- 1IRCCS Neuromed, Pozzilli, IS, Italy
- 2Department of Human Neurosciences, Sapienza University of Rome, Rome, Italy
- 3Parkinson and Movement Disorders Unit, Study Center on Neurodegeneration (CESNE), University of Padua, Padua, Italy
- 4Department of Clinical Neurosciences, University Hospital of Lausanne (CHUV), Lausanne, Switzerland
Background: Bradykinesia has been reported in patients with dystonia. Despite this, the pathophysiological mechanisms of bradykinesia in dystonia remain largely unknown.
Methods: We here performed a comprehensive literature search and reviewed clinical and experimental studies on bradykinesia in patients with dystonia.
Results: Many studies have documented the presence of bradykinesia in patients with idiopathic and inherited isolated dystonia, regardless of the presence of parkinsonism. In addition, bradykinesia has been observed as a side effect in dystonic patients who have undergone deep brain stimulation, in those with functional dystonia as well as in those with combined dystonia, e.g., dystonia-parkinsonism. These clinical and experimental findings support the hypothesis that dysfunction in a brain network involving the basal ganglia, primary sensorimotor cortex, and cerebellum may play a key role in the pathophysiology of both bradykinesia and dystonia.
Conclusion: Bradykinesia is frequently observed in dystonia. We may gain insights into the pathophysiological underpinnings of two distinct movement disorders by investigating this issue. Furthermore, a deeper understanding of bradykinesia in dystonia may have terminological implications in this field.
Introduction
Bradykinesia, along with other associated motor features such as hypokinesia, sequence effect, and hesitations/halts, is traditionally believed to be a motor symptom resulting from basal ganglia dysfunction and it is considered the hallmark feature of Parkinson’s disease (PD) and atypical parkinsonism [1–8]. However, bradykinesia has also been described in numerous clinical and experimental studies in non-parkinsonian conditions, including dystonia [9].
It is interesting to note from a historical perspective that Verger and Cruchet introduced the term “bradykinesia” in their early 20th-century treatise on spasmodic torticollis to describe the movement slowness observed in patients with dystonia, or what they referred to as “bradykinesie spasmodique” [10, 11]. After the original description, several clinical and experimental studies have documented the presence of movement slowness in patients with dystonia, often referred to as bradykinesia. Notably, one relevant, albeit under-recognized topic, is the presence of bradykinesia in patients with idiopathic and inherited isolated dystonia, where dystonia is the predominant motor feature [12]. The issue has been primarily explored through case reports, case series, and a range of clinical and experimental studies [13–25]. The occurrence of bradykinesia as a side effect of pallidal deep brain stimulation (DBS) has also been noted in more recent observations [26–29]. Furthermore, clinical studies have shown that bradykinesia has been observed in patients diagnosed with functional dystonia [30]. The issue of combined dystonia, which mainly refers to dystonia-parkinsonism syndrome has been extensively reviewed from both a phenomenological and pathophysiological perspective [31–34]. In this context, given the complex etiology of dystonia-parkinsonism, that can result from a variety of factors affecting the basal ganglia such as structural, metabolic, drug-induced, infectious, autoimmune, or genetic diseases, the presence of bradykinesia is not unexpected [31, 33, 35].
Observing bradykinesia in hyperkinetic movement disorders like dystonia offers a unique opportunity to gain insight into the underlying pathophysiological mechanisms shared by the coexistence of two distinct movement disorders characterized by opposing phenomenological features. From a pathophysiological perspective, bradykinesia in dystonia might seem at first sight a paradox as these are two disorders that were originally interpreted based on opposing patterns of basal ganglia dysfunction [1, 6, 31, 36–40]. However, interpretations concerning the dysfunction of the basal ganglia and other interconnected brain areas have been changing over the years [6, 41, 42]. In this regard, both bradykinesia and dystonia can now be interpreted as motor disorders resulting from network dysfunction, and there may therefore be an overlap between the mechanisms underlying both disorders [6, 9, 41–45].
This paper builds upon our previous work [9] and further explores the relationship between bradykinesia, here specifically referred to as movement slowness [7], and dystonia. We first focused our discussion on clinical and experimental studies investigating bradykinesia in patients with idiopathic and inherited isolated dystonia (both focal/segmental and generalized), and other intriguing and new aspects that have emerged in recent literature including bradykinesia in patients with dystonia treated with DBS and in patients with functional dystonia. While acknowledging the issue of dystonia-parkinsonism, we only provide a brief overview of this topic as it has already been extensively covered by other authors [31–34].
Our comprehensive literature search on PubMed included full-text papers such as original clinical and experimental studies and reviews, published in English until March 2023, using the search terms “bradykinesia,” “movement slowness” and “hypokinesia” in combination with “dystonia.” We also manually searched the reference lists of identified articles for additional relevant studies. We screened articles based on their title and abstract, excluding non-English papers and those with no available full text. Based on the available data, we discussed pathophysiological and terminological issues related to bradykinesia in dystonia.
Bradykinesia in idiopathic and inherited dystonia
Several clinical studies have reported that patients with idiopathic and inherited isolated dystonia exhibit slow movements and other related motor abnormalities, often specifically referred to as bradykinesia [9]. For instance, decreased arm swing [13, 18] as well as hypomimia, a type of facial bradykinesia [46], have been described in patients with cervical dystonia (CD), focal hand dystonia (FHD) and laryngeal dystonia [13, 18]. Anecdotally, bradykinesia and other parkinsonian signs have been reported during the disease course in 3 dystonic patients in whom the evolution of dystonia and bradykinesia was inversely proportional [47], as well as in larger series of patients with late-onset focal or segmental (mostly cervical) dystonia [20, 25]. Finally, bradykinesia and other associated motor features have been clinically reported in patients with pathogenic variants that typically cause isolated dystonia, such as KM2TB [48, 49] and ANO3 variants [50], as well as in one case of DYT1 dystonia, a monogenic generalized isolated dystonia, who exhibit clumsiness in foot tapping without decrement [51].
Building on clinical evidence, neurophysiological investigations have shown altered voluntary movement execution in idiopathic and inherited isolated dystonia [9]. Studies on voluntary movement execution demonstrated movement slowness, reduced movement amplitude and altered rhythm in patients with FHD, performing rapid wrist and elbow flexions [52, 53], and in patients with CD performing both horizontal arm extensions [17] and neck movements [19]. More complex movements, e.g., reaching arm movements, are slowed and characterized by altered trajectories in some studies [54, 55], including observations in DYT1 patients performing movements without visual feedback [54] and in other studies of patients with isolated dystonia [55]. However, some studies found different results in patients with FHD and CD [56]. Kinematic analysis of finger movements in patients with blepharospasm, CD, and FHD has provided variable results. Some studies have demonstrated altered timing parameters, but normal movement velocity and amplitude [57, 58]. Simultaneous and sequential upper limb and finger movements were characterized by movement slowness, irregular rhythm, and longer pauses, but no progressive reduction of amplitude and velocity during movement repetition was observed, indicating no sequence effect [15, 59–62]. Notably, a recent kinematic study that assessed finger tapping movements in patients with both focal or generalized dystonia, demonstrated that bradykinesia ameliorated when patients executed their ‘Geste Antagoniste’, which improved not only the dystonic muscle contraction but also voluntary movement velocity and rhythm [63]. Finally, a few studies have investigated neck movements in patients with CD and have consistently found evidence of slowness together with prolonged movement time and reduced amplitude [19, 56, 64, 65], and the impairment was higher when the patient moved toward the dystonic side [56, 64]. In addition, these studies have also reported longer pause durations between movements [19, 56, 65], as well as poor smoothness during neck movements in CD patients [66]. On the other hand, neck movements in FHD patients were found to be normal [56]. Relevant to the understanding of movement execution in dystonia several authors also investigated movement preparation. These studies have yielded varying results when measuring the reaction time (RT), which was normal in some reports [16, 54, 67–70] while abnormally prolonged in others [15, 23, 61].
In summary, clinical and experimental studies conducted on patients with dystonia have reported the occurrence of bradykinesia in this condition, either involving the body segments affected by dystonia and those not affected by this disorder. Clinical studies were mostly case series, and they did not provide a detailed description of the bradykinesia features. Neurophysiological studies provided evidence of slowed, irregular and low amplitude voluntary movements in dystonia, and some studies demonstrate the lack of sequence effect in these patients [15, 59].
Bradykinesia in dystonia patients treated with DBS
Deep brain stimulation (DBS) of the globus pallidus internus (GPi) has been demonstrated as an effective treatment for medically refractory dystonia, resulting in a reduction of motor impairment and disability in patients [28, 29, 71]. Despite its effectiveness, several clinical reports have described the occurrence of bradykinesia as a side effect in dystonia patients treated with GPi DBS (Table 1) [29, 72–79]. Namely, motor difficulty and slowing in previously non-dystonic extremities have been reported as possible side effects in some cases. For example, a relatively small sample of adult-onset CD and cranial-cervical dystonia patients who underwent GPi DBS reported such effects [73]. These patients also had difficulties with handwriting, getting up from a chair, and walking [73]. Other clinical studies have reported slowness in finger tapping movements, micrographia, and freezing of gait (FOG) have been reported in other clinical studies on patients with segmental dystonia after DBS [80–82]. Movement slowness has been observed in patients with dystonic head tremor after thalamic DBS [83]. Observations on a larger sample of patients with various forms of dystonia have confirmed that bradykinesia can be a side effect of GPi DBS [29, 78]. However, there is variability in the prevalence of this side effect. For instance, a large retrospective study that assessing long-term clinical outcomes and safety in 61 patients with idiopathic, inherited and acquired dystonia who underwent unilateral GPi-DBS reported no clinically overt bradykinesia over the 6–10 years follow-up [84]. Moreover, a recent study investigating the long-term effects of bilateral pallidal DBS in 36 consecutive patients with isolated generalized and cervical/segmental dystonia reported that bradykinesia was only present in two patients [28].
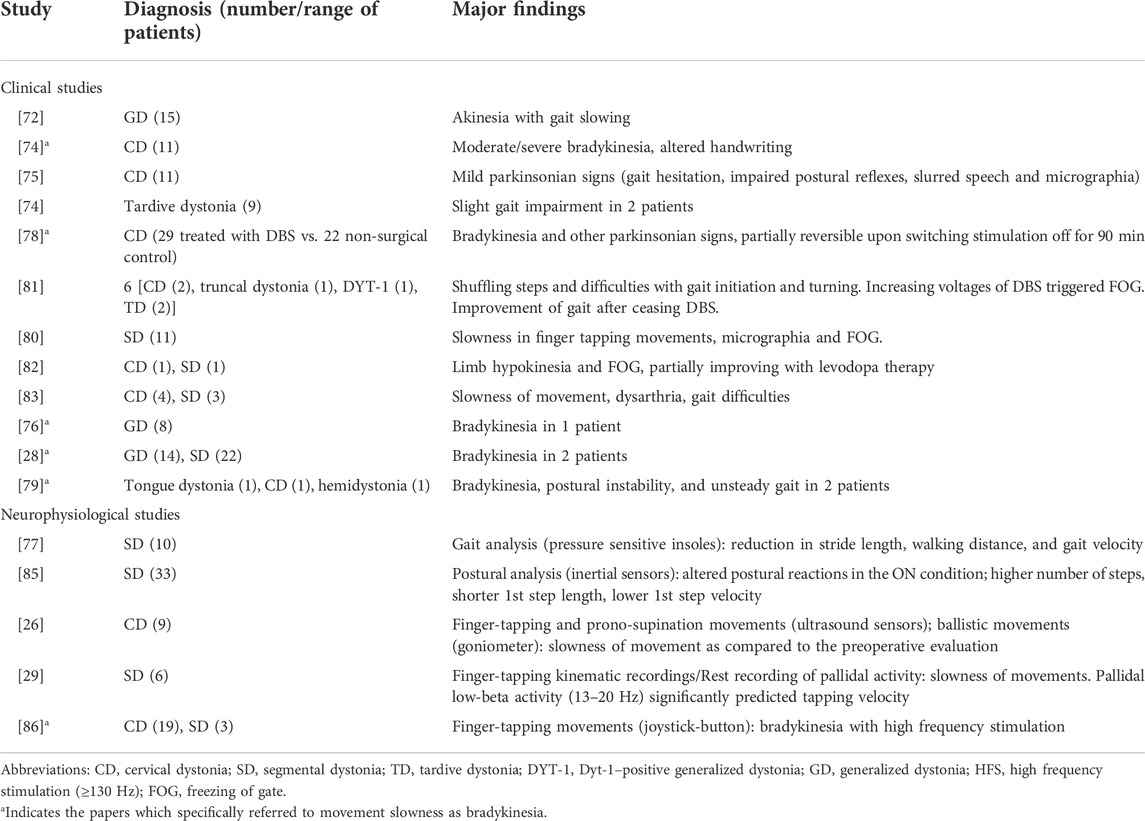
TABLE 1. Clinical and neurophysiological results of bradykinesia in dystonic patients treated with deep brain stimulation (DBS).
Some neurophysiological studies have objectively assessed the motor function alterations after pallidal DBS in dystonic patients [26, 29, 77, 85, 86]. Singh et al. conducted a comprehensive analysis of both distal (finger tapping and prono-supination) and proximal (ballistic) arm movements in dystonic patients who underwent GPi DBS and compared their performance to PD patients who received DBS targeting the subthalamic nucleus (STN) [26]. In contrast to PD patients, who show faster movements after surgery, patients with dystonia exhibit decreased finger tapping, prono-supination, and arm movement speed following GPi DBS, [26]. Another study on patients with cervical and segmental dystonia found that the tapping rate deteriorated when the DBS was set to a high stimulation frequency during 30-seconds of tapping a joystick button with their index finger [86]. The tapping speed after cessation of pallidal stimulation increased over time in another report on 6 patients with isolated dystonia [29]. GPi DBS may also impact posture, as demonstrated by a study that utilized gyroscopes to measure the velocity and amplitude of postural reactions [85]. Finally, gait changes were reported in a study that performed computerized gait analysis on 10 patients with segmental dystonia who underwent bilateral pallidal DBS [77].
The subthalamic nucleus (STN) has also been explored as a possible target for dystonia, based on intraoperative single unit recordings performed in primary dystonia that showed similar bursting and oscillatory activity in STN and GPi [87–90]. Concerning the possible occurrence of bradykinesia over time in these patients, in a 3-years follow-up study, Ostrem et al. clinically monitored 20 patients with medically refractory isolated dystonia treated with STN-DBS; they reported a worsened handwriting in 3 patients and the development of movement slowness in 2 patients [91]. These data might seem paradoxical given the strong efficacy of STN-DBS in improving bradykinesia in PD. However, to date, no neurophysiological study objectively assessed possible movements abnormalities in dystonia patients before and after undergoing STN-DBS.
In summary, bradykinesia may occur in patients affected by isolated dystonia treated with both GPi- and STN- DBS. The present observation may highlight the role of basal ganglia oscillations in bradykinesia pathophysiology.
Bradykinesia in functional dystonia
Functional movement disorders (FMDs) are defined as abnormal movements that are involuntary and do not have a clear neurologic cause or consistent neuroanatomy [30, 92–96]. Functional dystonia typically presents with fixed onset, inconsistent resistance, and absence of a sensory trick [96]. Functional bradykinesia is characterized by an abnormal slowness of movement that is not accompanied by a decrease in movement amplitude or complete movement arrest [94]. Other common features of functional bradykinesia may include fatigue, giveaway weakness, distractibility, and variability in movement [94]. Finally, gait may be slow and stiff with decreased arm swing, but the FOG is not typically observed. Interestingly, functional dystonia and bradykinesia often coexist in patients with FMDs, with up to 74% exhibiting two or more phenomena [97]. However, no studies have specifically investigated the co-occurrence of these two phenomena, and previous studies have not emphasized this issue.
Although no specific neurophysiological investigations have been carried out in patients with functional dystonia and bradykinesia, a recent study examined eyelid movements in a patient with functional eyelid opening apraxia (EOA) using kinematic methods, demonstrating more severely impaired kinematic features in functional EAO as compared to EAO in PD [98]. EOA is characterized by the inability to initiate eye opening [99, 100]. It can occur in isolation or be associated with various neurological conditions, including focal dystonia such as blepharospasm [100]. In another case, eyelid opening apraxia was observed as part of functional parkinsonism along with involuntary facial movements and was considered a type of facial bradykinesia [98, 101].
In summary, anecdotic cases have demonstrated the coexistence of dystonia and bradykinesia in patients with FMDs, although the present topic requires further investigation.
Bradykinesia in dystonia-parkinsonism
Numerous conditions can cause dystonia-parkinsonism, including genetic and acquired disorders, as recently highlighted in various review papers [31, 33, 35, 42, 102–109]. Bradykinesia is not unexpected, given the complex etiology of dystonia-parkinsonism, that can result from a variety of basal ganglia diseases. Notably, in dystonia-parkinsonism, the severity of bradykinesia and dystonia strongly correlates, thus supporting the hypothesis of a partially overlapping pathophysiological mechanisms underlying the two disorders. It is plausible, though, that bradykinesia in dystonia-parkinsonism may be at least in part influenced by coexisting symptoms, e.g., diplegia/hemiplegia, spasticity, and cognitive deficits [6, 9]. However, no pathophysiological studies have investigated this aspect in detail. Another critical aspect of dystonia-parkinsonism is that it is assumed that the bradykinesia in these cases is similar to that observed in PD. However, this assumption has not been well-supported by clinical and experimental evidence. So far, few neurophysiological studies have been conducted to evaluate motor disturbances in patients with dystonia-parkinsonism [110, 111]; when specifically investigated the sequence effect was not observed [111].
In summary, dystonia-parkinsonism is a clinically and etiologically heterogeneous syndrome. Notably, in these cases the characteristics of bradykinesia have not been investigated either clinically or experimentally. Therefore, the assumption that bradykinesia in dystonia-parkinsonism is comparable to that of PD is not supported by substantial evidence. Future studies will necessarily have to investigate this topic in more detail.
Pathophysiological insight
The coexistence of bradykinesia and dystonia is intriguing from a pathophysiological standpoint, as these two disorders have historically been interpreted as do to opposing patterns of basal ganglia dysfunction [1, 6, 31, 36–40].
One proposed explanation to reconcile this paradox is to view bradykinesia as a secondary effect of the co-contraction between agonist and antagonist muscles, a common dystonia feature as demonstrated by electromyographic (EMG) recordings [1, 6, 9]. In other words, co-contraction and impaired muscle relaxation may interfere with the execution of voluntary movement, as seen in FHD patients performing tasks that trigger cramps [14, 52], or in patients with various forms of focal dystonia performing isometric contraction and relaxation tasks [16]. However, bradykinesia in dystonia has also been observed during upper limb movements where co-contraction activity cannot be clearly identified [53] and in non-dystonic body segments where there is no co-contraction activity [9, 17]. Hence another plausible explanation is that bradykinesia and dystonia may have common underlying pathophysiological mechanisms, including abnormalities in the cortico-basal ganglia-thalamic and cerebellar networks, as well as alterations in dopaminergic neurotransmission [6, 6, 33, 35, 42].
Although the factors contributing to the differences in kinetic bradykinesia features between PD and dystonia have not been fully identified [22], one possibility is that variations in firing rate [112–115] and synchronization of oscillatory activity in the basal ganglia network [116–119] could be responsible for bradykinesia or dystonia, respectively. Also, studies have shown that patients with dystonia who undergo DBS to alleviate their symptoms may develop bradykinesia [72, 73, 77, 78, 86] suggesting that stimulation-induced changes in basal ganglia oscillatory activities may play a significant role in the development of bradykinesia in dystonia. In this regard, a recent neurophysiological study demonstrated that the objectively-measured bradykinesia induced by GPi-DBS in dystonia patients is paralleled by an increased low-beta activity in the GPi [29]. The positive relationship between low-beta oscillations power and bradykinesia severity resembles that observed in PD [120–123]. The authors speculated that in dystonia characterized by a direct pathway hyperactivity, GPi-DBS might imbalance brain rhythms by excessively suppressing pro-kinetic oscillations, which may lead to a relative increase of anti-kinetic beta activity [29]. However, more in general, the relationship between beta oscillations and slowness observed in dystonia may support the existence of common or related neurophysiological substrates in bradykinesia pathophysiology regardless of the disease condition (dystonia or PD). This view would also partially explain the observation that the evolution of dystonia and parkinsonism is inversely proportional [47], and that tapping speed has opposite response to GPi DBS in dystonic and PD patients [26, 29, 86]. Abnormalities in the primary motor cortex (M1) are another common factor in dystonia and bradykinesia [6, 9].
Abnormalities of intracortical excitability, as well as maladaptive plasticity, have been demonstrated in M1 through neurophysiological studies in patients with dystonia and parkinsonism [6, 124, 125]. Interestingly, reduced GABA-A-ergic inhibition at the M1 level, as assessed by short-interval intracortical inhibition (SICI), is a cardinal neurophysiological feature of dystonia and PD [6, 42, 126]. In PD, SICI changes correlate with movement slowness severity and are thought to reflect compensatory cortical mechanisms against motor dysfunction [127, 128]. To date, the precise functional significance of altered SICI in dystonia is unclear [42]. Abnormal sensory processing has also been identified as a possible sensorimotor cortex abnormality that may underlie dystonia and bradykinesia [129–134]. It has been found that somatosensorimotor integration mechanisms, as quantified by the somatosensory temporal discrimination threshold (STDT), are critically impaired in both dystonia and parkinsonisms. Dystonia patients exhibit abnormally increased STDT at rest, and changes in STDT during motor execution may worsen dystonia during voluntary movements [135]. Similarly, STDT is increased in PD, and this alteration correlates with the variability in movement amplitude and speed, objectively measured using sensors [136]. Furthermore, the analysis of movement-related modifications of STDT has demonstrated that the temporal coupling between tactile information and motor outflow is altered in PD patients [137]. Finally, although less commonly compared to nigrostriatal lesions, prefrontal lesions, including the supplementary motor area, may also lead to dystonia and parkinsonism, indicating that changes in motor integration at the cortical level may also be involved in the pathophysiology of both dystonia and bradykinesia [138, 139].
The cerebellum is another crucial node in the pathophysiology of both bradykinesia and dystonia [6, 140–142], even though the precise pathophysiological mechanisms underlying these motor disorders are not yet fully understood. Regarding bradykinesia, it is worth noting that the cerebellum is involved in encoding kinematic parameters such as movement direction and velocity, as shown by neurophysiological studies [143, 144]. Also, neuroimaging studies demonstrated that cerebellar activity was related to the severity of micrographia [145] and specific bradykinesia characteristics in PD [146].
A further intriguing aspect relates to the observation of dystonia and parkinsonism resulting from dopamine receptor blockade, supporting to the hypothesis that the dopaminergic system may play a role in the pathophysiology of these two motor disorders. This is further corroborated by genetic evidence demonstrating that disruption of dopamine synthesis leads to dystonia and parkinsonism, as seen in variants of PARKIN or GCH1 genes. Furthermore, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesion animal model, dystonia with a decline in striatal dopamine and dopamine 2-like receptors precedes the onset of parkinsonism [33]. Thus, extensive research has highlighted the role of dopaminergic dysfunction, abnormal basal ganglia circuitry, and altered cortical and cerebellar function in the pathophysiology of both bradykinesia and dystonia [1, 6, 9, 61, 124, 142, 147–150].
In summary, evidence suggests that both bradykinesia and dystonia can be interpreted as motor disorders resulting from network dysfunction, and there may be an overlap between the mechanisms underlying both disorders [6, 41–45, 142] (Figure 1).
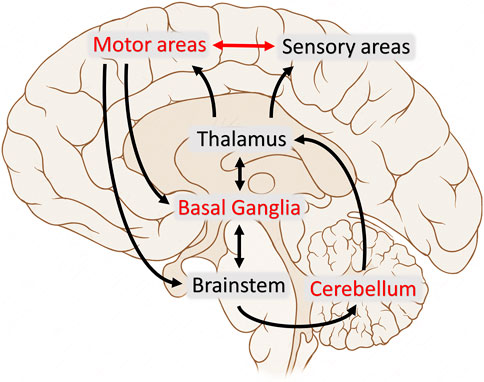
FIGURE 1. The figure illustrates the various brain areas and circuits involved in the motor control in humans. Red text and arrows reflect the nodes and pathways which demonstrated overlapping abnormalities between bradykinesia and dystonia.
Terminological issues
A critical point to consider is that bradykinesia in dystonia may not display the same motor characteristics as in PD, such as the sequence effect [6, 7, 9]. As a result, the term “bradykinesia” has been avoided in many studies on dystonia to describe movement slowness and other motor abnormalities in these patients. Some authors, for example, have highlighted the absence of “true bradykinesia” in dystonic patients [11, 51], arguing that the combination of movement slowness and the sequence effect, as defined by clinical criteria in PD, is not observed in dystonia [3]. The absence of the sequence effect in dystonia has led some researchers (Haggstrom et al.), suggesting using the term “non-decremental bradykinesia” to describe movement slowness in dystonia [22]. However, in other instances the term bradykinesia seemed more appropriate when referred to dystonia. This is the case of bradykinesia induced by DBS in dystonic patients [28, 29, 73, 78], due to a common pathophysiological background, e.g., beta-band oscillations [40]. Finally, it is important to note, that as in dystonia, the sequence effect may also be absent in advanced PD stages of and atypical parkinsonism [7, 151].
Inconsistencies in using the term bradykinesia extend beyond dystonia and are also present in other pathological conditions where motor disturbances, such as slowness of movement, have been observed [9]. The presence of bradykinesia in non-parkinsonian conditions and the possibility of common pathophysiological mechanisms underlying bradykinesia in pathophysiologically distinct conditions supports using the term bradykinesia in dystonia, as recently proposed [7]. Accordingly, the term bradykinesia should be used to describe the slowness of voluntary movements, as it is a non-specific finding that can be present in various conditions, including dystonia. Therefore, when there is a combination of motor alterations, such as bradykinesia with sequence effect and additional features, typical for the clinical picture of parkinsonism, all features should be spelled out individually and not implied. Further studies are needed to elucidate the relationship between the variable phenomenology of bradykinesia and the underlying etiology, including causes of dystonia [7]. In dystonia, once this aspect is clarified similarly to the tremor in dystonia [12, 152], one could adopt the term “bradykinesia in dystonia” when bradykinesia is present in a dystonic patient but involves a body segment not affected by dystonia, and the term ‘dystonic bradykinesia’ when bradykinesia involves the body segment affected by dystonia.
Concluding remarks
Despite the use of varied and heterogeneous terminology across studies, there is evidence to suggest the consistent occurrence of bradykinesia in patients with dystonia, including not only those with dystonia-parkinsonism [31, 33], but also those with isolated dystonia [13–20, 20–25]. The findings discussed in this paper have important implications for the pathophysiology of bradykinesia and dystonia, indicating that they may be related motor disorders resulting from network dysfunction. This perspective supports using of the term bradykinesia and other related terms in describing the phenomenology of voluntary movement alterations in patients with dystonia.
Author contributions
GP and MB contributed to the conception and design of the study. GP, AG, SG, and AC performed the literature review. GP wrote the first draft of the manuscript; AG, SG, AC, LA, TP, AB, and MB contributed to the manuscript revision, and read and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Italian Ministry of Health (Current Research 2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Laura Centonze (bGF1cmEuY2VudG9uemVAdW5pcm9tYTEuaXQ=) from the Department of Human Neurosciences, Sapienza University of Rome, for her support in the English-language editing of the manuscript.
Abbreviations
CD, cervical dystonia; DBS, deep brain stimulation; DAT, dopamine transporter; EMG, electromyographic; EOA, eyelid opening apraxia; FHD, focal hand dystonia; FOG, freezing of gait; FMDs, functional movement disorders; GPi, globus pallidus pars interna; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson’s disease; M1, primary motor cortex; RT, reaction time; SICI, short intracortical inhibition; STDT, somatosensory temporal discrimination threshold; STN, subthalamic nucleus.
References
1. Berardelli, A, Rothwell, JC, Thompson, PD, and Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain (2001) 124:2131–46. doi:10.1093/brain/124.11.2131
2. Armstrong, MJ, Litvan, I, Lang, AE, Bak, TH, Bhatia, KP, Borroni, B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology (2013) 80:496–503. doi:10.1212/WNL.0b013e31827f0fd1
3. Postuma, RB, Berg, D, Stern, M, Poewe, W, Olanow, CW, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord (2015) 30:1591–601. doi:10.1002/mds.26424
4. Höglinger, GU, Respondek, G, Stamelou, M, Kurz, C, Josephs, KA, Lang, AE, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord (2017) 32:853–64. doi:10.1002/mds.26987
5. McKeith, IG, Boeve, BF, Dickson, DW, Halliday, G, Taylor, J-P, Weintraub, D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology (2017) 89:88–100. doi:10.1212/WNL.0000000000004058
6. Bologna, M, Paparella, G, Fasano, A, Hallett, M, and Berardelli, A. Evolving concepts on bradykinesia. Brain (2020) 143:727–50. doi:10.1093/brain/awz344
7. Bologna, M, Espay, AJ, Fasano, A, Paparella, G, Hallett, M, and Berardelli, A. Redefining bradykinesia. Mov Disord (2023) 38:551–7. doi:10.1002/mds.29362
8. Wenning, GK, Stankovic, I, Vignatelli, L, Fanciulli, A, Calandra-Buonaura, G, Seppi, K, et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov Disord (2022) 37:1131–48. doi:10.1002/mds.29005
9. Paparella, G, Fasano, A, Hallett, M, Berardelli, A, and Bologna, M. Emerging concepts on bradykinesia in non-parkinsonian conditions. Eur J Neurol (2021) 28:2403–22. doi:10.1111/ene.14851
10. Verger, H, and Cruchet, R Trait e de torticolis spasmodiques. Paris: Masson & Cie (1907). p. 346.
11. Schilder, JCM, Overmars, SS, Marinus, J, van Hilten, JJ, and Koehler, PJ. The terminology of akinesia, bradykinesia and hypokinesia: Past, present and future. Parkinsonism Relat Disord (2017) 37:27–35. doi:10.1016/j.parkreldis.2017.01.010
12. Albanese, A, Bhatia, K, Bressman, SB, DeLong, MR, Fahn, S, Fung, VSC, et al. Phenomenology and classification of dystonia: A consensus update. Movement Disord (2013) 28:863–73. doi:10.1002/mds.25475
13. Sheehy, MP, and Marsden, CD. Writers’ cramp-a focal dystonia. Brain (1982) 105(Pt 3):461–80. doi:10.1093/brain/105.3.461
14. Cohen, LG, and Hallett, M. Hand cramps: Clinical features and electromyographic patterns in a focal dystonia. Neurology (1988) 38:1005–12. doi:10.1212/wnl.38.7.1005
15. Currà, A, Agostino, R, Galizia, P, Fittipaldi, F, Manfredi, M, and Berardelli, A. Sub-movement cueing and motor sequence execution in patients with Huntington’s disease. Clin Neurophysiol (2000) 111:1184–90. doi:10.1016/s1388-2457(00)00302-3
16. Buccolieri, A, Avanzino, L, Marinelli, L, Trompetto, C, Marchese, R, and Abbruzzese, G. Muscle relaxation is impaired in dystonia: A reaction time study. Mov Disord (2004) 19:681–7. doi:10.1002/mds.10711
17. Carboncini, MC, Manzoni, D, Strambi, S, Bonfiglio, L, Andre, P, and Rossi, B. Impaired agonists recruitment during voluntary arm movements in patients affected by spasmodic torticollis. Arch Ital Biol (2004) 142:113–24.
18. Schneider, SA, Edwards, MJ, Mir, P, Cordivari, C, Hooker, J, Dickson, J, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord (2007) 22:2210–5. doi:10.1002/mds.21685
19. Gregori, B, Agostino, R, Bologna, M, Dinapoli, L, Colosimo, C, Accornero, N, et al. Fast voluntary neck movements in patients with cervical dystonia: A kinematic study before and after therapy with botulinum toxin type A. Clin Neurophysiol (2008) 119:273–80. doi:10.1016/j.clinph.2007.10.007
20. Camargo, CHF, Camargos, ST, Becker, N, Munhoz, RP, Raskin, S, Cardoso, FEC, et al. Cervical dystonia: About familial and sporadic cases in 88 patients. Arq Neuropsiquiatr (2014) 72:107–13. doi:10.1590/0004-282X20130225
21. Katschnig-Winter, P, Schwingenschuh, P, Davare, M, Sadnicka, A, Schmidt, R, Rothwell, JC, et al. Motor sequence learning and motor adaptation in primary cervical dystonia. J Clin Neurosci (2014) 21:934–8. doi:10.1016/j.jocn.2013.08.019
22. Haggstrom, L, Darveniza, P, and Tisch, S. Mild parkinsonian features in dystonia: Literature review, mechanisms and clinical perspectives. Parkinsonism Relat Disord (2017) 35:1–7. doi:10.1016/j.parkreldis.2016.10.022
23. Choudhury, S, Roy, A, Mondal, B, Singh, R, Halder, S, Chatterjee, K, et al. Slowed movement stopping in Parkinson’s disease and focal dystonia is improved by standard treatment. Sci Rep (2019) 9:19504. doi:10.1038/s41598-019-55321-5
24. Shetty, AS, Bhatia, KP, and Lang, AE. Dystonia and Parkinson’s disease: What is the relationship? Neurobiol Dis (2019) 132:104462. doi:10.1016/j.nbd.2019.05.001
25. Balint, B, Mulroy, E, Gövert, F, Latorre, A, Di Lazarro, G, Erro, R, et al. Development of parkinsonism after long-standing cervical dystonia - a cohort. J Neurol Sci (2021) 427:117477. doi:10.1016/j.jns.2021.117477
26. Singh, A, Kammermeier, S, Mehrkens, JH, and Bötzel, K. Movement kinematic after deep brain stimulation associated microlesions. J Neurol Neurosurg Psychiatry (2012) 83:1022–6. doi:10.1136/jnnp-2012-302309
27. Volkmann, J, Mueller, J, Deuschl, G, Kühn, AA, Krauss, JK, Poewe, W, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: A randomised, sham-controlled trial. Lancet Neurol (2014) 13:875–84. doi:10.1016/S1474-4422(14)70143-7
28. Krause, P, Völzmann, S, Ewert, S, Kupsch, A, Schneider, GH, and Kühn, AA. Long-term effects of bilateral pallidal deep brain stimulation in dystonia: A follow-up between 8 and 16 years. J Neurol (2020) 267:1622–31. doi:10.1007/s00415-020-09745-z
29. Lofredi, R, Scheller, U, Mindermann, A, Feldmann, LK, Krauss, JK, Saryyeva, A, et al. Pallidal beta activity is linked to stimulation-induced slowness in dystonia. Mov Disord (2023) 38:894–9. doi:10.1002/mds.29347
30. Barbey, A, and Aybek, S. Functional movement disorders. Curr Opin Neurol (2017) 30:427–34. doi:10.1097/WCO.0000000000000464
31. Jankovic, J, and Tintner, R. Dystonia and parkinsonism. Parkinsonism Relat Disord (2001) 8:109–21. doi:10.1016/s1353-8020(01)00025-6
32. Balint, B, and Bhatia, KP. Dystonia: An update on phenomenology, classification, pathogenesis and treatment. Curr Opin Neurol (2014) 27:468–76. doi:10.1097/WCO.0000000000000114
33. Morales-Briceno, H, Fung, VSC, Bhatia, KP, and Balint, B. Parkinsonism and dystonia: Clinical spectrum and diagnostic clues. J Neurol Sci (2022) 433:120016. doi:10.1016/j.jns.2021.120016
34. Chin, HL, Lin, C-Y, and Chou, OH-I. X-Linked dystonia parkinsonism: Epidemiology, genetics, clinical features, diagnosis, and treatment. Acta Neurol Belg (2023) 123:45–55. doi:10.1007/s13760-022-02144-3
35. Jankovic, J, Truong, DD, and Bologna, M. Parkinsonism across the spectrum of movement disorders and beyond. J Neurol Sci (2022) 433:120013. doi:10.1016/j.jns.2021.120013
36. Marsden, CD, Obeso, JA, Zarranz, JJ, and Lang, AE. The anatomical basis of symptomatic hemidystonia. Brain (1985) 108(Pt 2):463–83. doi:10.1093/brain/108.2.463
37. Berardelli, A, Rothwell, JC, Hallett, M, Thompson, PD, Manfredi, M, and Marsden, CD. The pathophysiology of primary dystonia. Brain (1998) 121(7):1195–212. doi:10.1093/brain/121.7.1195
38. DeLong, MR, and Wichmann, T. Circuits and circuit disorders of the basal ganglia. Arch Neurol (2007) 64:20–4. doi:10.1001/archneur.64.1.20
39. Neychev, VK, Gross, RE, Lehéricy, S, Hess, EJ, and Jinnah, HA. The functional neuroanatomy of dystonia. Neurobiol Dis (2011) 42:185–201. doi:10.1016/j.nbd.2011.01.026
40. Lofredi, R, and Kühn, AA. Brain oscillatory dysfunctions in dystonia. Handb Clin Neurol (2022) 184:249–57. doi:10.1016/B978-0-12-819410-2.00026-6
41. Wichmann, T Changing views of the pathophysiology of Parkinsonism. Mov Disord (2019) 34:1130–43. doi:10.1002/mds.27741
42. Bologna, M, Valls-Solè, J, Kamble, N, Pal, PK, Conte, A, Guerra, A, et al. Dystonia, chorea, hemiballismus and other dyskinesias. Clin Neurophysiol (2022) 140:110–25. doi:10.1016/j.clinph.2022.05.014
43. Tewari, A, Fremont, R, and Khodakhah, K. It’s not just the basal ganglia: Cerebellum as a target for dystonia therapeutics. Mov Disord (2017) 32:1537–45. doi:10.1002/mds.27123
44. Corp, DT, Joutsa, J, Darby, RR, Delnooz, CCS, van de Warrenburg, BPC, Cooke, D, et al. Network localization of cervical dystonia based on causal brain lesions. Brain (2019) 142:1660–74. doi:10.1093/brain/awz112
45. Chen, R, Berardelli, A, Bhattacharya, A, Bologna, M, Chen, K-HS, Fasano, A, et al. Clinical neurophysiology of Parkinson’s disease and parkinsonism. Clin Neurophysiol Pract (2022) 7:201–27. doi:10.1016/j.cnp.2022.06.002
46. Bologna, M, Fabbrini, G, Marsili, L, Defazio, G, Thompson, PD, and Berardelli, A. Facial bradykinesia. J Neurol Neurosurg Psychiatry (2013) 84:681–5. doi:10.1136/jnnp-2012-303993
47. Katchen, M, and Duvoisin, RC. Parkinsonism following dystonia in three patients. Mov Disord (1986) 1:151–7. doi:10.1002/mds.870010210
48. Carecchio, M, Invernizzi, F, Gonzàlez-Latapi, P, Panteghini, C, Zorzi, G, Romito, L, et al. Frequency and phenotypic spectrum of KMT2B dystonia in childhood: A single-center cohort study. Mov Disord (2019) 34:1516–27. doi:10.1002/mds.27771
49. Feuerstein, JS, Taylor, M, Kwak, JJ, and Berman, BD. Parkinsonism and positive dopamine transporter imaging in a patient with a novel KMT2B variant. Mov Disord Clin Pract (2021) 8:279–81. doi:10.1002/mdc3.13140
50. Kuo, M-C, Lin, H-I, and Lin, C-H. Craniocervical dystonia with levodopa-responsive parkinsonism co-segregating with a pathogenic ANO3 mutation in a Taiwanese family. Parkinsonism Relat Disord (2019) 62:236–8. doi:10.1016/j.parkreldis.2019.01.020
51. Stamelou, M, Edwards, MJ, and Bhatia, KP. Late onset rest-tremor in DYT1 dystonia. Parkinsonism Relat Disord (2013) 19:136–7. doi:10.1016/j.parkreldis.2012.05.026
52. van der Kamp, W, Berardelli, A, Rothwell, JC, Thompson, PD, Day, BL, and Marsden, CD. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatry (1989) 52:1043–9. doi:10.1136/jnnp.52.9.1043
53. Prodoehl, J, Corcos, DM, Leurgans, S, Comella, CL, Weis-McNulty, A, and MacKinnon, CD. Changes in the relationship between movement velocity and movement distance in primary focal hand dystonia. J Mot Behav (2008) 40:301–13. doi:10.3200/JMBR.40.4.301-314
54. Inzelberg, R, Flash, T, Schechtman, E, and Korczyn, AD. Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry (1995) 58:312–9. doi:10.1136/jnnp.58.3.312
55. Pelosin, E, Bove, M, Marinelli, L, Abbruzzese, G, and Ghilardi, MF. Cervical dystonia affects aimed movements of nondystonic segments. Mov Disord (2009) 24:1955–61. doi:10.1002/mds.22693
56. Bologna, M, Paparella, G, Fabbrini, A, Leodori, G, Rocchi, L, Hallett, M, et al. Effects of cerebellar theta-burst stimulation on arm and neck movement kinematics in patients with focal dystonia. Clin Neurophysiol (2016) 127:3472–9. doi:10.1016/j.clinph.2016.09.008
57. Jabusch, H-C, Vauth, H, and Altenmüller, E. Quantification of focal dystonia in pianists using scale analysis. Mov Disord (2004) 19:171–80. doi:10.1002/mds.10671
58. Conte, A, Ferrazzano, G, Belvisi, D, Manzo, N, Battista, E, Li Voti, P, et al. Somatosensory temporal discrimination in Parkinson’s disease, dystonia and essential tremor: Pathophysiological and clinical implications. Clin Neurophysiol (2018) 129:1849–53. doi:10.1016/j.clinph.2018.05.024
59. Agostino, R, Berardelli, A, Formica, A, Accornero, N, and Manfredi, M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain (1992) 115(Pt 5):1481–95. doi:10.1093/brain/115.5.1481
60. Furuya, S, and Altenmüller, E. Finger-specific loss of independent control of movements in musicians with focal dystonia. Neuroscience (2013) 247:152–63. doi:10.1016/j.neuroscience.2013.05.025
61. Simonyan, K, Berman, BD, Herscovitch, P, and Hallett, M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci (2013) 33:14705–14. doi:10.1523/JNEUROSCI.0407-13.2013
62. Furuya, S, Uehara, K, Sakamoto, T, and Hanakawa, T. Aberrant cortical excitability reflects the loss of hand dexterity in musician’s dystonia. J Physiol (Lond) (2018) 596:2397–411. doi:10.1113/JP275813
63. Newby, R, Muhamed, S, Alty, J, Cosgrove, J, Jamieson, S, Smith, S, et al. Geste Antagoniste effects on motor performance in dystonia-A kinematic study. Mov Disord Clin Pract (2022) 9:759–64. doi:10.1002/mdc3.13505
64. Boccagni, C, Carpaneto, J, Micera, S, Bagnato, S, and Galardi, G. Motion analysis in cervical dystonia. Neurol Sci (2008) 29:375–81. doi:10.1007/s10072-008-1033-z
65. Shaikh, AG, Zee, DS, and Jinnah, HA. Oscillatory head movements in cervical dystonia: Dystonia, tremor, or both?: Head oscillations in cervical dystonia. Movement Disord (2015) 30:834–42. doi:10.1002/mds.26231
66. Caronni, A, Arcuri, P, Carpinella, I, Marzegan, A, Lencioni, T, Ramella, M, et al. Smoothness of movement in idiopathic cervical dystonia. Sci Rep (2022) 12:5090. doi:10.1038/s41598-022-09149-1
67. Murase, N, Kaji, R, Shimazu, H, Katayama-Hirota, M, Ikeda, A, Kohara, N, et al. Abnormal premovement gating of somatosensory input in writer’s cramp. Brain (2000) 123(Pt 9):1813–29. doi:10.1093/brain/123.9.1813
68. MacKinnon, CD, Velickovic, M, Drafta, C, Hesquijarosa, A, and Brin, MF. Corticospinal excitability accompanying ballistic wrist movements in primary dystonia. Mov Disord (2004) 19:273–84. doi:10.1002/mds.20017
69. Jankowski, J, Paus, S, Scheef, L, Bewersdorff, M, Schild, HH, Klockgether, T, et al. Abnormal movement preparation in task-specific focal hand dystonia. PLoS ONE (2013) 8:e78234. doi:10.1371/journal.pone.0078234
70. Kishore, A, Popa, T, James, P, Krishnan, S, Robert, S, and Meunier, S. Severity of writer’s cramp is related to faulty motor preparation. Cereb Cortex (2018) 28:3564–77. doi:10.1093/cercor/bhx228
71. Vidailhet, M, Vercueil, L, Houeto, J-L, Krystkowiak, P, Benabid, A-L, Cornu, P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med (2005) 352:459–67. doi:10.1056/NEJMoa042187
72. Tisch, S, Zrinzo, L, Limousin, P, Bhatia, KP, Quinn, N, Ashkan, K, et al. Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia. J Neurol Neurosurg Psychiatry (2007) 78:1314–9. doi:10.1136/jnnp.2006.109694
73. Berman, BD, Starr, PA, Marks, WJ, and Ostrem, JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg (2009) 87:37–44. doi:10.1159/000195718
74. Gruber, D, Trottenberg, T, Kivi, A, Schoenecker, T, Kopp, UA, Hoffmann, KT, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology (2009) 73:53–8. doi:10.1212/WNL.0b013e3181aaea01
75. Zauber, SE, Watson, N, Comella, CL, Bakay, RAE, and Metman, LV. Stimulation-induced parkinsonism after posteroventral deep brain stimulation of the globus pallidus internus for craniocervical dystonia. J Neurosurg (2009) 110:229–33. doi:10.3171/2008.6.17621
76. Krause, P, Lauritsch, K, Lipp, A, Horn, A, Weschke, B, Kupsch, A, et al. Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol (2016) 263:2319–26. doi:10.1007/s00415-016-8253-6
77. Wolf, ME, Capelle, HH, Bäzner, H, Hennerici, MG, Krauss, JK, and Blahak, C. Hypokinetic gait changes induced by bilateral pallidal deep brain stimulation for segmental dystonia. Gait Posture (2016) 49:358–63. doi:10.1016/j.gaitpost.2016.07.301
78. Mahlknecht, P, Georgiev, D, Akram, H, Brugger, F, Vinke, S, Zrinzo, L, et al. Parkinsonian signs in patients with cervical dystonia treated with pallidal deep brain stimulation. Brain (2018) 141:3023–34. doi:10.1093/brain/awy217
79. Horisawa, S, Kohara, K, Murakami, M, Fukui, A, Kawamata, T, and Taira, T. Deep brain stimulation of the forel’s field for dystonia: Preliminary results. Front Hum Neurosci (2021) 15:768057. doi:10.3389/fnhum.2021.768057
80. Blahak, C, Capelle, H-H, Baezner, H, Kinfe, TM, Hennerici, MG, and Krauss, JK. Micrographia induced by pallidal DBS for segmental dystonia: A subtle sign of hypokinesia? J Neural Transm (Vienna) (2011) 118:549–53. doi:10.1007/s00702-010-0544-y
81. Schrader, C, Capelle, H-H, Kinfe, TM, Blahak, C, Bäzner, H, Lütjens, G, et al. GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology (2011) 77:483–8. doi:10.1212/WNL.0b013e318227b19e
82. Amtage, F, Feuerstein, TJ, Meier, S, Prokop, T, Piroth, T, and Pinsker, MO. Hypokinesia upon pallidal deep brain stimulation of dystonia: Support of a GABAergic mechanism. Front Neurol (2013) 4:198. doi:10.3389/fneur.2013.00198
83. Pauls, KAM, Hammesfahr, S, Moro, E, Moore, AP, Binder, E, El Majdoub, F, et al. Deep brain stimulation in the ventrolateral thalamus/subthalamic area in dystonia with head tremor. Mov Disord (2014) 29:953–9. doi:10.1002/mds.25884
84. Meoni, S, Fraix, V, Castrioto, A, Benabid, AL, Seigneuret, E, Vercueil, L, et al. Pallidal deep brain stimulation for dystonia: A long term study. J Neurol Neurosurg Psychiatry (2017) 88:960–7. doi:10.1136/jnnp-2016-315504
85. Brecl Jakob, G, Pelykh, O, Košutzká, Z, Pirtošek, Z, Trošt, M, Ilmberger, J, et al. Postural stability under globus pallidus internus stimulation for dystonia. Clin Neurophysiol (2015) 126:2299–305. doi:10.1016/j.clinph.2015.01.022
86. Huebl, J, Brücke, C, Schneider, G-H, Blahak, C, Krauss, JK, and Kühn, AA. Bradykinesia induced by frequency-specific pallidal stimulation in patients with cervical and segmental dystonia. Parkinsonism Relat Disord (2015) 21:800–3. doi:10.1016/j.parkreldis.2015.04.023
87. Kleiner-Fisman, G, Liang, GSL, Moberg, PJ, Ruocco, AC, Hurtig, HI, Baltuch, GH, et al. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J Neurosurg (2007) 107:29–36. doi:10.3171/JNS-07/07/0029
88. Pahapill, PA, and O’Connell, B. Long-term follow-up study of chronic deep brain stimulation of the subthalamic nucleus for cervical dystonia. Neuromodulation (2010) 13:26–30. doi:10.1111/j.1525-1403.2009.00231.x
89. Ostrem, JL, Racine, CA, Glass, GA, Grace, JK, Volz, MM, Heath, SL, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology (2011) 76:870–8. doi:10.1212/WNL.0b013e31820f2e4f
90. Tisch, S. Deep brain stimulation in dystonia: Factors contributing to variability in outcome in short and long term follow-up. Curr Opin Neurol (2022) 35:510–7. doi:10.1097/WCO.0000000000001072
91. Ostrem, JL, San Luciano, M, Dodenhoff, KA, Ziman, N, Markun, LC, Racine, CA, et al. Subthalamic nucleus deep brain stimulation in isolated dystonia: A 3-year follow-up study. Neurology (2017) 88:25–35. doi:10.1212/WNL.0000000000003451
92. Stone, J, LaFrance, WC, Brown, R, Spiegel, D, Levenson, JL, and Sharpe, M. Conversion disorder: Current problems and potential solutions for DSM-5. J Psychosom Res (2011) 71:369–76. doi:10.1016/j.jpsychores.2011.07.005
93. Hallett, M, Weiner, WJ, and Kompoliti, K. Psychogenic movement disorders. Parkinsonism Relat Disord (2012) 18(Suppl. 1):S155–157. doi:10.1016/S1353-8020(11)70048-7
94. Thenganatt, MA, and Jankovic, J. Psychogenic (functional) parkinsonism. Handb Clin Neurol (2016) 139:259–62. doi:10.1016/B978-0-12-801772-2.00022-9
95. Tinazzi, M, Geroin, C, Marcuzzo, E, Cuoco, S, Ceravolo, R, Mazzucchi, S, et al. Functional motor phenotypes: To lump or to split? J Neurol (2021) 268:4737–43. doi:10.1007/s00415-021-10583-w
96. Kola, S, and LaFaver, K. Updates in functional movement disorders: From pathophysiology to treatment advances. Curr Neurol Neurosci Rep (2022) 22:305–11. doi:10.1007/s11910-022-01192-9
97. Hinson, VK, Cubo, E, Comella, CL, Goetz, CG, and Leurgans, S. Rating scale for psychogenic movement disorders: Scale development and clinimetric testing. Mov Disord (2005) 20:1592–7. doi:10.1002/mds.20650
98. Hophing, L, Bologna, M, Berardelli, A, and Fasano, A. Functional eyelid opening apraxia: A kinematic study. Eur J Neurol (2018) 25:e95–e97. doi:10.1111/ene.13682
100. Boghen, D. Apraxia of lid opening: A review. Neurology (1997) 48:1491–4. doi:10.1212/wnl.48.6.1491
101. Fasano, A, Valadas, A, Bhatia, KP, Prashanth, LK, Lang, AE, Munhoz, RP, et al. Psychogenic facial movement disorders: Clinical features and associated conditions. Mov Disord (2012) 27:1544–51. doi:10.1002/mds.25190
102. Bhatia, KP, and Marsden, CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain (1994) 117(Pt 4):859–76. doi:10.1093/brain/117.4.859
103. Martikainen, MH, Ng, YS, Gorman, GS, Alston, CL, Blakely, EL, Schaefer, AM, et al. Clinical, genetic, and radiological features of extrapyramidal movement disorders in mitochondrial disease. JAMA Neurol (2016) 73:668–74. doi:10.1001/jamaneurol.2016.0355
104. Balint, B, Vincent, A, Meinck, H-M, Irani, SR, and Bhatia, KP. Movement disorders with neuronal antibodies: Syndromic approach, genetic parallels and pathophysiology. Brain (2018) 141:13–36. doi:10.1093/brain/awx189
105. Joutsa, J, Horn, A, Hsu, J, and Fox, MD. Localizing parkinsonism based on focal brain lesions. Brain (2018) 141:2445–56. doi:10.1093/brain/awy161
106. Galosi, S, Nardecchia, F, and Leuzzi, V. Treatable inherited movement disorders in children: Spotlight on clinical and biochemical features. Mov Disord Clin Pract (2020) 7:154–66. doi:10.1002/mdc3.12897
107. Leuzzi, V, Nardecchia, F, Pons, R, and Galosi, S. Parkinsonism in children: Clinical classification and etiological spectrum. Parkinsonism Relat Disord (2021) 82:150–7. doi:10.1016/j.parkreldis.2020.10.002
108. Menozzi, E, Mulroy, E, Akbarian-Tefaghi, L, Bhatia, KP, and Balint, B. Movement disorders in systemic autoimmune diseases: Clinical spectrum, ancillary investigations, pathophysiological considerations. Parkinsonism Relat Disord (2021) 88:116–28. doi:10.1016/j.parkreldis.2021.05.026
109. Corp, DT, Greenwood, CJ, Morrison-Ham, J, Pullinen, J, McDowall, GM, Younger, EFP, et al. Clinical and structural findings in patients with lesion-induced dystonia: Descriptive and quantitative analysis of published cases. Neurology (2022) 99:e1957–e1967. doi:10.1212/WNL.0000000000201042
110. Becker, LF, Tunc, S, Murphy, P, Bäumer, T, Weissbach, A, Pauly, MG, et al. Time estimation and arousal responses in dopa-responsive dystonia. Sci Rep (2022) 12:14279. doi:10.1038/s41598-022-17545-w
111. Passaretti, M, Pollini, L, Paparella, G, De Biase, A, Colella, D, Angelini, L, et al. Neurophysiological assessment of juvenile parkinsonism due to primary monoamine neurotransmitter disorders. J Neural Transm (Vienna) (2022) 129:1011–21. doi:10.1007/s00702-022-02527-z
112. Sanghera, MK, Grossman, RG, Kalhorn, CG, Hamilton, WJ, Ondo, WG, and Jankovic, J. Basal ganglia neuronal discharge in primary and secondary dystonia in patients undergoing pallidotomy. Neurosurgery (2003) 52:1358–70. doi:10.1227/01.neu.0000064805.91249.f5
113. Starr, PA, Rau, GM, Davis, V, Marks, WJ, Ostrem, JL, Simmons, D, et al. Spontaneous pallidal neuronal activity in human dystonia: Comparison with Parkinson’s disease and normal macaque. J Neurophysiol (2005) 93:3165–76. doi:10.1152/jn.00971.2004
114. Tang, JKH, Moro, E, Mahant, N, Hutchison, WD, Lang, AE, Lozano, AM, et al. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. J Neurophysiol (2007) 98:720–9. doi:10.1152/jn.01107.2006
115. Alam, M, Sanghera, MK, Schwabe, K, Lütjens, G, Jin, X, Song, J, et al. Globus pallidus internus neuronal activity: A comparative study of linear and non-linear features in patients with dystonia or Parkinson’s disease. J Neural Transm (Vienna) (2016) 123:231–40. doi:10.1007/s00702-015-1484-3
116. Vitek, JL. Deep brain stimulation for Parkinson’s disease. A critical re-evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg (2002) 78:119–31. doi:10.1159/000068959
117. Vitek, JL, Chockkan, V, Zhang, JY, Kaneoke, Y, Evatt, M, DeLong, MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol (1999) 46:22–35. doi:10.1002/1531-8249(199907)46:1<22:aid-ana6>3.0.co;2-z
118. Obeso, JA, Rodríguez-Oroz, MC, Rodríguez, M, Lanciego, JL, Artieda, J, Gonzalo, N, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci (2000) 23:S8–19. doi:10.1016/s1471-1931(00)00028-8
119. Weinberger, M, Hutchison, WD, Alavi, M, Hodaie, M, Lozano, AM, Moro, E, et al. Oscillatory activity in the globus pallidus internus: Comparison between Parkinson’s disease and dystonia. Clin Neurophysiol (2012) 123:358–68. doi:10.1016/j.clinph.2011.07.029
120. Kühn, AA, Tsui, A, Aziz, T, Ray, N, Brücke, C, Kupsch, A, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol (2009) 215:380–7. doi:10.1016/j.expneurol.2008.11.008
121. Little, S, Pogosyan, A, Kuhn, AA, and Brown, P. β band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol (2012) 236:383–8. doi:10.1016/j.expneurol.2012.04.024
122. Neumann, W-J, Degen, K, Schneider, G-H, Brücke, C, Huebl, J, Brown, P, et al. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord (2016) 31:1748–51. doi:10.1002/mds.26759
123. Eisinger, RS, Cagle, JN, Opri, E, Alcantara, J, Cernera, S, Foote, KD, et al. Parkinsonian beta dynamics during rest and movement in the dorsal pallidum and subthalamic nucleus. J Neurosci (2020) 40:2859–67. doi:10.1523/JNEUROSCI.2113-19.2020
124. Quartarone, A, and Hallett, M. Emerging concepts in the physiological basis of dystonia. Mov Disord (2013) 28:958–67. doi:10.1002/mds.25532
125. Bologna, M, Guerra, A, Paparella, G, Giordo, L, Alunni Fegatelli, D, Vestri, AR, et al. Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain (2018) 141:2432–44. doi:10.1093/brain/awy155
126. Berardelli, A, Abbruzzese, G, Chen, R, Orth, M, Ridding, MC, Stinear, C, et al. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul (2008) 1:183–91. doi:10.1016/j.brs.2008.06.005
127. Blesa, J, Trigo-Damas, I, Dileone, M, Del Rey, NL-G, Hernandez, LF, and Obeso, JA. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp Neurol (2017) 298:148–61. doi:10.1016/j.expneurol.2017.10.002
128. Guerra, A, Colella, D, Giangrosso, M, Cannavacciuolo, A, Paparella, G, Fabbrini, G, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson’s disease. Brain (2021) 145:224–36. doi:10.1093/brain/awab257
129. Tinazzi, M, Priori, A, Bertolasi, L, Frasson, E, Mauguière, F, and Fiaschi, A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain (2000) 123(Pt 1):42–50. doi:10.1093/brain/123.1.42
130. Abbruzzese, G, and Berardelli, A. Sensorimotor integration in movement disorders. Mov Disord (2003) 18:231–40. doi:10.1002/mds.10327
131. Kaji, R, Urushihara, R, Murase, N, Shimazu, H, and Goto, S. Abnormal sensory gating in basal ganglia disorders. J Neurol (2005) 252(4):IV13–IV16. doi:10.1007/s00415-005-4004-9
132. Scontrini, A, Conte, A, Defazio, G, Fiorio, M, Fabbrini, G, Suppa, A, et al. Somatosensory temporal discrimination in patients with primary focal dystonia. J Neurol Neurosurg Psychiatry (2009) 80:1315–9. doi:10.1136/jnnp.2009.178236
133. Stamelou, M, Edwards, MJ, Hallett, M, and Bhatia, KP. The non-motor syndrome of primary dystonia: Clinical and pathophysiological implications. Brain (2012) 135:1668–81. doi:10.1093/brain/awr224
134. Patel, N, Jankovic, J, and Hallett, M. Sensory aspects of movement disorders. Lancet Neurol (2014) 13:100–12. doi:10.1016/S1474-4422(13)70213-8
135. Conte, A, Belvisi, D, De Bartolo, MI, Manzo, N, Cortese, FN, Tartaglia, M, et al. Abnormal sensory gating in patients with different types of focal dystonias: Sensory Gating in Focal Dystonias. Movement Disord (2018) 33:1910–7. doi:10.1002/mds.27530
136. Lee, MS, Lyoo, CH, Lee, MJ, Sim, J, Cho, H, and Choi, YH. Impaired finger dexterity in patients with Parkinson’s disease correlates with discriminative cutaneous sensory dysfunction. Mov Disord (2010) 25:2531–5. doi:10.1002/mds.23304
137. Conte, A, Belvisi, D, Tartaglia, M, Cortese, FN, Baione, V, Battista, E, et al. Abnormal temporal coupling of tactile perception and motor action in Parkinson’s disease. Front Neurol (2017) 8:249. doi:10.3389/fneur.2017.00249
138. Nishimura, K, Uehara, T, and Toyoda, K. Early-onset dystonia after supplementary motor area infarction. J Stroke Cerebrovasc Dis (2014) 23:1267–8. doi:10.1016/j.jstrokecerebrovasdis.2013.09.028
139. Dhakar, MB, Watson, C, and Rajamani, K. Acute onset dystonia after infarction of premotor and supplementary motor cortex. J Stroke Cerebrovasc Dis (2015) 24:2880–2. doi:10.1016/j.jstrokecerebrovasdis.2015.09.016
140. Wu, T, and Hallett, M. The cerebellum in Parkinson’s disease. Brain (2013) 136:696–709. doi:10.1093/brain/aws360
141. Bologna, M, and Berardelli, A. Cerebellum: An explanation for dystonia? Cerebellum Ataxias (2017) 4:6. doi:10.1186/s40673-017-0064-8
142. Bologna, M, and Berardelli, A. The cerebellum and dystonia. Handbook Clin Neurol (2018) 155:259–72. doi:10.1016/B978-0-444-64189-2.00017-2
143. Ebner, TJ. A role for the cerebellum in the control of limb movement velocity. Curr Opin Neurobiol (1998) 8:762–9. doi:10.1016/s0959-4388(98)80119-0
144. Ebner, TJ, Hewitt, AL, and Popa, LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum (2011) 10:683–93. doi:10.1007/s12311-010-0243-0
145. Wu, T, Zhang, J, Hallett, M, Feng, T, Hou, Y, and Chan, P. Neural correlates underlying micrographia in Parkinson’s disease. Brain (2016) 139:144–60. doi:10.1093/brain/awv319
146. Lee, E, Lee, JE, Yoo, K, Hong, JY, Oh, J, Sunwoo, MK, et al. Neural correlates of progressive reduction of bradykinesia in de novo Parkinson’s disease. Parkinsonism Relat Disord (2014) 20:1376–81. doi:10.1016/j.parkreldis.2014.09.027
147. Perlmutter, JS, Tempel, LW, Black, KJ, Parkinson, D, and Todd, RD. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology (1997) 49:1432–8. doi:10.1212/wnl.49.5.1432
148. Naumann, M, Pirker, W, Reiners, K, Lange, KW, Becker, G, and Brücke, T. Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: A SPECT study using [123I] epidepride and [123I] beta-CIT. Mov Disord (1998) 13:319–23. doi:10.1002/mds.870130219
149. Balcioglu, A, Kim, M-O, Sharma, N, Cha, J-H, Breakefield, XO, and Standaert, DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem (2007) 102:783–8. doi:10.1111/j.1471-4159.2007.04590.x
150. Schirinzi, T, Sciamanna, G, Mercuri, NB, and Pisani, A. Dystonia as a network disorder: A concept in evolution. Curr Opin Neurol (2018) 31:498–503. doi:10.1097/WCO.0000000000000580
151. Bologna, M, Leodori, G, Stirpe, P, Paparella, G, Colella, D, Belvisi, D, et al. Bradykinesia in early and advanced Parkinson’s disease. J Neurol Sci (2016) 369:286–91. doi:10.1016/j.jns.2016.08.028
Keywords: bradykinesia, dystonia, neurophysiology, motor control, basal ganglia
Citation: Paparella G, Guerra A, Galosi S, Cannavacciuolo A, Angelini L, Popa T, Berardelli A and Bologna M (2023) Bradykinesia and dystonia. Dystonia 2:11448. doi: 10.3389/dyst.2023.11448
Received: 06 April 2023; Accepted: 06 July 2023;
Published: 08 August 2023.
Edited by:
Aasef Shaikh, Case Western Reserve University, United StatesCopyright © 2023 Paparella, Guerra, Galosi, Cannavacciuolo, Angelini, Popa, Berardelli and Bologna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Bologna, bWF0dGVvLmJvbG9nbmFAdW5pcm9tYTEuaXQ=
 Giulia Paparella1,2
Giulia Paparella1,2 Andrea Guerra
Andrea Guerra Matteo Bologna
Matteo Bologna