Abstract
Globus pallidus internus (GPi) deep brain stimulation (DBS) was used to treat a stiff-person syndrome (SPS) patient. Prior to implantation, microelectrode recordings measured firing frequencies and patterns (burst index). Contralateral and ipsilateral hemispheres relative to myoclonic jerks on the left side of the body were compared. Analysis revealed significantly lower firing frequencies and significantly higher and more variable burst index in the right contralateral GPi compared to the left ipsilateral GPi.
Introduction
Deep Brain Stimulation (DBS) is chronic indwelling high frequency electrical stimulation of subcortical nuclei for movement disorders and in some instances psychiatric disorders. This therapy has been widely applied to Parkinson’s disease, dystonia and tremor with remarkable success. One of the main targets employed for hyperkinetic movement disorders is the internal segment of the globus pallidus (GPi). Overall, lower firing rates of GPi neurons have been suggested to be associated with dystonia but sedative drugs and anesthetics may be a confound [1]. However, less is known about rare diseases such as Stiff-Person Syndrome (SPS). SPS is a rare neurological immune disorder characterized by progressive rigidity and stiffness of the lower back and lower limbs. With 1 to 2 cases per million and no available treatment, it becomes challenging to obtain and analyze patient data to better understand disease etiology. In many cases, SPS can progressively worsen affecting many areas of the body, and impairing routine tasks. Impairments can present as difficulty in walking, hunched posture, uncontrolled tremors, and greater sensitivity to sensory input. Moreover, like other autoimmune disorders, SPS affects twice as many females as males, and can sometimes presents with other forms of autoimmune disease (e.g., type 1 diabetes, thyroiditis, and anemia) [2]. While the cause of the disease remains unknown, certain markers associated with the condition can facilitate its diagnosis. For example, previous literature found that SPS patients tend to have abnormally high levels of anti-glutamic acid decarboxylase (GAD) antibodies in the blood and cerebrospinal fluid [3]. Anti-GAD antibodies, produced by B lymphocytes, inhibit the conversion of glutamate to GABA by blocking the actions of the GAD enzyme [4]. Consequently, patients suffer from low GABA levels, leading to motor and cognitive problems [5, 6]. Other studies have highlighted the association of SPS with other forms of antibodies such as amphiphysin. However, out of 11 SPS patients that presented with amphiphysin, Murinson and Guarnaccia found that amphiphysin -associated SPS showed significantly different stiffness patterns compared to GAD-associated SPS, with a higher risk for cervical region stiffness [7]. Additionally, all 11 patients were females and 10 presented with breast cancer, indicating a high association between amphiphysin -associated SPS, female sex, and breast cancer. That said, in a recent case study, GPi DBS demonstrated positive effects on a SPS patient’s stiffness, spasms and ability to communicate [8]. To our knowledge, no other case report has reported the neurophysiological features of GPi prior to DBS in the treatment of SPS patients. Our group identified a case of SPS and applied GPi stimulation, with the reasoning that motor symptoms of rigidity would be ameliorated.
Case
This is a case report of a 58-year-old female SPS patient who reported 7 years of progressive compromise and stiffness in the left hand. The case was defined by uncontrolled myoclonic jerks and abnormal positioning of the thumb (Figure 1A). Relative to the left hand, the right hand and face did not suffer any form of stiffness or uncontrolled movements. Moreover, no form of rigidity was reported in the lower extremities. The patient underwent 120 h of electroencephalogram (EEG) without any seizures and revealed normal EEG data. After undergoing diagnostic workup, the patient’s data revealed no tumors in the body. Yet, the patient tested positive for amphiphysin antibodies. At 51 years of age, the patient also experienced progressive paresthesia associated with progressive insomnia for 4 months. After that, she saw a doctor that diagnosed her with Parkinson’s disease and took levodopa for 18 months without improvement. Further details on timeline of main events can be found in Table 1. Data measurements were conducted before and after posteroventral GPi DBS and quantification of results was based on input from the patient. Informed and free consent was obtained from the patient for recording of brain data and use of photos and videos for publication.
FIGURE 1
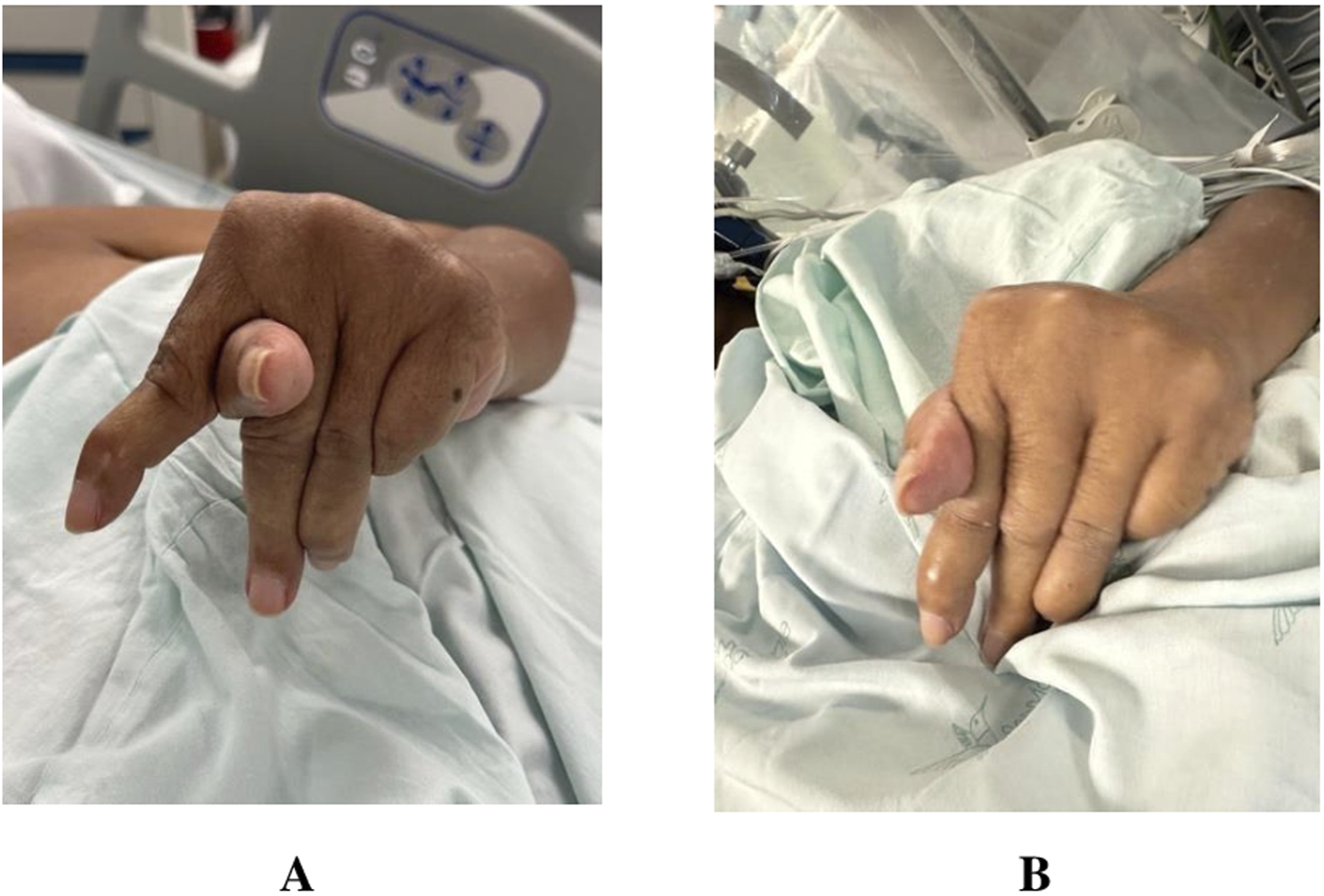
Images captured of patient left hand before (A) and after (B) Deep Brain Stimulation (DBS) procedure.
TABLE 1
| Event | Timeline |
|---|---|
| Paresthesia and tingling sensation in the fingers of the left hand | 51 years of age |
| Evolution of paresthesia progressing proximally till reaching the palm. Associated with progressive insomnia (over 4 months) and an inversion of cycle | 52 years of age |
| Physician diagnosis of Parkinson’s Disease and ordering levodopa for 18 months without any improvement | 52 years of age |
| Progressive tremors and difficulty in using left hand, which worsens with emotional stress and cold | 57 years of age |
| Deformities in hand posturing and abnormal thumb placement leading to difficulty grasping objects | 57 years of age |
Main events timeline before Deep brain stimulation (DBS) surgery on patient.
Different diagnostic tests were conducted on the patient, including whole-body and brain PET-CT tests. The latter demonstrated moderate to severe hypometabolism in the right parietal lobe and caudate nucleus. These results suggested corticobasal syndrome, yet the patient lacked any cortical deficits or parkinsonism and other clinical criteria for that disorder.
The patient received three injections of botulinum toxin in the extremity, which all failed. After amphiphysin positivity, a high dose of methylprednisolone (1gr/day × 5 days), rituximab, and IV immune globin were administered to the patient with no clear improvement. Clonazepam was the only medication that demonstrated some benefit to the patient, decreasing myoclonic jerks.
Diagnostic assessment
The implants were made using stereotactic targeting using a 3D coordinate Leksell frame applied under local anesthetic to the cranium. A tentative target was identified on T1 and T2 MRI scans using a 3-Tesla magnet, with 1-mm non-overlapping slices in all three planes: axial, sagittal, and coronal. Direct targeting using the optic tract was used as well as indirect targeting from an atlas sagittal map 20 mm lateral to the midline. The GPi indirect target was 20 mm lateral, 3 mm posterior to MCP and 5 mm below ACPC line. A microelectrode guide tube was inserted 10 mm above the intended target and recordings were taken with fine microelectrodes along the 10 mm trajectory to the target. After mapping was completed, a Medtronic Sensight DBS directional lead was implanted. The 1.36 mm directional lead diameter, and unique electrode configuration allows steering of DBS stimulation current to better avoid side effects. Further description of the methods is included in previous work from the lab [9].
Forty segments were recorded in the right hemisphere, of which 8 were selected for data analysis based on quality of single unit isolation and sufficient length of time. Thirty-six were included in the left hemisphere, of which 10 were further analyzed. Data was sampled at a frequency of 32 kHz, bandpass filtered 300–3,000 Hz, and an average segment length was 9.2 and 6.7 s in the right and left hemispheres, respectively. Mean firing frequencies and burst indices were calculated. Both variables were compared using a t-test. The patient was experiencing left sided myoclonic jerks. The right hemisphere was consequently determined as the contralateral side to the affected left rigid limb. Mean firing frequencies were significantly lowered in the right hemisphere of the patient compared to the left hemisphere (49.38 ± 10.11 vs. 63.65 ± 13.40; p = 0.017) (Figure 2). Burst index was calculated as the quotient of the mean interspike interval (ISI)/modal ISI. Similar to the mean frequencies, burst index also revealed a significantly high value in the left hemisphere compared to the right. Values of 0.76 ± 0.35 and 1.39 ± 0.41 were revealed for the right and left hemispheres, respectively. The burst index was calculated as mean burst divided by the mean frequency for both hemispheres and mean values of all included segments were analyzed and compared. Significantly elevated mean burst index was determined in the left hemispheres compared to the right hemisphere (3.52 ± 0.61 vs. 4.66 ± 1.37; p = 0.04). Altogether, the left hemispheres reported significantly elevated values in all calculated variables. Lastly, no correlation was found between firing rates and depth in the right (R2 = 0.014) or left (R2 = 0.110) hemispheres, ruling out any effect of anesthetics on firing rates. Thus, we did not find evidence that firing rates were recovering as time progressed (and anesthetic effect wear off) indicated by the absence of difference in values in superior and inferior positions of the track. The abnormal thumb placement between the index and middle finger was improved post-surgery and DBS treatment (Figure 1B). The left hand posture was fixed and lacked typical dystonic phenomenology. While myoclonus persisted in the patient’s arm and hand, patient reported a reduction in pain scores from 7 to 2, on a scale of 1–10 (Table 2). This indicates an improvement in the dystonic hand posturing without an amelioration in the myoclonic muscle jerks in the hand, suggesting a different locus for both purposes. Lastly, the patient reported no pain or stiffness in the right arm. Over time, the patient’s myoclonus has shown minimal improvement, while posture has remained unresponsive. Low-frequency stimulation attempts were conducted initially, exacerbating the jerks, and necessitating the adjustment to higher frequencies. Based on a subjective report, the patient reported a 90% improvement immediately after surgery. However, 1 year after surgery, the patient reported the appearance of symptoms again.
FIGURE 2
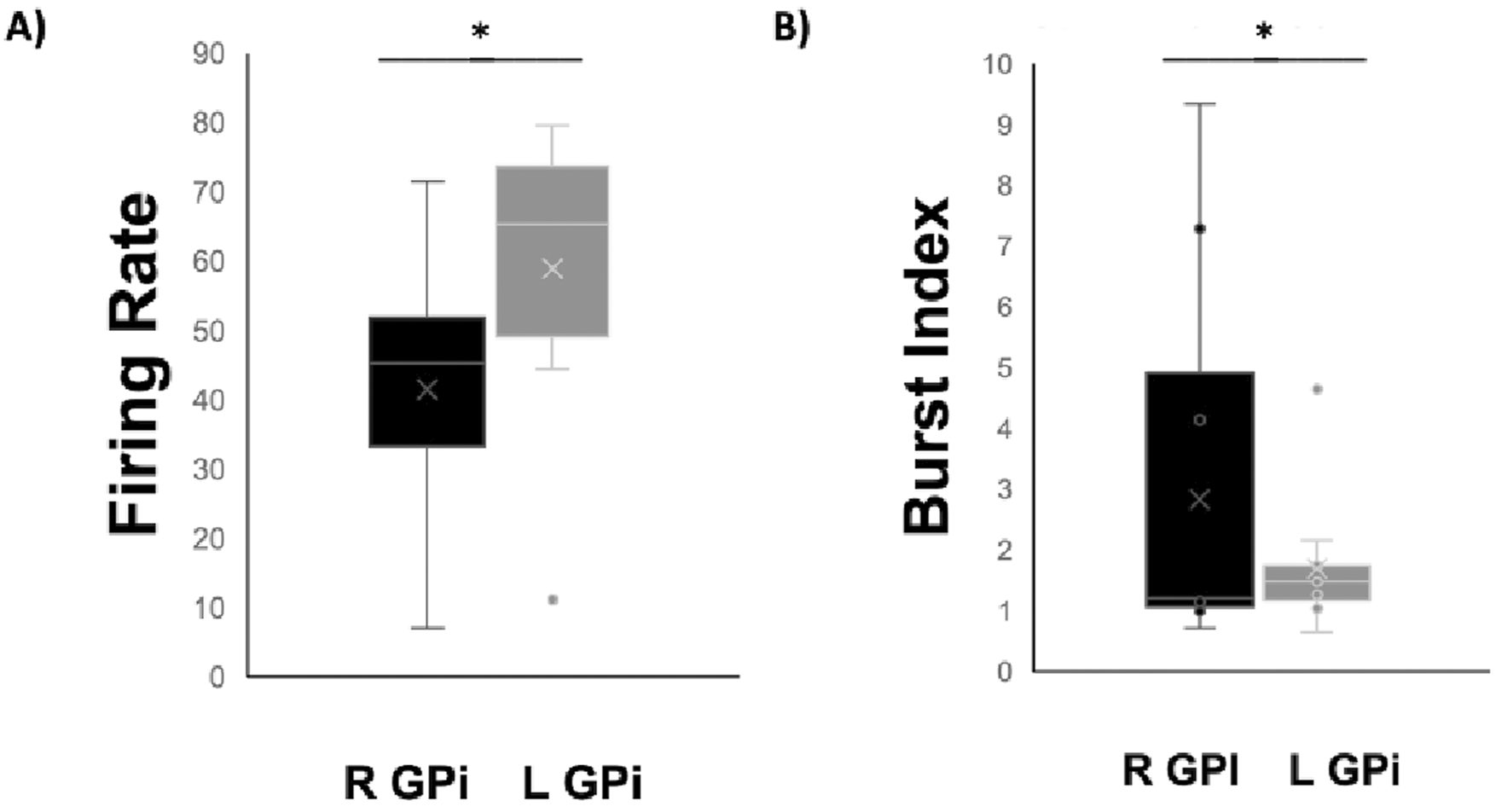
Boxplots comparing mean firing rates (A) and mean burst index (B) of left (L) and right (R) GPi cells in a Stiff-person syndrome (SPS) patient (n = 1). Error bars represent standard deviation values. * = p < 0.05.
TABLE 2
| Pre-DBS | Post-DBS | |
|---|---|---|
| Pain Severity | 7 | 2–5 |
| Myoclonic Movement | Uncontrollable muscle jerks present | Uncontrollable muscle jerks still present |
| Hand Posture | Awkward thumb positioning between index and middle finger | Thumb position back to normal and hand posturing improved |
Pain and Myoclonic movement assessment before and after deep brain stimulation (DBS) surgery on the globus pallidus internus (GPi).
Pain ratings are dependent on patient input from Supplementary Video S1.
Our Power Spectrum Density (PSD) analysis revealed that the right hemisphere (contralateral to the stiff limb) exhibited elevated peak powers (average power = 215 µV2), approximately 10 times higher in power compared to the left hemisphere (ipsilateral) (average power = 12.9 µV2), with the beta range (15–18 Hz) displaying the most prominent oscillatory activity. This is consistent with other movement disorders, such as Parkinson’s disease, where elevated beta oscillations are strongly associated with motor rigidity. Additionally, we occasionally observed delta/theta-band peaks (3–6 Hz), though these were less frequent. In terms of frequency, the left hemisphere demonstrated lower peak positions (lower by 2–7 Hz) within the beta range and exhibited significantly lower oscillatory activity compared to the right hemisphere. The standard deviation for the right hemisphere was 95.4 µV2, and for the left hemisphere, it was 11.01 µV2. A t-test comparing the power between hemispheres showed a t-statistic of 8.42 and a p-value<0.001, indicating a highly significant difference between the two sides.
Discussion
Our results revealed a significantly lower firing rate and a significantly higher burst index in the right contralateral GPi when compared to the left ipsilateral GPi. Results from this case are in line with previous literature. In a prospective multicenter study of 22 patients, GPi DBS was evaluated at four time points: before surgery, 3, 6, and 12 months after surgery. Motor disability scores were significantly lower in the stimulation group compared to no stimulation [10]. Another study evaluated the effects of GPi DBS at 130 Hz in five patients with generalized (n = 4) or segmental (n = 1) dystonia [11]. Using the Burke-Fahn-Marsden-Dystonia Scale (BFMDS), patients showed a 43% improvement 6–12 months post-operatively. Additionally, quality of life measures were enhanced by roughly 60%. Patients also showed a frequency-dependent pattern, with higher frequencies (>130 Hz) correlating with clinical improvement, and lower frequencies (<130 Hz) associated with deterioration. In the present study, the left hemisphere firing rate was similar to previous measurements in GPi in humans. In a paper discussing neuronal firing rates in the GPi of patients with cervical dystonia, a mean firing rate of 71.4 ± 2.2 Hz was determined for cervical dystonia patients [12].
The similar lower GPi firing rates between PD patients and our patient with SPS may imply adherence to similar activation pathways as increases in GPi activation may provide proof for the initiation of the indirect pathway (GPi activation through GPe and STN). Additionally, it holds promise for enhancing our comprehension of the underlying pathophysiological mechanisms governing this disorder, potentially accelerating our diagnosis of SPS and intervention time.
These findings underscore the importance of beta-band oscillations in the contralateral hemisphere, which are likely contributing to the patient’s stiff limb. Furthermore, these oscillations are in line with existing literature on Parkinson’s disease, suggesting common underlying mechanisms in movement disorders.
While DBS may improve disease management, it is crucial to combine it with other forms of therapy to optimize patient treatment outcomes. Immunotherapy has been proposed as a form of treatment for SPS patients since 80% are known to have elevated titer antibodies against glutamic acid decarboxylase (GAD-65), and approximately 15% of SPS patient have antibodies to glycine receptor ⍺-subunit. However, a previous large, controlled trial showed no differences between SPS patients who underwent immunotherapy (Rituximab) and controls after 6 months [13].
DBS remains as an attractive potential treatment to ensure reduction in patient symptoms and unwanted muscle tremors. Our results combined with results from former literature may prove this. In a previous case report, Shah et al. revealed that DBS was indeed useful for a SPS patient [8]. The 22-year-old female was suffering from uncontrollable hyperextension of the trunk and flexion of the arms and legs that slowly worsened over time. Eventually, the patient was unable to walk due to increased rigidity in the lower extremities. DBS was then conducted and the GPi was designated as the target for the operation. After DBS installation and battery replacement, the case reported revealed that the patient’s stiffness and spasms significantly improved. Moreover, her ability to sit up and communicate was regained post-operatively. This promising finding shows the value that GPi DBS may offer to SPS patients as it may help them regain their independence and functioning. It is important to note, however, that SPS may present in many forms. For example, the patient in this case report was positive for GAD-65 antibodies while our patient is positive for amphiphysin antibodies.
Amphiphysin antibodies-associated SPS differs from GAD-associated SPS in a few points. First, amphiphysin-associated SPS is typically paraneoplastic, although our patient did not have any forms of tumors present in her body [14]. Second, previous literature has suggested that amphiphysin-associated SPS does not respond as well to immunosuppression therapy relative to GAD-associated SPS [13]. In fact, steroids and cancer treatment may be required for treatment of amphiphysin -associated SPS. While this form of SPS is much rarer than GAD-associated SPS, our patient was able to demonstrate characteristics similar to previous amphiphysin-associated SPS patients in literature. Murinson and Guarnaccia evaluated 11 amphiphysin-associated SPS patient and found that 100% were females with 10 having breast cancer [7]. More importantly, the study suggested that the average of amphiphysin-associated SPS characteristics was 58 years and that clinical features involving neck or arm were present in 80% of amphiphysin-associated SPS compared to only 20% in GAD-associated SPS patients (n = 112). Lastly, the age range of Amphiphysin-associated SPS was 39–75 years while GAD-associated SPS patients reported an age range of 14–82, suggesting amphiphysin-associated SPS may be more common amongst older adults. These reported characteristics are almost entirely in line with the characteristics of the patient in our case report, indicating a common pattern of stiffness among the thoracolumbar musculature. Understanding how these clinical markers may be associated with disease characteristics is of extreme importance in detecting, diagnosing, and managing SPS patients’ syndromes in a timely manner to ensure optimal patient outcomes. Moreover, it allows researchers to better understand disease etiology and participate in building a solid knowledge foundation to develop long-term treatment for SPS. Lastly, it may suggest that amphiphysin-associated SPS patients like ours, without positive breast cancer, may be at high risk of developing tumors.
Limitations of our case study include our inability to generalize the potential of DBS to all amphiphysin-associated SPS patients. Although our results point to an improvement in the dystonic movement and thumb placement of our patient, amphiphysin-associated SPS may present in many other forms. Therefore, the effectiveness of DBS in these patients may differ and it is unknown if it will improve or worsen symptoms of SPS. Moreover, throughout our measurements, a standard scale for pain and dystonia severity was not used. Hence, information obtained for this case report was largely dependent on patient perspective, leading to potential bias such as recall bias when the patient reports their insight.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Research Ethics Board of Hospital San Vincente Fundación. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Research was conducted by CR, JS, LB-P, and AL. Patient diagnosis was conducted by JS and DBS implant was conducted by AL. Data analysis was conducted by AM, YC, FA, and WH. Manuscript preparation and revision was conducted by AM, YC, FA, and WH. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/dyst.2024.13549/full#supplementary-material.
References
1.
Hutchison WD Lang AE Dostrovsky JO Lozano AM . Pallidal neuronal activity: implications for models of dystonia. Ann Neurol (2003) 53(4):480–8. 10.1002/ana.10474
2.
Baer AN . Paraneoplastic muscle disease. Rheum Dis Clin North America (2011) 37(2):185–200. 10.1016/j.rdc.2011.01.011
3.
Ciccotto G Blaya M Kelley RE . Stiff person syndrome. Neurol Clin (2013) 31(1):319–28. 10.1016/j.ncl.2012.09.005
4.
Dalakas MC Li M Fujii M Jacobowitz DM . Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology (2001) 57(5):780–4. 10.1212/wnl.57.5.780
5.
Najjar S Pearlman D Zagzag D Golfinos J Devinsky O . Glutamic acid decarboxylase autoantibody syndrome presenting as schizophrenia. The Neurologist (2012) 18(2):88–91. 10.1097/NRL.0b013e318247b87d
6.
Farooqi MS Lai Y Lancaster E Schmitt SE Sachais BS . Therapeutic plasma exchange and immunosuppressive therapy in a patient with anti-GAD antibody-related epilepsy: quantification of the antibody response. J Clin Apher (2015) 30(1):8–14. 10.1002/jca.21342
7.
Murinson BB Guarnaccia JB . Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology (2008) 71(24):1955–8. 10.1212/01.wnl.0000327342.58936.e0
8.
Shah E Isfahani S Swope D . Case report: deep brain stimulation as a potential treatment for stiff-person syndrome [abstract]. Mov Disord (2022) 37(Suppl. 2). 10.3389/fimmu.2023.1297340
9.
Lozano A Hutchison W Kiss Z Tasker R Davis K Dostrovsky J . Methods for microelectrode-guided posteroventral pallidotomy. J Neurosurg (1996) 84(2):194–202. 10.3171/jns.1996.84.2.0194
10.
Vidailhet M Vercueil L Houeto JL Krystkowiak P Benabid AL Cornu P et al Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med (2005) 352(5):459–67. 10.1056/NEJMoa042187
11.
Kupsch A Klaffke S Kuehn AA Meissner W Arnold G Schneider GH et al The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol (2003) 250(10):1201–5. 10.1007/s00415-003-0179-0
12.
Tang JK Moro E Mahant N Hutchison WD Lang AE Lozano AM et al Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. J Neurophysiol (2007) 98(2):720–9. 10.1152/jn.01107.2006
13.
Dalakas MC Rakocevic G Dambrosia JM Alexopoulos H McElroy B . A double‐blind, placebo‐controlled study of rituximab in patients with stiff person syndrome. Ann Neurol (2017) 82(2):271–7. 10.1002/ana.25002
14.
Antoine JC Absi L Honnorat J Boulesteix JM De Brouker T Vial C et al Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol (1999) 56(2):172–7. 10.1001/archneur.56.2.172
Summary
Keywords
dystonia, tremor, deep brain stimulation, myoclonus, globus pallidus internus
Citation
Mohamed A, Chu Y, Alanazi FI, Restrepo Bravo CA, Saavedra Moreno JS, Botero-Posada LF, Lopez Rios AL and Hutchison WD (2024) Case report: Effective globus pallidus internus deep brain stimulation for patient with stiff-person syndrome. Dystonia 3:13549. doi: 10.3389/dyst.2024.13549
Received
16 July 2024
Accepted
29 November 2024
Published
20 December 2024
Volume
3 - 2024
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Updates
Copyright
© 2024 Mohamed, Chu, Alanazi, Restrepo Bravo, Saavedra Moreno, Botero-Posada, Lopez Rios and Hutchison.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Mohamed, amoha423@uwo.ca
† Present address: Ahmed Mohamed, Schulich School of Medicine and Dentistry, University of Western Ontario, London, ON, Canada
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.