Abstract
Dystonia is an uncommon complication of relapsing-remitting multiple sclerosis (MS) and related disorders. The types of dystonia described associated with MS are heterogenous, and the relationship between dystonia and MS remains unclear. Moreover, the anatomical correspondence between MS lesions and the site of dystonia has not been determined. Onset, treatment response, and outcomes of dystonia associated with MS still remain largely uncharacterized. Here, we report a case series of 14 patients with dystonia affecting different body parts in people with MS and neuromyelitis optica (NMO). We characterize the brain regions associated with this form of secondary dystonia and the response to botulinum toxin injections.
Introduction
Dystonia comprises a heterogenous group of movement abnormalities characterized by sustained or intermittent muscle contractions causing abnormal postures, repetitive movements and postures, or both [1]. MS and NMO are inflammatory conditions of the central nervous system caused by immune dysregulation. The clinical diagnosis of MS requires the dissemination of lesions in space and time [2]. Diagnostic criteria for NMO have been established for individuals with and without NMO-IgG antibody seropositivity [3]. Although dystonia has been reported in people with MS, the clinical-anatomic relationship between the body part affected by dystonia and the location of demyelinating plaques in people with either MS or NMO has not been well-described [4].
The primary goal of this case series is to determine the relationship between the location of demyelinating plaques in the brain or spinal cord and body part affected by dystonia. Our secondary goal is to report the response to botulinum toxin. We include patients with dystonia and either MS or NMO that were seen at the Washington University Movement clinic between 1 January 1996, and 1 August 2022.
Methods
A retrospective chart review was performed. All clinical data from the Washington University Movement Disorders Center had been stored in an electronic medical record system implemented in 1995. We searched all outpatient records collected between 1 January 1996 and 1 April 2022 for the diagnosis of dystonia and MS using the McDonald Diagnostic Criteria [2, 5] and the International Consensus for NMO Spectrum Disorders [6]. When available, we recorded age at diagnosis of MS or NMO, age at onset of dystonia, relevant family history, past neuroleptic exposure, neurological examination, botulinum toxin used for treatment and response to injection. In addition, we reviewed available brain and spine MRIs, and neuroradiology reports where original imaging was unavailable. All patients with the diagnosis of MS had evidence of periventricular, juxtacortical, or infratentorial lesions. Approval for this study was obtained from Washington University Institutional Review Board (IRB ID #:202201182).
Cases
We found 14 patients with dystonia and MS or NMO between ages 13 to 49 (average age 32, Table 1). In two patients (3 and 7), dystonia began at the onset of MS, and in patient 10, bilateral arm dystonia occurred 1 year prior to other MS manifestations. Twelve patients had MS and two had NMO (5 and 6), as diagnosed by their primary neurologist (1, 3, and 9) or by a neuroimmunologist/MS specialist. Dystonia was diagnosed by a movement disorders specialist. Types of dystonia included focal (n = 8), multifocal (n = 5), and segmental (n = 1). No individual had a family history of dystonia. Of note, patient 4 had a 1 year exposure to an anti-dopaminergic agent prior to the onset of dystonia but had MS for 18 years prior to dystonia onset. This was discontinued at the time of diagnosis of dystonia but the dystonia did not improve over the next 15 years. For all participants, we summarized the regions of demyelination identified on MRI (Figure 1). Table 2 shows the Tsui torticollis rating score for those patients with cervical dystonia (before and after Botulinum toxin treatment). 2 of 7 cervical dystonia patients did not have Tsui torticollis rating score performed. In addition, it shows the treatment including units of botulinum toxin type A, the number of chemodenervation sessions, and the response to treatment.
TABLE 1
| Patient | Age of DD | Demyelinating diagnosis (DD) | Years to onset of dystonia symptom | Movement diagnosis; dystonia (focal, segmental, multifocal, generalized) | Exposure to anti-DA or toxins prior to onset of dystonia | Available MRI (Y/N) (Figure 1) |
|---|---|---|---|---|---|---|
| 1 | 39 | MS | 6 | Meige Syndrome (blepharospasm, cervical dystonia) — segmental | none | N |
| 2 | 43 | MS | 17 | Cervical dystonia — focal | none | Y |
| 3 | 27 | MS | 0 | Cervical dystonia — focal | none; h/o of encephalitis | Y (Read only) |
| 4 | 27 | MS | 18 | Cervical dystonia with tremor — focal | Prochlorperazine for 1 year | Y |
| 5 | 40 | NMO | 2 | Cervical dystonia — focal | none | Y (Read only) |
| 6 | 37 | NMO | 8 | Cervical dystonia — focal | none | Y |
| 7 | 48 | MS | 0 | L foot dystonia, cervical dystonia with tremor — multifocal | none | Y |
| 8 | 14 | MS | 18 | R arm and leg dystonia, bilateral upper and lower bilateral facial dystonia — multifocal | none | Y (Read only) |
| 9 | 34 | MS | 4 | L arm dystonia — focal | none | N |
| 10 | 27 | MS | −1 | Bilateral arm dystonia — multifocal | none | Y |
| 11 | 13 | MS | 26 | L>R foot dystonia (toe curling) — multifocal | none | Y |
| 12 | 24 | MS | 20 | Bilateral foot dystonia — multifocal | none | N |
| 13 | 49 | MS | 2 | R LE dystonia (toe curling) — focal | none | Y |
| 14 | 38 | MS | 15 | Adductor laryngeal dystonia — focal | none | N |
Demographic and clinical characteristics of patients.
FIGURE 1
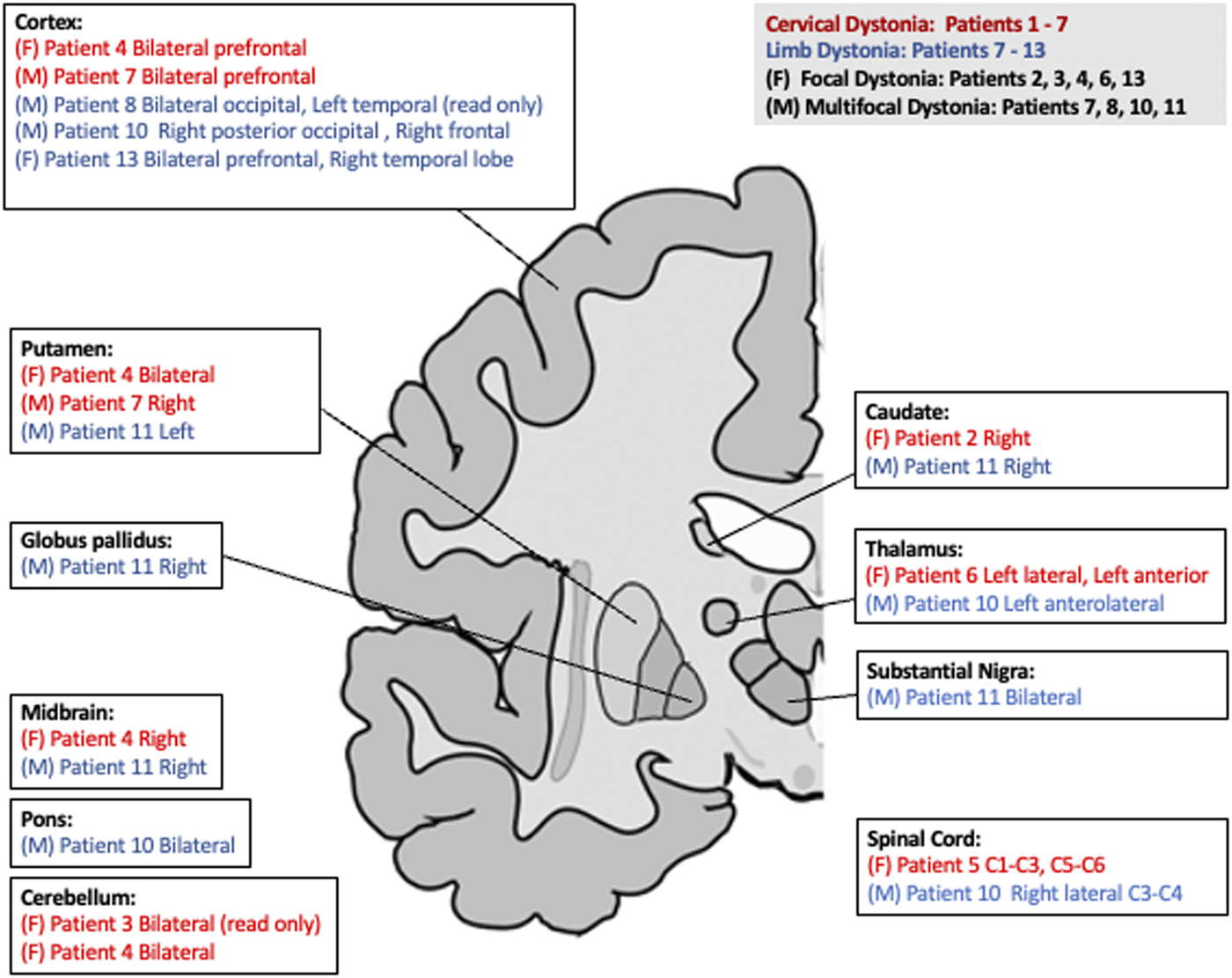
Simplified representation highlighting basal ganglia and critical brain regions of the corticostriato- thalamo circuit. Red color represents cervical dystonia, while blue represents limb dystonia. (F) is for focal dystonia. (M) is for multifocal. (L) Represents left and (R) represents right.
TABLE 2
| Patient | Movement diagnosis | Tsui torticollis rating scale before and after Btx (if applicable) | MS/NMO immunotherapy | Systemic anti-spasmodics | Botulinum injections (units) | Botulinum sessions (n) | Response to Btx (good, partial, poor) |
|---|---|---|---|---|---|---|---|
| 1 | Cervical dystonia, blepharospasm | not rated | None | 40–60 (face) | 6 | Good | |
| 2 | Cervical dystonia | 15, 15 | Dimethyl fumarate | 120–270 (neck) | 5; ongoing | Partial | |
| 3 | Cervical dystonia | 18, 12 | None | 280 (neck) | 2 | Good | |
| 4 | Cervical dystonia with tremor | 12, 9 | Dimethyl Fumarate | Intrathecal baclofen | 60–200 (neck) | 2 | Poor |
| 5 | Cervical dystonia | 7, 4 | None | 200 (neck) | 2 | Good | |
| 6 | Cervical dystonia, bilateral upper extremity spasticity | 8, 8 | Interferon-beta 1b | 500 (neck 200, limbs 300) | 8 | Good | |
| 7 | cervical dystonia with tremor, L foot dystonia, | not rated | None | 0 | |||
| 8 | R arm and R leg dystonia, facial dystonia with excessive grimacing | n/a | Glatiramer acetate | 300 (R forearm 50, R leg 250) | 3 | Partial | |
| 9 | L arm dystonia | n/a | Teriflunomide | 320 (L shoulder, arm, forearm) | 7 | Poor | |
| 10 | Bilateral arm dystonia | n/a | Fingolimod | 0 | |||
| 11 | L>R foot dystonia (toe curling), spasticity | n/a | Siponimod | Baclofen, tizanidine | 600 (L leg 150, R leg 150, L thigh 140, R thigh 140, L forearm 20) | 30 + | Good |
| 12 | Bilateral foot dystonia | n/a | None | 275 (R leg) | 1 | Poor | |
| 13 | R LE dystonia (toe curling) | n/a | Glatiramer acetate | 400 (R thigh 100, R leg 300) | 17 | Good | |
| 14 | Adductor laryngeal dystonia with pseudobulbar component | n/a | Interferon-beta-1b | 7.5 (bilateral vocal cords) | 2 | Poor |
History of MS/NMO, dystonia treatment, Tsui torticollis rating scale, and response to Botulinum treatment.
Discussion
We present a case series of 14 patients who had dystonia secondary to multiple sclerosis and NMO. This is the most extensive case series to date with clinical presentation and MRI imaging. The locations of the demyelinating lesions were variable and included the caudate and putamen as well as thalamus, cortex, brainstem, and cerebellum, which supports the idea that disruption in either the cortico-ponto-cerebello-thalamo-cortical or the cortico-basal ganglia-thalamo-cortical could lead to dystonia. We also reported treatment responses from botulinum injections.
The extant relevant literature includes few cases but does provide some clinical-anatomic correlations of secondary dystonias associated with demyelinating disease. One patient with hemidystonia involving the left upper and lower extremities was attributed to demyelinating plaques in the posterolateral spine at C2 and C3 [7]. Another report noted blepharospasm in two patients with cortical parietal lobe lesions that improved after IV methylprednisolone [8]. To date, the largest case series published describes nine patients with dystonia and MS, of which three had lesions in the basal ganglia [9]. A cohort of seven people with dystonia and MS had a variety of demyelinating plaques found most frequently in the thalamus [10].
Both generalized dystonia from genetic causes and isolated, idiopathic cervical dystonia could be due to disruption in the cortico-ponto-cerebello-thalamo-cortical or the cortico-basal ganglia-thalamo-cortical pathways [11, 12]. Interestingly, our patients with dystonia and either MS or NMO also had demyelinating disease involving the basal ganglia, thalamus, midbrain, cerebellum, or the spinal cord supporting the notion that this form of secondary dystonia may share dysfunction of similar brain networks as idiopathic or genetic dystonia. Specifically, we found two patients with cerebellar lesions, four with lesions in basal ganglia, and two with thalamic lesions. Two cases with cervical dystonia had lesions in the cerebellum. In addition, all 4 patients with dystonia in either upper or lower extremities on both sides, had plaques located in more than one area. Of note, one person (4) with cervical dystonia had lesions in multiple brain areas including bilateral putamen, bilateral prefrontal lesions, right posterior midbrain, and right cerebellum, but we could not identify which of these lesions specifically contributed to dystonia. We did not know the temporal relationship between the onset of these plaques and the onset of dystonia which could have provided stronger evidence of the clinical pathologic relationships in these cases. Anatomical or functional lesions may fall below the sensitivity of MRI detection so visualization on MR imaging likely does not reveal all relevant pathology.
Dystonia, in general, has traditionally been regarded as dysfunction of the basal ganglia, implicating the cortico-basal ganglia-thalmo-cortical circuity. Clinical anatomic studies initially focused on secondary dystonias found defects of basal ganglia nuclei. For example, discrete focal lesions often involving the putamen or striatum may accompany dystonia [13–15]. Resting state functional connectivity also identifies dysfunction of specific basal ganglia networks [16]. When compared to healthy volunteers, FDG-PET studies have shown abnormal activity of posterior putamen, globus pallidus, cerebellum, and supplementary motor areas in patients with dystonia [17].
The cerebellum and related networks may also play a role in the pathophysiology of dystonia suggesting dysfunction of the cortico-ponto-cerebello-thalmo-cortical loop [18, 19]. Patients with secondary focal neck dystonia often have lesions in the cerebellum [20–22]. The cerebellum plays a role in networks involving the striatum so these are not functionally independent structures; regions of cerebellum project to the cortex via ventrolateral thalamus and connect to the striatum via intralaminar thalamic nuclei [23]. Disruption of cerebellum circuits have been associated with motor manifestation of dystonia [24]. Functional magnetic resonance imaging studies demonstrate abnormal activation in the cerebellum during tasks of blepharospasm and writer’s cramps [25–27]. Moreover, histopathological studies from autopsy of cervical dystonia patients and blepharospasm patients found loss of Purkinje neurons in the cerebellum [28–30].
Idiopathic dystonia is often treated with botulinum toxin with good response [31–34]. However, few studies have reported the efficacy of treating dystonia secondary to demyelinating diseases. Moreover, there are no randomized clinical trials of any treatments for dystonia in MS. In 8 of 12 of our patients with plaques involving the cortico-striato-thalamo tract, clinical observations suggest that botulinum toxin provided partial to good clinical benefit. Thus dystonia related to MS or NMO may respond to botulinum toxin, similar to isolated, idiopathic dystonia. We recognize that this is not a treatment study with our findings being limited to clinical observations and self-reports. However, this is similar to the previously reported 50%–90% of patients with substantial improvement of dystonic symptoms and dystonia-related pain [35–38]. Failure of response to botulinum toxin may also be due to failure to find an optimal dose without side effects (i.e., head drop in patient 9) which may reflect exacerbation of antecedent weakness related to MS. These limited observations would support at least a trial of treatment with botulinum toxin in secondary cases of dystonia attributed to MS or NMO lesion if no contraindications exist.
Improved dystonia symptoms after corticosteroid treatment could suggest that the inflammatory process in MS or NMO contributed to dystonia [39]. In our case series, we do not have sufficient evidence to address this. Although some patients received corticosteroids, we do not have imaging at the time of symptom onset that demonstrates contrast enhancement and active demyelination in regions that may lead to dystonia if damaged. We also do not have evidence that cervical dystonia improves after MS treatment, as all these patients were sent to be evaluated in the movement disorder clinic due to dystonia that was not alleviated by MS treatment, reflecting the sampling biases of this report.
Conclusion
Demyelinating lesions associated with MS or NMO involving either the cortico-striato-thalamo or the cerebello-cortical-loop network circuit can contribute to dystonia. The involvement of different brain and spinal regions matches that found with other forms of secondary dystonia (i.e., stroke), and relates to dysfunction of brain networks found in idiopathic or genetic forms of dystonia. These cases reveal dysfunction or anatomic lesions of motor networks involving the cortex, basal ganglia, midbrain, thalamus, and cerebellum. Treatment of this form of secondary dystonia in the setting of MS and NMO seems to respond to botulinum toxin injections similar to idiopathic, isolated focal and segmental dystonia.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Washington University Institutional Review Board (IRB ID #:202201182). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HH devised the research project from conception, organization, and execution. HH, VL, and JP wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
JP is supported by grants from the National Institutes of Health (NINDS, NIA) (NS075321, U54 NS116025), the American Parkinson Disease Association (APDA), the Greater St. Louis Chapter of the APDA, the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and the Parkinson Disease Research Fund), the Paula and Rodger Riney Fund, and the Sam & Barbara Murphy Fund. HH is supported by NIH T32 EB021955 TOP TIER (Training Opportunities in Translational Imaging Education and Research) and American Academy of Neurology (AAN) Clinical Research Training Scholarship in Parkinson’s disease. VL is supported by Sylvia Lawry Physician Fellowship (FP-2006-36668) from National Multiple Sclerosis Society.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Albanese A Bhatia K Bressman SB Delong MR Fahn S Fung VSC et al Phenomenology and classification of dystonia: a consensus update. Mov Disord (2013) 28:863–73. 10.1002/mds.25475
2.
Thompson AJ Banwell BL Barkhof F Carroll WM Coetzee T Comi G et al Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2018) 17:162–73. 10.1016/S1474-4422(17)30470-2
3.
Wingerchuk DM . Diagnosis and treatment of neuromyelitis optica. Neurologist (2007) 13:2–11. 10.1097/01.nrl.0000250927.21903.f8
4.
Mehanna R Jankovic J . Movement disorders in multiple sclerosis and other demyelinating diseases. J Neurol Sci (2013) 328:1–8. 10.1016/j.jns.2013.02.007
5.
McDonald WI Compston A Edan G Goodkin D Hartung HP Lublin FD et al Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol (2001) 50:121–7. 10.1002/ana.1032
6.
Wingerchuk DM Banwell B Bennett JL Cabre P Carroll W Chitnis T et al International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology (2015) 85:177–89. 10.1212/WNL.0000000000001729
7.
Yucesan C Tuncel D Akbostanci MC Yücemen N Mutluer N . Hemidystonia secondary to cervical demyelinating lesions. Eur J Neurol (2000) 7:563–6. 10.1046/j.1468-1331.2000.t01-1-00120.x
8.
Minagar A Sheremata WA Weiner WJ . Transient movement disorders and multiple sclerosis. Park Relat Disord (2002) 9:111–3. 10.1016/s1353-8020(02)00009-3
9.
Tranchant C Bhatia KP Marsden CD . Movement disorders in multiple sclerosis. Mov Disord (1995) 10:418–23. 10.1002/mds.870100403
10.
Potulska-Chromik A Rudzinska M Nojszewska M Podlecka-Piętowska A Szczudlik A Zakrzewska-Pniewska B et al Clinical and neuroimaging correlation of movement disorders in multiple sclerosis: case series and review of the literature. Folia Neuropathol (2014) 52:92–100. 10.5114/fn.2014.41747
11.
Argyelan M Carbon M Niethammer M Ulug AM Voss HU Bressman SB et al Cerebellothalamocortical connectivity regulates penetrance in dystonia. Soc Neurosci (2009) 29:9740–7. 10.1523/JNEUROSCI.2300-09.2009
12.
Kaňovsk´y PK Bares M Streitová H Klajblová H Daniel P Rektor I . Abnormalities of cortical excitability and cortical inhibition in cervical dystonia Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol (2003) 250:42–50. 10.1007/s00415-003-0942-2
13.
Burton K Farrell K Li D Calne DB . Lesions of the putamen and dystonia: CT and magnetic resonance imaging. Neurology (1984) 34:962–5. 10.1212/wnl.34.7.962
14.
Bhatia KP Marsden CD . The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain (1994) 117:859–76. 10.1093/brain/117.4.859
15.
Perlmutter JS Raichle ME . Pure hemidystonia with basal ganglion abnormalities on positron emission tomography. Ann Neurol (1984) 15:228–33. 10.1002/ana.410150303
16.
Norris SA Morris AE Campbell MC Karimi M Adeyemo B Paniello RC et al Regional, not global, functional connectivity contributes to isolated focal dystonia. Neurology (2020) 95:e2246–58. 10.1212/WNL.0000000000010791
17.
Poston KL Eidelberg D . Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage (2012) 62:2261–70. 10.1016/j.neuroimage.2011.12.021
18.
Jinnah HA Neychev V Hess EJ . The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinet Mov (N Y) (2017) 7:506. 10.7916/D8V69X3S
19.
Balint B Mencacci N Valente E Antonio P-N Rothwell J Jankovic J et al Dystonia. Nat Rev Dis Prim (2018) 4:25–3. 10.1038/s41572-018-0023-6
20.
LeDoux MS Brady KA . Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord (2003) 18:60–9. 10.1002/mds.10301
21.
Boisen E . Torticollis caused by an infratentorial tumour: three cases. Br J Psychiatry (1979) 134:306–7. 10.1192/bjp.134.3.306
22.
Plant GT Kermode AG Du Boulay E McDonald WI . Spasmodic torticollis due to a midbrain lesion in a case of multiple sclerosis. Mov Disord (1989) 4:359–62. 10.1002/mds.870040413
23.
Hoshi E Tremblay L Féger J Carras PL Strick PL . The cerebellum communicates with the basal ganglia. Nat Neurosci (2005) 8:1491–3. 10.1038/nn1544
24.
Chen CH Fremont R Arteaga-Bracho EE Khodakhah K . Short latency cerebellar modulation of the basal ganglia. Nat Neurosci (2014) 17:1767–75. 10.1038/nn.3868
25.
Zhou B Wang J Huang Y Yang Y Gong Q Zhou D . A resting state functional magnetic resonance imaging study of patients with benign essential blepharospasm. J Neuroophthalmol (2013) 33:235–40. 10.1097/WNO.0b013e31828f69e5
26.
Hu X Wang L Liu H Zhang S . Functional magnetic resonance imaging study of writer’s cramp. Chin Med J (Engl) (2006) 119:1263–71. 10.1097/00029330-200608010-00006
27.
Gallea C Herath P Voon V Lerner A Ostuni J Saad Z et al Loss of inhibition in sensorimotor networks in focal hand dystonia. Neuroimage Clin (2018) 17:90–7. 10.1016/j.nicl.2017.10.011
28.
Prudente CN Pardo CA Xiao J Hanfelt J Hess EJ Ledoux MS et al Neuropathology of cervical dystonia. Exp Neurol (2013) 241:95–104. 10.1016/j.expneurol.2012.11.019
29.
Ma K Babij R Cortés E Vonsattel JG Louis ED . Cerebellar pathology of a dual clinical diagnosis: patients with essential tremor and dystonia. Tremor and Other Hyperkinetic Movements (2012) 2:12. 10.5334/tohm.94
30.
Fagan M Scorr L Bernhardt D Hess EJ Perlmutter JS Pardo CA et al Neuropathology of blepharospasm. Exp Neurol (2021) 346:113855. 10.1016/j.expneurol.2021.113855
31.
Hefter H Schomaecker I Schomaecker M Samadzadeh S . Disease progression of idiopathic cervical dystonia in spite of improvement after botulinum toxin therapy. Front Neurol (2020) 11:588395. 10.3389/fneur.2020.588395
32.
Jinnah H Comella C Perlmutter J Lungu C Hallett M, Dystonia Coalition Investigators. Longitudinal studies of botulinum toxin in cervical dystonia: why do patients discontinue therapy?Toxicon (2018) 147:89–95. 10.1016/j.toxicon.2017.09.004
33.
Jankovic J . Treatment of cervical dystonia with botulinum toxin. Mov Disord (2004) 19:S109–S115. 10.1002/mds.20024
34.
Tassorelli C Mancini F Balloni L Pacchetti C Sandrini G Nappi G et al Botulinum toxin and neuromotor rehabilitation: an integrated approach to idiopathic cervical dystonia. Mov Disord (2006) 21:2240–3. 10.1002/mds.21145
35.
Brans JWM Lindeboom R Snoek JW Zwarts MJ van Weerden TW Brunt ER et al Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double-blind controlled trial. Neurology (1996) 46:1066–72. 10.1212/wnl.46.4.1066
36.
Berardelli A Abbruzzese G Bertolasi L Cantarella G Carella F Currà A et al Guidelines for the therapeutic use of botulinum toxin in movement disorders. Italian study group for movement disorders, Italian society of Neurology. Neurol Sci (1997) 18:261–9. 10.1007/BF02083302
37.
Greene P Kang U Fahn S Brin M Moskowitz C Flaster E . Double-blind, placebo-controlled trial of botulinum toxin injections for the treatment of spasmodic torticollis. Neurology (1990) 40:1213–8. 10.1212/wnl.40.8.1213
38.
Comella CL Jankovic J Brin MF . Use of botulinum toxin type A in the treatment of cervical dystonia. Neurology (2000) 55:S15–21.
39.
Rüegg SJ Bühlmann M Renaud S Steck AJ Kappos L Fuhr P . Cervical dystonia as first manifestation of multiple sclerosis. J Neurol (2004) 251:1408–10. 10.1007/s00415-004-0544-7
Summary
Keywords
dystonia, multiple sclerosis, secondary dystonia, neuromyelitis optica, botulinum toxin
Citation
Hwang H, Levasseur VA and Perlmutter JS (2023) Case Series: Dystonia with multiple sclerosis and neuromyelitis optica. Dystonia 2:11678. doi: 10.3389/dyst.2023.11678
Received
12 June 2023
Accepted
31 August 2023
Published
01 December 2023
Volume
2 - 2023
Edited by
Sanjay Pandey, Amrita Hospitals Faridabad, India
Updates
Copyright
© 2023 Hwang, Levasseur and Perlmutter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helen Hwang, helenhwang@wustl.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.