Abstract
Background:
Cervical dystonia is the most common form of focal dystonia and is the most studied neurological condition in patients receiving botulinum toxin. In clinical trials of botulinum toxin, there is a placebo response, but this has not been studied systematically.
Objectives:
A systematic review and meta-analysis were conducted to assess and compare the peak and duration of placebo response in people with cervical dystonia receiving botulinum toxin in randomized, blinded controlled clinical trials.
Methods:
Three databases (Embase, PubMed, Scopus) and two trial registers (Cochrane CENTRAL and Cochrane Movement Disorders Trials Register) were searched by a biomedical librarian in May 2022 and September 2023. Covidence was used for screening titles and abstracts and full text. Two authors independently screened each record and collected data from the included articles. Microsoft Excel was used for data collection. The Cochrane Collaboration’s Risk of Bias tool in randomized trials was used by two authors for each included study. A third author resolved conflicts during screening and discrepancies during data collection and risk of bias assessment. We conducted a random-effects meta-analysis of the mean reduction in the TWSTRS total score at 4 weeks post injection, to assess the clinical change in the placebo arm overall and by TWSTRS pain, severity, and disability scales.
Results:
Twelve studies, with moderate overall risk of bias, that included 2,145 participants with 580 randomized to placebo, were included in the systematic review. Clinical changes in the placebo arm were mild with a mean reduction of Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score of 3.5 [95% confidence interval (CI): 2.6–4.4] 4 weeks post injection. TWSTRS pain score at week four was 0.9 (95% CI: 0.6 - 1.1; I2 = 59%). TWSTRS severity score at week four was 1.7 (95% CI: 1.3–2.0; I2 = 63%). TWSTRS disability score at week four was 0.7 (95% CI: 0.3–1.0; I2 = 63%). Duration of benefit ranged from 45.1 to 76.3 days with a mean duration of 57.6 days. Dropouts in patients randomized to placebo were due to lack of efficacy, pain, neck weakness, fatigue, and tiredness.
Conclusion:
This information on placebo response will be useful in estimating sample size and interpreting participant benefit in future interventional studies of cervical dystonia.
Introduction
Cervical dystonia (CD) is the most common form of focal dystonia in adults. It is defined by the involuntary contraction of muscles in the head and neck that result in abnormal movements and postures. It may be classified as either isolated or combined with other neurological disorders and predominantly affects women in the 4th to 5th decades of life. It is associated with significant disease morbidity, including pain, physical disability, impaired employment, depression, and social isolation [1]. Disease burden of CD is often defined by TWSTRS (Toronto Western Spasmodic Torticollis Rating Scale), which includes a total sum in addition to scores assessing pain, disability and severity [2, 3].
A well-defined treatment modality for CD is botulinum toxin injection. Both botulinum toxin types A and B have been extensively investigated for utility in CD patients and are currently the first-line therapeutic modalities for CD [4–6]. However, toxin injections are costly, and treatment usually requires four procedures per year. In addition, there is a significant proportion of patients who discontinue therapy, most commonly for poor response, adverse events, remission, relocation, inconvenience, and cost [7]. Because of the potency of these agents some have postulated a nocebo response, or negative response to treatment, as a reason for discontinuing participation [8]. There is a need for new treatments or the development of toxins with a longer duration of benefit.
Placebo response to botulinum toxin injections has not been previously defined in the literature, although Cochrane reviews of toxin efficacy comparing active and placebo arms are available [9, 10]. The objective of this systematic review is to define the placebo response of botulinum toxin injections by comparing the TWSTRS scores, including pain, severity total and scores, between study and placebo arms to determine if placebo injections affect patient perceptions of disease burden. Having good information about placebo response in these patients will be valuable in designing future clinical trials.
Methods
A protocol was written a priori using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P), and the PRISMA Checklist was used for reporting the completed review.
Eligibility criteria
Studies included peer-reviewed, double-blinded, randomized placebo-controlled trials that investigated the use of botulinum toxin injections in patients previously diagnosed with isolated CD of unknown cause. All studies had to include TWSTRS as an outcome reported either as TWSTRS total mean data at baseline and week 4 or the change in TWSTRS total mean data between baseline and week 4. Studies were excluded if they were not in English, were published prior to 1977, or included patients under the age of 18. No restrictions were placed on patient demographics, prior exposure to botulinum toxin injections, or study setting.
Information sources and search strategy
A biomedical librarian (AAL) searched three databases: Embase (Elsevier), PubMed (US National Library of Medicine), and Scopus (Elsevier), and two trial registers: Cochrane Library: CENTRAL (Wiley & Sons) and Cochrane Movement Disorders Trials Register (Wiley & Sons) in May 2022 and September 2023. A combination of keywords and controlled vocabulary terms (i.e., EMTREE for Embase and MeSH for PubMed and Cochrane CENTRAL) were used for each concept of interest (i.e., cervical dystonia, botulinum toxin, and clinical trial). The search terms were reviewed by the team and feedback provided. All searches were run prior to January 2024 [11–19], but later two clinical trial summaries were found in clinicaltrials.gov [20, 21] and the publications were added to the original search [22]. Additionally search strategies were used to exclude animal studies and specific publication types as specified in the exclusion criteria [23].
The results of the searches were exported to EndNote 20 (Clarivate Analytics) and duplicate records identified. The unique records were exported by the biomedical librarian into Covidence (Veritas Health Innovation) which was used for screening.
Selection process
The unique records from the database searches were screened at two stages using Covidence. First, two authors (CE, TC) independently screened each record title and abstract using the predefined eligibility criteria. Second, the full texts of those articles included after stage one were retrieved and uploaded into Covidence. Then two authors (CE, TC) independently screened each record using the same eligibility criteria. At both stages, a different third review author (MS) resolved any disagreements or conflicts.
Before commencing the title and abstract screening process, a pilot of 25 records randomly selected by the biomedical librarian was conducted with the review team. Then for the articles included for full text review, those were also screened by the same individuals using the same process and eligibility criteria. As necessary, the eligibility criteria were revised to reflect clarifications and questions addressed during the pilot.
Data collection process and data items
For each included article, data collection was performed independently by two review authors (MS, EW) utilizing a standardized data collection spreadsheet in Microsoft Excel (Microsoft Corporation). All data collected were reviewed by a separate third reviewer to resolve discrepancies and errors (HR).
The data items collected were: demographics (participant mean age, gender, and race), study duration, study sponsor, number of participants, type of botulinum toxin used, dose of botulinum toxin, TWSTRS scores including total mean, subtype (pain, severity, and disability) mean, and/or total change in mean at baseline and week 4, and Visual Analog Scale (VAS) pain mean or mean change at baseline and week 4. If additional data were required, requests were made to study sponsors or senior authors via email.
Study risk of bias assessment
To assess the risk of bias for each included study, two authors independently used the specified checklist. A separate third author reviewed the results and resolved disagreements between the two authors; if needed, consensus discussion was used with the two authors who completed the assessment. Risk of bias was assessed with the Cochrane Collaboration tool for assessing the risk of bias [21].
The following domains were assessed for possible bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, incomplete outcome data, for-profit bias, and enriched population bias. To evaluate reporting bias, we assessed whether the study protocol was published before patient recruitment, selective reporting of outcomes, and possible presence of small sample bias. The risk of bias for each domain was classified as high, low, or unclear, and the overall assessment for the individual study as high or low.
Synthesis methods and statistical analysis
For the systematic review, we present a narrative summary and descriptive statistics for our findings. To analyze the clinical change in the placebo arm, we conducted a random-effects meta-analysis using Comprehensive Meta-Analysis version 4 [24]. A random-effects statistical model was used to calculate the mean reduction in the TWSTRS total score at 4 weeks post injection and 95% confidence interval [95% CI]. A descriptive analysis of the I2 statistic was performed to measure the degree of heterogeneity present across multiple studies included in the meta-analysis [25, 26]. Similar analyses were conducted to analyze the mean reduction in the TWSTRS: pain, severity, and disability scores.
Results
Study selection
The database searches resulted in 3,008 records of which 644 were duplicates and 2,364 were unique. Of these 2,364 records screened at title and abstract, 2,181 were excluded and 19 proceeded to full text screening. Of the 19 screened at full text, 10 were excluded and 9 included as represented in Figure 1. Two additional studies, that have not been published, were discovered by searching the terms “cervical dystonia” and “botulinum toxin” in the clinicaltrials.gov [20, 21] database, and an additional study was published after the original data searches [22] to reach a total of 12 studies for review.
FIGURE 1
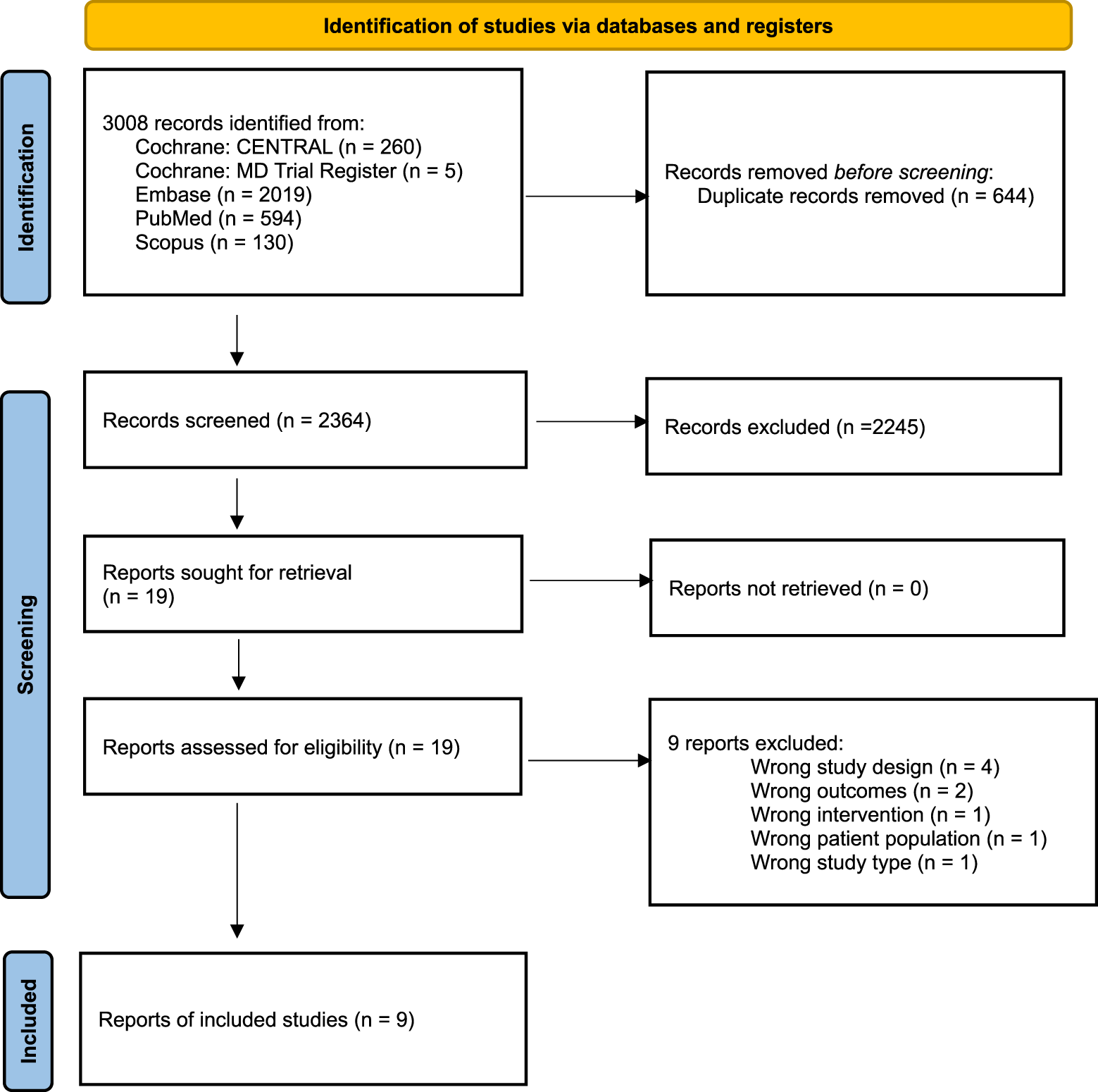
PRISMA Flow Diagram.
Risk of bias in studies
Risk bias assessment is provided in Table 1. There were 5 instances in which allocation concealment could not be determined [15, 17, 18, 20, 21]; in all other instances risk of bias was scored as low.
TABLE 1
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Objective outcomes blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Enriched population – preferential enrolment of positive responders | Enriched population – exclusion of poor responders | For-profit bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Allergan [21] | L | U | L | L | L | L | L | L | L | L |
| Brashear et al [11] | L | L | L | L | L | L | L | L | L | L |
| Brin et al [12] | L | L | L | L | L | L | L | L | L | L |
| Comella et al [22] | L | L | L | L | L | L | L | L | L | L |
| Comella et al [13] | L | L | L | L | L | L | L | L | L | L |
| Esai [20] | L | U | L | L | L | L | L | L | L | L |
| Lew et al [14] | L | U | L | L | L | L | L | L | L | L |
| Lew et al [15] | L | L | L | L | L | L | L | L | L | L |
| Patel et al [19] | L | L | L | L | L | L | L | L | L | L |
| Poewe et al [16] | L | L | L | L | L | L | L | L | L | L |
| Truong et al [18] | L | U | L | L | L | L | L | L | L | L |
| Truong et al [20] | L | U | L | L | L | L | L | L | L | L |
| Overall | L | L | L | L | L | L | L | L | L | L |
Risk bias of all included studies.
Footnote: L, low risk of bias; U, unknown risk of bias.
Meta-analysis results
Randomization to placebo ranged from 14.6% to 47.4% with a mean assignment of 31.7%. In general, dynamic of change in placebo and active groups correlated within the scope of response per protocol.
All TWSTRS subcomponent scores showed improvement at 4 weeks with the total score averaging a 3.5-point reduction. Duration of benefit was tracked in five of the twelve studies, ranging from 45 to 73 days with average duration of response of 56.7 days (Table 2).
TABLE 2
| Study | N (total) | N (placebo) | TWSTRS (total score at 4 w) | TWSTRS (pain score at 4 w) | TWSTRS (disability score at 4 w) | TWSTRS (severity score at 4 w) | Duration of response (days) |
|---|---|---|---|---|---|---|---|
| Allergan [21] | 242 | 64 | −5.6 (8.6) | −1.3 (3.6) | -- | -- | |
| Brashear et al [11] | 109 | 36 | −3.3 (11.7) | −0.5 | −1.6 | −1.8 | 63 days |
| Brin et al [12] | 114 | 37 | −2.0 (12.3) | −0.1 (4.3) | −0.8 (5.1) | −1.1 (5.5) | 52 days |
| Comella et al [22] | 301 | 46 | −4.3 (1.82) | −1.1 (0.6) | −1 (0.66) | −2 (0.8) | -- |
| Comella et al [13] | 243 | 74 | −2.2 (7.3) | −0.3 (3) | 0 (3.4) | −0.9 (4) | -- |
| Esai [20] | 95 | 33 | −3 (1.3) | −0.8 (0.4) | −0.6 ((0.7) | −1.6 (0.6) | -- |
| Lew et al [14] | 122 | 30 | −3.3 | −1 | −0.7 | −1.6 | 45.1 d |
| Lew et al [15] | 134 | 45 | −3 | -- | -- | -- | |
| Patel et al [19] | 129 | 45 | −2.5 (12.5) | -- | -- | -- | 52.3 days |
| Poewe et al [16] | 369 | 54 | −3.4 | −1.2 | −0.8 | −1.9 | -- |
| Truong et al [18] | 171 | 61 | −6.7 | −0.6 (4.9) | −0.6 (11.4) | −2.1 (4.8) | 76.3 days |
| Truong et al [20] | 116 | 55 | −3.8 (12.2) | -- | -- | -- | -- |
| Total [mean] [95% CI] [I2] |
2,145 | 580 | −3.5a (−4.4, −2.6) 66% |
−0.9a (−1.1, −0.6) 59% |
−0.7a (−1.0, −0.3) 63% |
−1.7a (−2.0,-1.3) 63% |
[57.6 days] |
Placebo response outcome.
Random Effects Mean Reduction in TWSTR scores; -- data not available.
Other outcome measures included: PSAC (1 study), C-PEGR (1), CPGAC (2), C-VAS change (2), C-VAS pain (1), Pt GA-VAS (2), PGA VAS (2). Except for the C-VAS change, all showed some improvement at 4 weeks.
Discontinuations occurred in 8 of twelve studies and totaled 22 of 580 (3.8%) participants randomized to placebo [11–13, 16–18, 20]. Reasons for withdrawal, where stated, included lack of efficacy (5), protocol violation (2), relocation (2), carcinoma, withdrawal of consent and lost to follow up. The most common adverse events include pain, bulbar disturbances, or infection (Table 3).
TABLE 3
| Study | N | Withdrew N (%) |
Adverse events | ||||
|---|---|---|---|---|---|---|---|
| Lack of efficacy | Pain | Infection | Dysphagia | Other | |||
| Allergan [21] | 64 | 5 | 27 | 9 | 21 | 10 | |
| Brashear et al [11] | 36 | 2 | 2 | 17 | 10 | 2 | |
| Brin et al [12] | 37 | 1 | 11 | 6 | 3 | 4 | |
| Comella et al [22] | 46 | 2 | 3 | 1 | |||
| Comella et al [13] | 74 | 6 | 3 | 8 | 7 | 4 | |
| Esai [20] | 33 | 0 | 2 | 8 | 1 | ||
| Lew et al [14] | 30 | 0 | 1 | ||||
| Lew et al [15] | 45 | 0 | 11 | 1 | 1 | ||
| Patel et al [19] | 45 | 0 | 1 | ||||
| Poewe et al [16] | 54 | 2 | 4 | 3 | 2 | ||
| Truong et al [18] | 61 | 3 | 2 | 5 | 5 | ||
| Truong et al [20] | 55 | 1 | 36 | 4 | 16 | 31 | |
| Total | 580 | 22 | 7 | 119 | 38 | 63 | 55 |
Participant discontinuation in placebo arm.
Discussion
Summary of main results
This review included twelve randomized, parallel-designed trials. These trials enrolled 2,145 participants with cervical dystonia, with 580 randomized to placebo, 74% of whom had been previously treated with botulinum toxin for this condition. Clinical changes in the placebo arm were mild with a mean reduction of TWSTRS total score of 3.5 at 4 weeks post injection. Additionally, TWSTRS pain score at week four was 0.9, TWSTRS severity score at week four was 1.7, and TWSTRS disability score at week four was 0.7. Duration of benefit ranged from 45.1 to 76.3 days with a mean duration of 57.6 days. Dropouts in participants randomized to placebo were lack of efficacy, pain, neck weakness, fatigue, and tiredness.
Overall completeness and applicability of evidence
All trials listed data to assess placebo response, using TWSTRS and other outcomes. However, in some instances cross-comparisons were limited, the confidence in overall conclusions for under-reported outcomes. The participants did not represent the overall population of people with cervical dystonia and conclusions from these studies are most pertinent to a predominantly white study population from North America and Europe.
Quality of the evidence
Risk bias assessment found low or undetermined risk for all studies and for all questions. Risk could not be determined for five studies and only when assessing allocation concealment or selection bias.
Implications for practice
This analysis suggests that a mild benefit is seen in participants randomized to receive placebo botulinum toxin in a clinical trial setting. The duration of benefit averaged almost 60 days. In a clinical setting, given that placebo injection of botulinum toxin would not be considered, this mild benefit for six to 10 weeks may represent the floor response from this therapy. Patient reports of pain, fatigue or tiredness since a prior injection may also suggest a need for a different anatomic approach or dosage increase.
Implications for research
Placebo response is an important consideration when designing randomized clinical trials for cervical dystonia, whether this includes a focal injection of toxin or systemic therapy. There is some variability in the trials reviewed for this analysis and how TWSTRS response was reported. Power calculation parameters were stated specifically in three instances and included a TWSTRS response of Placebo/Active of 4.0/10.0 [13, 22], and 5.5/8.8 [14]. Others used difference in from placebo to active arm response: 10.0. [18]; 5.5 (low dose), 8.8 (high dose) [15], 5.9 [16], and 9.0 [17]. In all but one, the observed difference from placebo exceeded the expected response.
The three trials assessing response to rimabotulinumtoxinB used these criteria: The level of significance for the main effects (i.e., center and treatment) was set at a 5 0.05, and the level of significance for interactions was set at a 5.10 [14]. The reported results found 3.3, 11.5, 12.6, 16.4 [14], 2.0, 11.1 [12] and 4.3, 9.3, 11.7 [11].
Differences between placebo and active responses using total TWSTRS were less when randomization was at 2 active: 1 placebo with change ranging from 6.0–9.8. Studies with 1:1 randomization ratio demonstrated a 8.1–1.9 and studies with >2:1 range from 6.6–10.1.
All primary efficacy assessments were at 4 weeks and did not include duration of response. Because of this a decline in benefit using TWSTRS could not be provided. While this may not be necessary in toxin evaluation, it may be more important in the study of systemic treatments. Given one study reported a 76-day response from placebo, a 12-week study period seems prudent.
Placebo response in medicine has been recognized for more than 70 years [26]. Besides treatment type, such as medical therapy or surgical outcome, it has also been linked to medication adherence [27], religious practice [28] and medication cost [29]. Neuroimaging study of subjects randomized to placebo suggests that expectation of reward increases dopamine release in the basal ganglia [30, 31]. There may be differences in placebo response, based on the type of intervention: oral medication, injected medication or surgical intervention. In the setting of injection, it is also possible that an intramuscular stimulation may be similar to “dry needling,” or acupuncture [32, 33]. These interventions have not been utilized in the setting of dystonia but are reported to improve pain in other settings. This meta-analysis did not demonstrate any response differences between pain, disability, severity or total TWSTRS scores.
Conclusion
Botulinum toxin has been proven effective in treating cervical dystonia and is now regarded as a first line therapy. Since the approval of onabotulinumtoxinA for this indication, three additional A-type toxins (incobotulinumtoxinA, abobotulinumtoxinA, daxxybotulinumtoxinA) and one B-type toxin (rimabotulinumtoxinB) have also been approved. In double-blind placebo-controlled trials, a small benefit in the TWSTRS total score 3.5 [95% (CI): 2.6–4.4] was seen at 4 weeks after placebo toxin injection. In designing future therapeutic protocols, sample size determination may be better informed using this 4-week baseline change as a meaningful interval. However, the duration of response and efficacy assessments should extend beyond 11 weeks.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: data were extracted from primary manuscripts or clinicaltrials.gov submissions.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
EW: Concept design, data extraction, author of first draft, manuscript review. TC: Concept design, drafting of protocol, completed screening and data extraction, risk bias assessment, manuscript review. CE: Concept design, completed screening and data extraction, data dictionary preparation, database development of extracted data, risk bias assessment, manuscript review. AL: Concept design, drafting of protocol, developed and completed literature searches, assisted with managing Covidence, contribution to first draft, manuscript review. GN: Statistical development and direction regarding data entry, manuscript review. HR: Statistical support and analysis, contribution to first draft, manuscript review. MH: Concept design, supervisor of all project activities, manuscript review. MS: Concept design, supervised, database development, data requests, data entry, risk bias assessment, manuscript review. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. AL contribution is supported by the National Institutes of Health Library, Office of Research Services in support of the Intramural Research Program of the NIH. This work has been supported by the NIH Intramural Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Stacy M . Epidemiology, clinical presentation, and diagnosis of cervical dystonia. Neurol Clin (2008) 26(Suppl. 1):23–42. 10.1016/s0733-8619(08)80003-5
2.
Comella CL Stebbins GT Goetz CG Chmura TA Bressman SB Lang AE . Teaching tape for the motor section of the Toronto western spasmodic Torticollis scale. Mov Disord (1997) 12(4):570–5. 10.1002/mds.870120414
3.
Jost WH Hefter H Stenner A Reichel G . Rating scales for cervical dystonia: a critical evaluation of tools for outcome assessment of botulinum toxin therapy. J Neural Transm (Vienna) (2013) 120(3):487–96. 10.1007/s00702-012-0887-7
4.
Bledsoe IO Comella CL . Botulinum toxin treatment of cervical dystonia. Semin Neurol (2016) 36(1):47–53. 10.1055/s-0035-1571210
5.
Hallett M Albanese A Dressler D Segal KR Simpson DM Truong D et al Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon (2013) 67:94–114. 10.1016/j.toxicon.2012.12.004
6.
Contarino MF Van Den Dool J Balash Y Bhatia K Giladi N Koelman JH et al Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol (2017) 8:35. 10.3389/fneur.2017.00035
7.
Jinnah HA Comella CL Perlmutter J Lungu C Hallett M , Investigators Dystonia Coalition. Longitudinal studies of botulinum toxin in cervical dystonia: why do patients discontinue therapy?Toxicon (2018) 147:89–95. 10.1016/j.toxicon.2017.09.004
8.
Duarte GS Rodrigues FB Ferreira JJ Costa J . Adverse events with botulinum toxin treatment in cervical dystonia: how much should we blame placebo?Parkinsonism Relat Disord (2018) 56:16–9. 10.1016/j.parkreldis.2018.06.017
9.
Duarte GS Castelão M Rodrigues FB Marques RE Ferreira J Sampaio C et al Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev (2016) 10(10):CD004314. 10.1002/14651858.CD004314.pub3
10.
Rodrigues FB Duarte GS Marques RE Castelão M Ferreira J Sampaio C et al Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev (2020) 11(11):CD003633. 10.1002/14651858.CD003633.pub4
11.
Brashear A Lew MF Dykstra DD Comella CL Factor SA Rodnitzky RL et al Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-responsive cervical dystonia. Neurology (1999) 53(7):1439–46. 10.1212/wnl.53.7.1439
12.
Brin MF Lew MF Adler CH Comella CL Factor SA Jankovic J et al Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-resistant cervical dystonia. Neurology (1999) 53(7):1431–8. 10.1212/wnl.53.7.1431
13.
Comella CL Jankovic J Truong DD Hanschmann A Grafe S , U.S. XEOMIN Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci (2011) 308(1-2):103–9. 10.1016/j.jns.2011.05.041
14.
Lew MF Brashear A Dashtipour K Isaacson S Hauser RA Maisonobe P et al A 500 U/2 mL dilution of abobotulinumtoxinA vs. placebo: randomized study in cervical dystonia. Int J Neurosci (2018) 128(7):619–26. 10.1080/00207454.2017.1406935
15.
Lew MF Adornato BT Duane DD Dykstra DD Factor SA Massey JM et al Botulinum toxin type B: a double-blind, placebo-controlled, safety and efficacy study in cervical dystonia. Neurology (1997) 49(3):701–7. 10.1212/wnl.49.3.701
16.
Poewe W Burbaud P Castelnovo G Jost WH Ceballos-Baumann AO Banach M et al Efficacy and safety of abobotulinumtoxinA liquid formulation in cervical dystonia: a randomized-controlled trial. Mov Disord (2016) 31(11):1649–57. 10.1002/mds.26760
17.
Truong D Duane DD Jankovic J Singer C Seeberger LC Comella CL et al Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double-blind, placebo-controlled study. Mov Disord (2005) 20(7):783–91. 10.1002/mds.20403
18.
Truong D Brodsky M Lew M Brashear A Jankovic J Molho E et al Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord (2010) 16(5):316–23. 10.1016/j.parkreldis.2010.03.002
19.
Patel AT Lew MF Dashtipour K Isaacson S Hauser RA Ondo W et al Sustained functional benefits after a single set of injections with abobotulinumtoxinA using a 2-mL injection volume in adults with cervical dystonia: 12-week results from a randomized, double-blind, placebo-controlled phase 3b study. PLoS One (2021) 16(2):e0245827. 10.1371/journal.pone.0245827
20.
ClinicalTrials.gov. Randomized, double-blind, placebo-controlled, parallel, group dose-response, study of E2014 in patients WIth spasmodic Torticollis (2024). Available from: https://clinicaltrials.gov/study/NCT00165776?cond=Cervical%20Dystonia&intr=botulinum%20toxin&page=3&rank=24&tab=results (Accessed April 24, 2024).
21.
ClinicalTrials.gov. Study to evaluate safety, efficacy of botulinum toxin type A in patients with cervical dystonia (2024). Available from: https://clinicaltrials.gov/search?cond=Cervical%20Dystonia&intr=botulinum%20toxin&term=NCT00564681 (Accessed April 24, 2024).
22.
Comella CL Jankovic J Hauser RA Patel AT Banach MD Ehler E et al Efficacy and safety of daxibotulinumtoxinA for injection in cervical dystonia: ASPEN-1 phase 3 randomized controlled trial. Neurology (2024) 102(4):e208091. 10.1212/WNL.0000000000208091
23.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. 10.1136/bmj.l4898
24.
Borenstein M Hedges L Higgins J Rothstein H . Comprehensive meta-analysis version 4. Englewood, NJ: Biostat. (2022).
25.
Higgins JP Thompson S . Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. 10.1002/sim.1186
26.
Higgins JP Thompson S Deeks J Altman DG . Measuring inconsistency in meta-analyses. Br Med J (2003) 327:557–60. 10.1136/bmj.327.7414.557
27.
Beecher HK . The powerful placebo. J Am Med Assoc (1955) 159(17):1602–6. 10.1001/jama.1955.02960340022006
28.
Simpson SH Eurich DT Majumdar SR Padwal RS Tsuyuki RT Varney J et al A meta-analysis of the association between adherence to drug therapy and mortality. BMJ (2006) 333(7557):15. 10.1136/bmj.38875.675486.55
29.
Koenig HG . Religion and medicine IV: religion, physical health, and clinical implications. Int J Psychiatry Med (2001) 31(3):321–36. 10.2190/X28K-GDAY-75QV-G69N
30.
Espay AJ Norris MM Eliassen JC Dwivedi A Smith MS Banks C et al Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology (2015) 84(8):794–802. 10.1212/WNL.0000000000001282
31.
Quattrone A Barbagallo G Cerasa A Stoessl AJ . Neurobiology of placebo effect in Parkinson's disease: what we have learned and where we are going. Mov Disord (2018) 33(8):1213–27. 10.1002/mds.27438
32.
Lew J Kim J Nair P . Comparison of dry needling and trigger point manual therapy in patients with neck and upper back myofascial pain syndrome: a systematic review and meta-analysis. J Man Manip Ther (2021) 29(3):136–46. 10.1080/10669817.2020.1822618
33.
Jang S Ko Y Sasaki Y Park S Jo J Kang NH et al Acupuncture as an adjuvant therapy for management of treatment-related symptoms in breast cancer patients: systematic review and meta-analysis (PRISMA-compliant). Medicine (Baltimore) (2020) 99(50):e21820. 10.1097/MD.0000000000021820
Summary
Keywords
cervical dystonia, botulinum toxin, meta-analysis, placebo response, systematic review
Citation
Wetmore E, Roberts H, Livinski AA, Camacho T, Eaton C, Norato G, Hallett M and Stacy M (2025) Clinical response to placebo botulinum toxin injection in cervical dystonia—a systematic review and meta-analysis. Dystonia 4:14297. doi: 10.3389/dyst.2025.14297
Received
03 January 2025
Accepted
14 February 2025
Published
28 February 2025
Volume
4 - 2025
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Updates
Copyright
© 2025 Wetmore, Roberts, Livinski, Camacho, Eaton, Norato, Hallett and Stacy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Stacy, stacym@musc.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.