients with TST to patients with ET or DTS. However, numerous studies have discussed the structural, functional, and metabolic presentations between patients with ET and patients with tremors associated with dystonia. Through understanding the difference between ET and DTS in the MRI images may shed lights on the pathophysiology of TST. Findings from DTI studies suggest an increased mean diffusivity and a decreased fractional anisotropy of the cerebellum in patients with ET, indicating possible microstructural tissue damage and a loss of cellular integrity [72–74]. fMRI studies in ET patients have further clearly demonstrated abnormal cerebellar function and altered connectivity in the cerebello-thalamico-cortical circuitry [75]. Another recent MRI study demonstrated grey matter hypertrophy of the thalamus and motor cortex in the cerebello-thalamo-cortical circuit among patients with DTS [76]. The author concluded that deficient input from the cerebellum towards the thalamo-cortical circuit with hypertrophy of the thalamus, may play a key role in the generation of DTS. To compare patients with ET and DTS, a functional MRI during a grip-force task as a proxy of tremor-related cerebral activity showed similar reduction of functional connectivity in the cerebellum in both patients with ET and DTS [77]. Nevertheless, when the region of interest was outside the cerebellum, compared to patients with ET, those with DTS have more widespread areas of reduced functional connectivity in the cortical regions when the seed regions were placed either in cortical regions, such as the sensorimotor cortex and inferior parietal lobule or subcortical areas, such as globus pallidus interna. Another study using multi-modal imaging combining resting-state functional MRI and DTI showed reduced functional connectivity between the cerebellum and dentate nucleus bilaterally for the ET group but not the DTS group, compared to healthy subjects [78]. From the treatment response viewpoint, both ET and DTS improved after deep brain stimulation were significantly correlated to the stimulation of the dentato-rubro-cortical tract, while only DTS, but not ET, presented a significant additional correlation to the pallidothalamic tract [79]. These findings point towards a second pathophysiological mechanism involving the basal ganglia in patients with DTS. Taken together, connectivity dysfunction of both the cerebello-thalamo-cortical and the basal ganglia-thalamo-cortical networks may both be involved in driving the pathophysiology of DTS [80, 81] which was different from ET who presented mainly cerebello-thalamo-cortical connectivity impairment.
Discussion and conclusion
There is an ongoing debate about whether TST is a distinct disease entity, a variant form of ET, or a focal task-specific dystonia with dystonic tremor. Based on current evidence, it is reasonable to classify TST as a subtype of DTS, rather than a subtype of ET. Clinically, TST occurs when the patient performs a specific task, similar to patients with writer’s cramp who present with dystonic postures when they are writing. Moreover, TST usually affects the dominant hand only, unlike ET, which involves both sides bilaterally. On the contrary, the findings of electrophysiological studies suggest that TST showed normal spinal inhibitory circuits and motor cortical excitability, but a disinhibited brain stem inhibitory circuitry is evident from the reduced EBCC and R2BRrc. The loss of LICI modulation by PAS and reduced SICI are present in both TST and DTS patients. Nevertheless, patients with dystonia usually demonstrate other forms of hyperexcitability of the motor cortex, for example, a reduced CSP, or hyperexcitability of the spinal cord and a loss of reciprocal inhibition. Therefore, the overall electrophysiological characteristics of TST imply that the underlying pathophysiology is not entirely identical to dystonia.
Due to the variable methodologies used in fMRI studies and the sparsity of fMRI studies in patients with TST, it remains inconclusive whether TST is distinct from ET or DTS. Although cerebellar functional connectivity impairments were observed in PWT, it could also represent a fundamental abnormality for any tremor syndrome, since patients with ET and DTS also demonstrate a decreased connectivity in the cerebello-thalamo-cortical circuits. Whether the additional basal ganglion-thalamo-cortical circuits are involved, or whether a more widespread reduction in functional connectivity in the cortical regions occurs in the patients with TST is still uncertain. From the structural point of view, whether patients with TST presented thalamic hypertrophy, which implied dystonia characteristics, may be another clue to interpret the pathophysiology of TST in the future. All these aspects may be critical to distinguishing the underlying pathophysiology between TST, ET and DTS. Table 1 compares the different features, such as the clinical presentation, electrophysiological findings and fMRI results, between TST, ET, and DTS.
TABLE 1
| TST | ET | DTS | |
|---|---|---|---|
| Clinical aspects | |||
| Symptoms | Task-specific, focal, non-progressive (most induced by writing or playing specific instruments) | Posture-related, bilateral involved | During postural holding and reaching tasks, focal or segmental, gestes antagonistes |
| Electrophysiological studies | |||
| Surface EMG | Alternating EMG bursts activity at 3–8 Hz (some reports with co-contraction, overflow activity) [4, 28, 33] | Rhythmic EMG burst at a 4–12 Hz bilaterally, without overflow or co-contractions [31] | Rhythmic EMG burst at 4–10 Hz, co-contractions, overflow, and mirror dystonia [34, 35, 81] |
| H reflex | Normal reciprocal inhibition of H reflex [4, 38] | Normal reciprocal inhibition [41] or attenuation of 2nd phase of reciprocal inhibition [39, 40] | Diminished reciprocal inhibition [42–44] |
| Blink reflex | Reduced blink recovery cycle, reduced EBCC [53] | Normal blink recovery cycle, reduced EBCC [46, 51, 53] | Reduced blink recovery cycle, reduced EBCC [52, 53] |
| TMS | Equivocal normal or slightly reduced SICI [38, 53, 57] | Normal SICI [64] | Reduced SICI [58, 59] |
| Normal CSP [62] | Normal CSP [65] | Reduced CSP [63] | |
| Neuroimaging studies | |||
| Functional MRI | Decreased functional connectivity in cerebellum to other cortical areas [69–71] | Decreased connectivity in cerebello-thalamico-cortical circuitry [72–75] | Decreased connectivity in cortical-basal ganglia-cerebellar pathway [79–81] |
| Reduced functional connectivity between cortical and subcortical regions [77] | |||
| Structural MRI | Gray matter atrophy in the cerebellum [69] | Loss of cerebellar integrity [72–74] | Thalamic hypertrophy [76] |
A summary of the differences between task-specific tremor (TST), dystonic tremor syndrome (DTS) and essential tremor (ET), from clinical, electrophysiological and neuroimage aspects.
CSP, cortical silent period; DTI, diffuse tensor image; EBCC, eyeblink classical conditioning; EMG, electromyography; MRI, magnetic resonance image; SICI, short-interval intracortical inhibition; TMS, transcranial magnetic stimulation.
Although the association between TST and Parkinson disease (PD) is less depicted in the previous literature, especially in the electrophysiological assessment, however, a recent study of eight patients with TST who later developed into PD showed an optimal response to apomorphine but was refractory to other dopaminergic agents [82]. Therefore, TST responses to the apomorphine test might provide an early hint to indicate that TST may be full-blown to PD in the future. Figure 1 delineated the current position of TST in the tremor syndrome by integrating electrophysiological and fMRI findings, and indicated the knowledge gap that might help clinicians to better understand the pathophysiology of TST in the future.
FIGURE 1
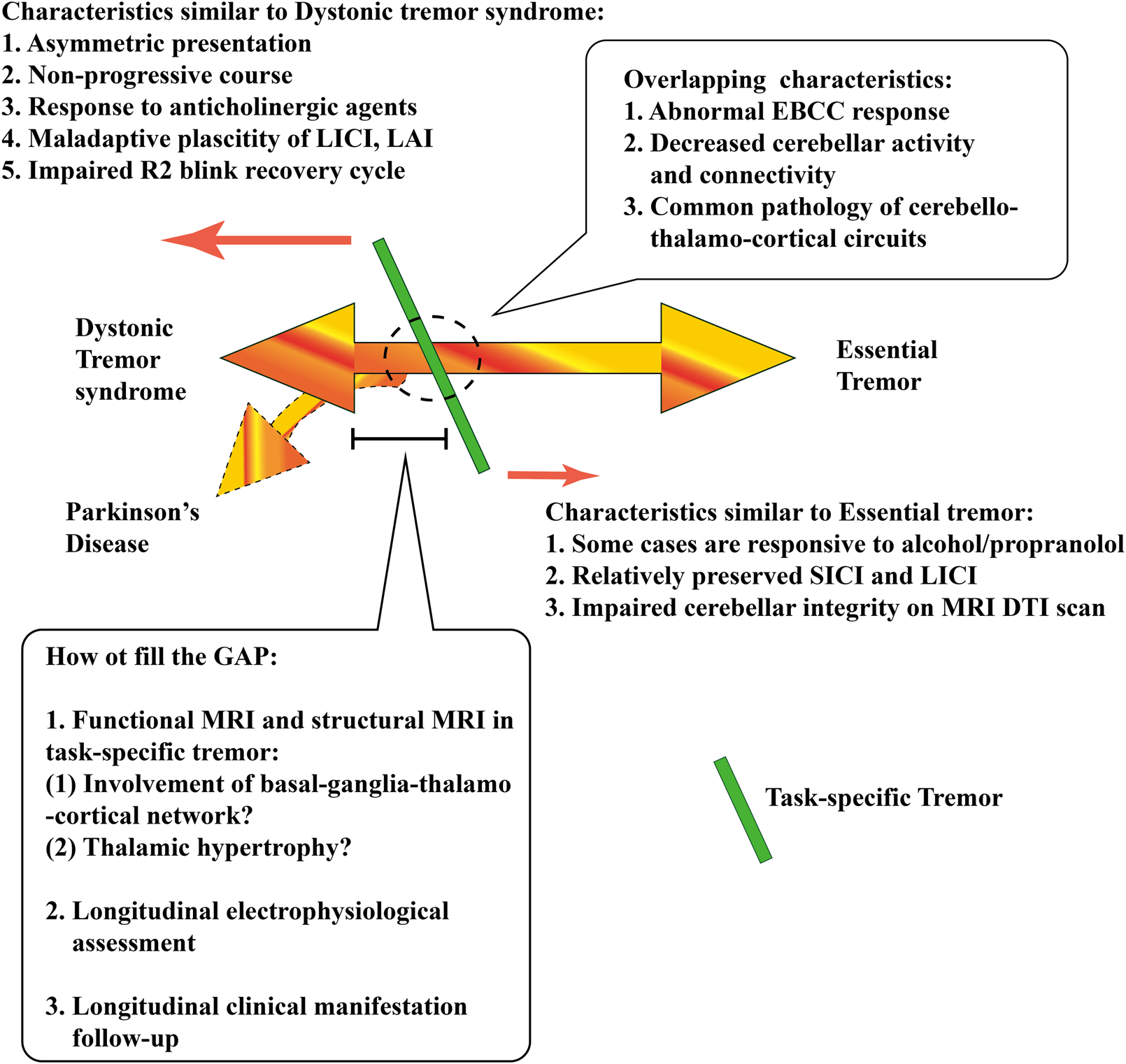
The current position of task-specific tremor (TST) in the tremor syndrome by integrating electrophysiological and fMRI findings, including the characteristics similar to or overlapped with dystonic tremor syndrome (DTS) and essential tremor (ET). There is still a knowledge gap, which might help clinicians understand the pathophysiology of TST in the future. DTI, diffuse tensor image; EBCC, eyeblink classical conditioning; LAI, long-latency afferent inhibition; LICI, long-Interval Intracortical Inhibition; MRI, magnetic resonance image; SICI, short-interval intracortical inhibition.
There is still a lack of comprehensive and consistent understanding of TST due to limitations in the currently available studies. First, most studies have a small sample size, with a large intra-subject variability. Second, the inclusion criteria in each study are varied, and some studies conducted even before the development of tremor classification and the definitions for the patient groups are ambiguous and non-standardized in some studies. For example, dystonic tremor or tremor with dystonia may not be necessarily shared the same pathophysiology, although they both can be sorted in the same disease population as “dystonic tremor syndromes” in most of the studies. A significant portion of the studies were conducted before the development of tremor classification criteria [83]. Third, the different methodologies and paradigm designs used in each study, including both electrophysiological and neuroimage aspects, have led to inconclusive results. Fourth, most of the TST studies mentioned in this review focused on PWT patients, which might only represent a specific subtype of TST though still giving us an insight of the picture of the underlying pathophysiology. Moreover, most studies lack long-term follow-up. Thus, additional neurological signs that emerge over time may be left undetected (e.g., Parkinson’s disease), which may have led to unreported but critical findings, misinterpretations, or incorrect inferences.
Findings from the available electrophysiological and fMRI studies on patients with PWT suggest that TST may be an isolated tremor entity or a spectrum of DTS, rather than an ET variant. This is consistent with the latest consensus statement on tremor classification from the task force on tremors of the International Parkinson and Movement Disorder Society [83], in which TST has been separately classified as a specific action-induced tremor, different from DTS or ET. Regular follow-ups and comprehensive symptoms documentation with longitudinal electrophysiological and neuroimaging assessment are the keys to fully understanding the underlying pathophysiology of each individual patient with TST.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Bain PG . Chapter 50 - task-specific tremor. In: WeinerWJTolosaE, editors. Handbook of clinical neurology. Amsterdam, Netherlands: Elsevier (2011). p. 711–8.
2.
Lenka A Jankovic J . Tremor syndromes: an updated review. Front Neurol (2021) 12:684835. 10.3389/fneur.2021.684835
3.
Bhidayasiri R Tarsy D . Primary writing tremor. In: BhidayasiriRTarsyD, editors. Movement disorders: a video atlas: a video atlas. Totowa, NJ: Humana Press (2012). p. 62–3.
4.
Bain PG Findley LJ Britton TC Rothwell JC Gresty MA Thompson PD et al Primary writing tremor. Brain (1995) 118(6):1461–72. 10.1093/brain/118.6.1461
5.
Lederman R . Primary bowing tremor a task-specific movement disorder of string instrumentalists. Med Probl performing artists (2012) 27:219–23. 10.21091/mppa.2012.4040
6.
Lee A Chadde M Altenmüller E Schoonderwaldt E . Characteristics of task-specific tremor in string instrument players. Tremor and other hyperkinetic movements (2014) 4:198. 10.7916/D86Q1V9W
7.
Lee A Furuya S Altenmüller E . Epidemiology and treatment of 23 musicians with task specific tremor. J Clin Mov Disord (2014) 1(1):5. 10.1186/2054-7072-1-5
8.
Lee J-E Kim J-S . Task-specific hand tremor during embouchure in a flutist. Neurol Sci (2018) 39(8):1501–2. 10.1007/s10072-018-3317-2
9.
Kahathuduwa CN Weerasinghe VS Dassanayake TL Priyadarshana R Dissanayake AL Perera C . Task-specific kinetic finger tremor affects the performance of carrom players. J Sports Sci (2016) 34(10):923–8. 10.1080/02640414.2015.1078487
10.
O'Gorman CM Bower JH Matsumoto JY Kantarci OH Kumar N . When drinking makes the tremor worse: a task-specific orolingual tremor. Mov Disord Clin Pract (2014) 1(3):237–9. 10.1002/mdc3.12041
11.
Macerollo A Meppelink AM Teodoro T Ricciardi L Cordivari C Edwards MJ . Isolated task-specific lip tremor. Parkinsonism Relat Disord (2016) 29:138–9. 10.1016/j.parkreldis.2016.04.019
12.
Stampanoni Bassi M Casciato S Gilio L Pavone L Cafolla D Sforza E et al Subclinical dysphagia in task-specific mouth tremor triggered by drinking. Clin Neurophysiol (2019) 130(8):1289–91. 10.1016/j.clinph.2019.05.009
13.
Benedek K Thomsen CE Bakke M . Task-specific drinking tremor. J Mov Disord (2023) 16(1):98–100. 10.14802/jmd.22103
14.
Yoo SW Lee M Ho SH Lee KS Kim JS . Task-specific focal chin tremor in idiopathic Parkinson's disease: is it an isolated phenomenon or a part of parkinsonism?Neurol Sci (2019) 40(3):649–51. 10.1007/s10072-018-3627-4
15.
Oh YS Ma HI Kim YJ Kim JS . Task-specific tremor with use of scissors. Mov Disord (2012) 27(7):921–2. 10.1002/mds.24984
16.
Lang AE Jog M Ashby P . “Weight-holding tremor”: an unusual task-specific form of essential tremor?Mov Disord (1995) 10(2):228–9. 10.1002/mds.870100220
17.
Yong SW Park DG Yoon JH Baik JS . Is an isolated weight-holding tremor a new subtype of isometric tremor?Yonsei Med J (2020) 61(7):644–6. 10.3349/ymj.2020.61.7.644
18.
Villa-López M Oh E Chen R Lang AE Masellis M Hopyan JJ . Teaching video neuroImage: “weighing” in on an unusual tremor. Neurology (2021) 97(9):e970–e971. 10.1212/WNL.0000000000012141
19.
Ondo WG Satija P . Task-specific writing tremor: clinical phenotypes, progression, treatment outcomes, and proposed nomenclature. Int J Neurosci (2012) 122(2):88–91. 10.3109/00207454.2011.630544
20.
Smith K Alawi A Ramiro J Chand P et al Pronounced task specific writing tremor in Parkinson's disease (P3.079). Neurology (2014) 82:P3.079.
21.
Koneru V Ondo WG . Task specific tremor subsequently developing into Parkinson's disease: case series. Mov Disord Clin Pract (2021) 8(1):111–3. 10.1002/mdc3.13109
22.
Schreglmann SR Baumann CR Waldvogel D . Mirror writing tremor: dystonic clues. Mov Disord Clin Pract (2015) 2:316–7. 10.1002/mdc3.12182
23.
Elble RJ Moody C Higgins C . Primary writing tremor. a form of focal dystonia?Mov Disord (1990) 5(2):118–26. 10.1002/mds.870050205
24.
Papapetropoulos S Singer C . Treatment of primary writing tremor with botulinum toxin type a snjections: report of a case series. Clin Neuropharmacology (2006) 29(6):364–7. 10.1097/01.WNF.0000236765.00785.9C
25.
Bagella CF Romito LM Scaioli V Elia AE . Sensory trick in task-specific tremor. Neurol Sci (2017) 38(7):1341–2. 10.1007/s10072-017-2913-x
26.
Jiménez-Jiménez FJ Cabrera-Valdivia F Orti-Pareja M Gasalla T Tallon-Barranco A Zurdo M . Bilateral primary writing tremor. Eur J Neurol (1998) 5(6):613–4. 10.1046/j.1468-1331.1998.560613.x
27.
Koller WC Martyn B . Writing tremor: its relationship to essential tremor. J Neurol Neurosurg Psychiatry (1986) 49:220. 10.1136/jnnp.49.2.220
28.
Kachi T Rothwell JC Cowan JM Marsden CD . Writing tremor: its relationship to benign essential tremor. J Neurol Neurosurg & Psychiatry (1985) 48:545–50. 10.1136/jnnp.48.6.545
29.
Hai C Yu-ping W Hua W Ying S . Advances in primary writing tremor. Parkinsonism Relat Disord (2010) 16(9):561–5. 10.1016/j.parkreldis.2010.06.013
30.
Pita Lobo P Quattrocchi G Jutras MF Sangla S Apartis E Vidailhet M et al Primary writing tremor and writer's cramp: two faces of a same coin? Mov Disord (2013) 28(9):1306–7. 10.1002/mds.25340
31.
Vial F Kassavetis P Merchant S Haubenberger D Hallett M . How to do an electrophysiological study of tremor. Clin Neurophysiol Pract (2019) 4:134–42. 10.1016/j.cnp.2019.06.002
32.
Ravits J Hallett M Baker M Wilkins D . Primary writing tremor and myoclonic writer's cramp. Neurology (1985) 35(9):1387–91. 10.1212/wnl.35.9.1387
33.
Lee A Tominaga K Furuya S Miyazaki F Altenmüller E . Electrophysiological characteristics of task-specific tremor in 22 instrumentalists. J Neural Transm (2015) 122(3):393–401. 10.1007/s00702-014-1275-2
34.
Hughes M McLellan DL . Increased co-activation of the upper limb muscles in writer's cramp. J Neurol Neurosurg & Psychiatry (1985) 48:782–7. 10.1136/jnnp.48.8.782
35.
Cohen LG Hallett M . Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology (1988) 38(7):1005–12. 10.1212/wnl.38.7.1005
36.
Fuhr P Hallett M . Reciprocal inhibition of the H-reflex in the forearm: methodological aspects. Electroencephalography Clin Neurophysiology/Evoked Potentials Section (1993) 89(5):319–27. 10.1016/0168-5597(93)90071-v
37.
Knikou M . The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods (2008) 171(1):1–12. 10.1016/j.jneumeth.2008.02.012
38.
Modugno N Nakamura Y Bestmann S Curra A Berardelli A Rothwell J . Neurophysiological investigations in patients with primary writing tremor. Mov Disord (2002) 17(6):1336–40. 10.1002/mds.10292
39.
Mercuri B Berardelli A Modugno N Vacca L Ruggieri S Manfredi M . Reciprocal inhibition in forearm muscles in patients with essential tremor. Muscle & Nerve (1998) 21(6):796–9. 10.1002/(sici)1097-4598(199806)21:6<796::aid-mus13>3.0.co;2-r
40.
Modugno N Priori A Berardelli A Vacca L Mercuri B Manfredi M . Botulinum toxin restores presynaptic inhibition of group Ia afferents in patients with essential tremor. Muscle & Nerve (1998) 21(12):1701–5. 10.1002/(sici)1097-4598(199812)21:12<1701::aid-mus12>3.0.co;2-k
41.
Münchau A Schrag A Chuang C MacKinnon CD Bhatia KP Quinn NP et al Arm tremor in cervical dystonia differs from essential tremor and can be classified by onset age and spread of symptoms. Brain (2001) 124(9):1765–76. 10.1093/brain/124.9.1765
42.
Panizza ME Hallett M Nilsson J . Reciprocal inhibition in patients with hand cramps. Neurology (1989) 39(1):85–9. 10.1212/wnl.39.1.85
43.
Nakashima K Rothwell JC Day BL Thompson PD Shannon K Marsden CD . Reciprocal inhibition between forearm muscles in patients with writer's cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain (1989) 112(3):681–97. 10.1093/brain/112.3.681
44.
Chen RS Tsai CH Lu CS . Reciprocal inhibition in writer's cramp. Mov Disord (1995) 10(5):556–61. 10.1002/mds.870100505
45.
Schwingenschuh P Katschnig P Edwards MJ Teo JTH Korlipara LVP Rothwell JC et al The blink reflex recovery cycle differs between essential and presumed psychogenic blepharospasm. Neurology (2011) 76(7):610–4. 10.1212/WNL.0b013e31820c3074
46.
Nisticò R Salsone M Vescio B Morelli M Trotta M Barbagallo G et al Blink reflex recovery cycle distinguishes essential tremor with resting tremor from de novo Parkinson's disease: an exploratory study. Parkinsonism Relat Disord (2014) 20(2):153–6. 10.1016/j.parkreldis.2013.10.006
47.
Nisticò R Pirritano D Salsone M Valentino P Novellino F Condino F et al Blink reflex recovery cycle in patients with dystonic tremor: a cross-sectional study. Neurology (2012) 78(17):1363–5. 10.1212/WNL.0b013e3182518316
48.
Nisticò R Pirritano D Novellino F Salsone M Morelli M Valentino P et al Blink reflex recovery cycle in patients with essential tremor associated with resting tremor. Neurology (2012) 79(14):1490–5. 10.1212/WNL.0b013e31826d5f83
49.
Yeo CH Hesslow G . Cerebellum and conditioned reflexes. Trends Cogn Sci (1998) 2(9):322–30. 10.1016/s1364-6613(98)01219-4
50.
Gerwig M Dimitrova A Maschke M Kolb FP Forsting M Timmann D . Amplitude changes of unconditioned eyeblink responses in patients with cerebellar lesions. Exp Brain Res (2004) 155(3):341–51. 10.1007/s00221-003-1731-y
51.
Kronenbuerger M Gerwig M Brol B Block F Timmann D . Eyeblink conditioning is impaired in subjects with essential tremor. Brain (2007) 130(6):1538–51. 10.1093/brain/awm081
52.
Antelmi E Di Stasio F Rocchi L Erro R Liguori R Ganos C et al Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Parkinsonism Relat Disord (2016) 31:23–7. 10.1016/j.parkreldis.2016.06.011
53.
Latorre A Rocchi L Batla A Berardelli A Rothwell JC Bhatia KP . The signature of primary writing tremor is dystonic. Mov Disord (2021) 36(7):1715–20. 10.1002/mds.28579
54.
Triggs WJ Cros D Macdonell RA Chiappa KH Fang J Day BJ . Cortical and spinal motor excitability during the transcranial magnetic stimulation silent period in humans. Brain Res (1993) 628(1):39–48. 10.1016/0006-8993(93)90935-g
55.
Inghilleri M Berardelli A Cruccu G Manfredi M . Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol (1993) 466(1):521–34. 10.1113/jphysiol.1993.sp019732
56.
Kimiskidis VK Papagiannopoulos S Sotirakoglou K Kazis DA Kazis A Mills KR . Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res (2005) 163(1):21–31. 10.1007/s00221-004-2134-4
57.
Byrnes ML Mastaglia FL Walters SE Archer SAR Thickbroom GW . Primary writing tremor: motor cortex reorganisation and disinhibition. J Clin Neurosci (2005) 12(1):102–4. 10.1016/j.jocn.2004.08.004
58.
Ridding MC Sheean G Rothwell JC Inzelberg R Kujirai T . Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry (1995) 59(5):493–8. 10.1136/jnnp.59.5.493
59.
Stinear CM Byblow WD . Elevated threshold for intracortical inhibition in focal hand dystonia. Mov Disord (2004) 19(11):1312–7. 10.1002/mds.20160
60.
Byrnes ML Thickbroom GW Wilson SA Sacco P Shipman JM Stell R et al The corticomotor representation of upper limb muscles in writer's cramp and changes following botulinum toxin injection. Brain (1998) 121(5):977–88. 10.1093/brain/121.5.977
61.
Meunier S Russmann H Shamim E Lamy JC Hallett M . Plasticity of cortical inhibition in dystonia is impaired after motor learning and paired-associative stimulation. Eur J Neurosci (2012) 35(6):975–86. 10.1111/j.1460-9568.2012.08034.x
62.
Ljubisavljevic M Kacar A Milanovic S Svetel M Kostic VS . Changes in cortical inhibition during task-specific contractions in primary writing tremor patients. Mov Disord (2006) 21(6):855–9. 10.1002/mds.20807
63.
Filipović SR Ljubisavljević M Svetel M Milanović S Kacar A Kostić VS . Impairment of cortical inhibition in writer's cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett (1997) 222(3):167–70. 10.1016/s0304-3940(97)13370-5
64.
Romeo S Berardelli A Pedace F Inghilleri M Giovannelli M Manfredi M . Cortical excitability in patients with essential tremor. Muscle Nerve (1998) 21(10):1304–8. 10.1002/(sici)1097-4598(199810)21:10<1304::aid-mus9>3.0.co;2-f
65.
Khedr EM El Fawal B Abdelwarith A Nasreldein A Rothwell JC Saber M . TMS excitability study in essential tremor: absence of gabaergic changes assessed by silent period recordings. Neurophysiol Clin (2019) 49(4):309–15. 10.1016/j.neucli.2019.05.065
66.
Shukla G Bhatia M Pandey RM Behari M . Cortical silent period in essential tremor. Electromyogr Clin Neurophysiol (2003) 43(6):329–33.
67.
Chuang W-L Huang YZ Lu CS Chen RS . Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov Disord (2014) 29(4):501–7. 10.1002/mds.25809
68.
Berg D Preibisch C Hofmann E Naumann M . Cerebral activation pattern in primary writing tremor. J Neurol Neurosurg Psychiatry (2000) 69(6):780–6. 10.1136/jnnp.69.6.780
69.
Sahni H Jayakumar PN Pal PK . Functional magnetic resonance imaging in primary writing tremor and writer's cramp: a pilot study. Ann Indian Acad Neurol (2010) 13(3):192–7. 10.4103/0972-2327.70884
70.
Jhunjhunwala K George L Kotikalapudi R Gupta PK Lenka A Stezin A et al A preliminary study of the neuroanatomical correlates of primary writing tremor: role of cerebellum. Neuroradiology (2016) 58(8):827–36. 10.1007/s00234-016-1700-3
71.
Lenka A Jhunjhunwala KR Panda R Saini J Bharath RD Yadav R et al Altered brain network measures in patients with primary writing tremor. Neuroradiology (2017) 59(10):1021–9. 10.1007/s00234-017-1895-y
72.
Tikoo S Pietracupa S Tommasin S Bologna M Petsas N Bharti K et al Functional disconnection of the dentate nucleus in essential tremor. J Neurol (2020) 267(5):1358–67. 10.1007/s00415-020-09711-9
73.
Saini J Bagepally BS Bhatt MD Chandran V Bharath RD Prasad C et al Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism Relat Disord (2012) 18(5):477–82. 10.1016/j.parkreldis.2012.01.006
74.
Shin DH Han BS Kim HS Lee PH . Diffusion tensor imaging in patients with essential tremor. Am J Neuroradiology (2008) 29(1):151–3. 10.3174/ajnr.A0744
75.
Holtbernd F Shah NJ . Imaging the pathophysiology of essential tremor—a systematic review. Front Neurol (2021) 12:680254. 10.3389/fneur.2021.680254
76.
Nieuwhof F Toni I Dirkx MF Gallea C Vidailhet M Buijink AWG et al Cerebello-thalamic activity drives an abnormal motor network into dystonic tremor. Neuroimage Clin (2022) 33:102919. 10.1016/j.nicl.2021.102919
77.
DeSimone JC Archer DB Vaillancourt DE Wagle Shukla A . Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain (2019) 142(6):1644–59. 10.1093/brain/awz085
78.
Bédard P Panyakaew P Cho HJ Hallett M Horovitz SG . Multimodal imaging of essential tremor and dystonic tremor. Neuroimage Clin (2022) 36:103247. 10.1016/j.nicl.2022.103247
79.
Tsuboi T Wong JK Eisinger RS Okromelidze L Burns MR Ramirez-Zamora A et al Comparative connectivity correlates of dystonic and essential tremor deep brain stimulation. Brain (2021) 144(6):1774–86. 10.1093/brain/awab074
80.
Rothkirch I Granert O Knutzen A Wolff S Gövert F Pedersen A et al Dynamic causal modeling revealed dysfunctional effective connectivity in both, the cortico-basal-ganglia and the cerebello-cortical motor network in writers' cramp. Neuroimage Clin (2018) 18:149–59. 10.1016/j.nicl.2018.01.015
81.
Panyakaew P Jinnah HA Shaikh AG . Clinical features, pathophysiology, treatment, and controversies of tremor in dystonia. J Neurol Sci (2022) 435:120199. 10.1016/j.jns.2022.120199
82.
Ondo WG Koneru V Arif C . Task specific tremor in Parkinson's disease responds to apomorphine. Tremor Other Hyperkinet Mov (N Y) (2023) 13:20. 10.5334/tohm.764
83.
Bhatia KP Bain P Bajaj N Elble RJ Hallett M Louis ED et al Consensus statement on the classification of tremors. from the task force on tremor of the international Parkinson and movement disorder society. Mov Disord (2018) 33(1):75–87. 10.1002/mds.27121
Summary
Keywords
task-specific tremor, primary writing tremor, dystonic tremor syndrome, essential tremor, electrophysiology, transcranial magnetic stimulation, neuroimage, functional magnetic resonance imaging
Citation
Kuo Y-CJ and Chen K-HS (2023) A mini-review of the pathophysiology of task-specific tremor: insights from electrophysiological and neuroimaging findings. Dystonia 2:11347. doi: 10.3389/dyst.2023.11347
Received
09 March 2023
Accepted
24 October 2023
Published
07 November 2023
Volume
2 - 2023
Edited by
Aparna Wagle Shukla, University of Florida, United States
Updates
Copyright
© 2023 Kuo and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai-Hsiang Stanley Chen, stanleychen1230@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.