- Department of Dermatology, Teikyo University School of Medicine, Tokyo, Japan
Baricitinib demonstrated efficacy and tolerable safety in clinical trials for atopic dermatitis (AD); however, real-world data are limited. We examined effectiveness and safety of baricitinib, and laboratory data in AD patients treated with baricitinib in our department. We also evaluated baseline clinical severity in responders and non-responders. All adult AD patients treated with baricitinib in our department between January 2021 and February 2023 were included. Data on 30 Japanese AD patients were analyzed. Objective severity scores and patient-reported outcomes improved at one and 3 months, except for the affected body surface area at 1 month. The proportions of patients who achieved eczema area and severity index-50% improvement were 30.0% (9/30) at 1 month and 53.3% (16/30) at 3 months. There were no significant changes in AD biomarkers. No significant difference was observed in baseline clinical severity between responders and non-responders. No significant changes were observed in laboratory results except for increased serum creatine phosphokinase levels at 3 months. One case of herpes zoster and one case of ocular herpes were observed. Baricitinib showed mild effectiveness and favorable safety including laboratory findings. Biomarkers did not reflect clinical improvement. Further study is needed to identify characteristics of responders.
Introduction
Recently, several systemic therapies have been approved for atopic dermatitis (AD) [1]. Among them, baricitinib demonstrated efficacy and tolerable safety in clinical trials for AD [2–4]; however, real-world data are limited. Real-world data can be different from results of clinical trials due to their strict inclusion and exclusion criteria [5, 6]. We examined the effectiveness and safety of baricitinib, and laboratory data in AD patients treated with baricitinib in our department. In addition, we also evaluated baseline clinical severity in responders and non-responders in order to explore which patients could benefit most from baricitinib.
Methods
All adult AD patients treated with baricitinib in our department between January 2021 and February 2023 were included. Under the Japanese insurance system, baricitinib is indicated only for AD patients meeting certain criteria, which are the same as the criteria for dupilumab [5]. Data were collected retrospectively from patients’ charts. This study was approved by the Teikyo University Institutional Review Board (22-015). As for statistical analyses, GraphPad Prism 8.4.3 (GraphPad Software, Boston, MA) was utilized. Shapiro-Wilk test was performed for normality. For multiple comparisons, ordinary one-way analysis of variance or Kruskal-Wallis test was performed. Then, if significant difference was observed, Dunnett’s multiple comparisons test or Dunn’s multiple comparisons test was further conducted. For comparisons for two groups, unpaired t-test or Mann-Whitney test was conducted.
Results
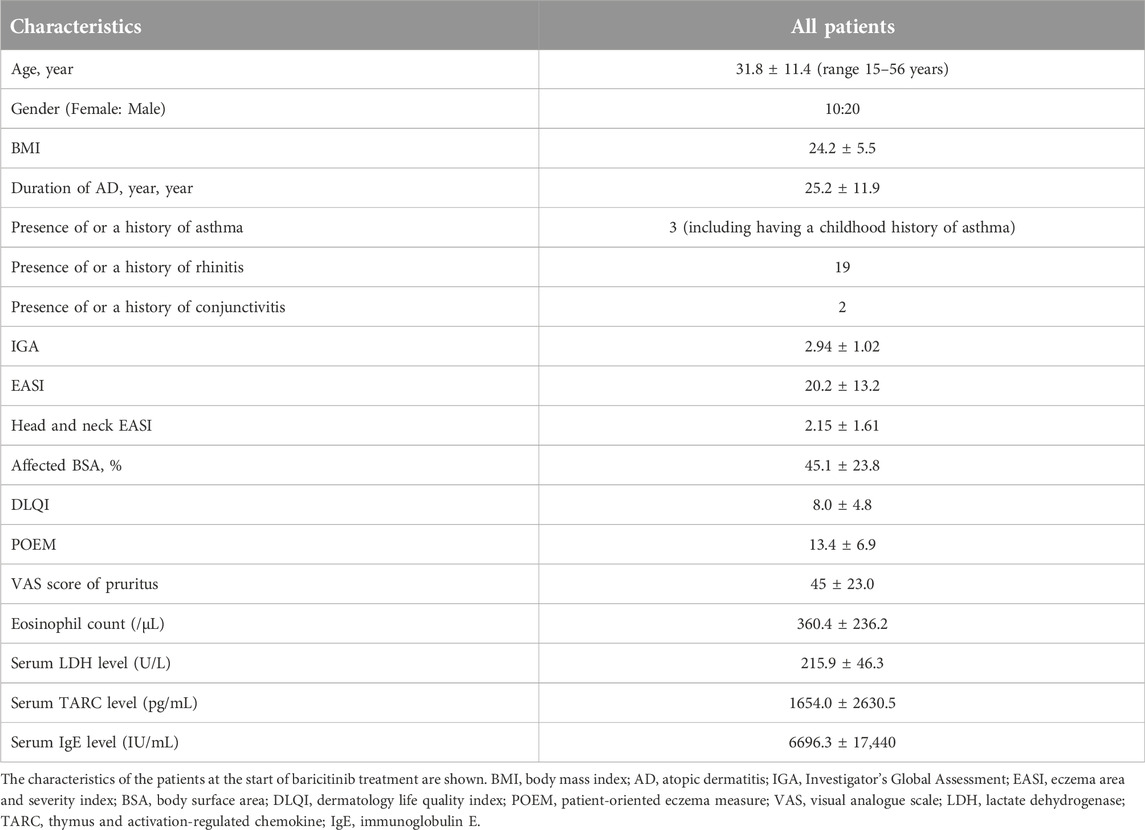
Data on 30 Japanese AD patients were analyzed. Baseline characteristics are summarized in Table 1. Regarding systemic therapies within 6 months before initiating baricitinib, nine patients had received dupilumab, three had received cyclosporine, and one had received upadacitinib. The reasons for switching from those systemic therapies to baricitinib included the following: persistent facial rash after long-term use of dupilumab, reluctance to continue long-term systemic therapy and wishing to discontinue systemic therapy by switching to baricitinib and tapering it, concomitant alopecia areata, and acne during upadacitinib treatment. Twenty-eight patients received 4 mg/day of baricitinib, while two received 2 mg/day.
Objective severity scores and patient-reported outcomes significantly improved at one and 3 months, except for the affected body surface area (BSA) at 1 month in all patents and in patients who did not receive any systemic therapy within 6 months before initiating baricitinib (naïve) (Dunnett’s multiple comparisons test or Dunn’s multiple comparisons test; Figure 1). In patients who switched from other systemic therapy to baricitinib within 6 months before initiating baricitinib (switch), tendency of improvement in objective severity scores and patient-reported outcomes was observed, although there was no significant difference (ordinary one-way analysis of variance or Kruskal-Wallis test). The proportions of patients who achieved eczema area and severity index (EASI)-50% improvement were 30.0% (9/30) at 1 month and 53.3% (16/30) at 3 months; the proportions who achieved EASI-75% improvement were 20.0% (6/30) at 1 month and 23.3% (7/30) at 3 months. There were no significant changes in AD biomarkers (Mann Whitney test for the serum IgE level and Kruskal-Wallis test for the others; Figure 2).
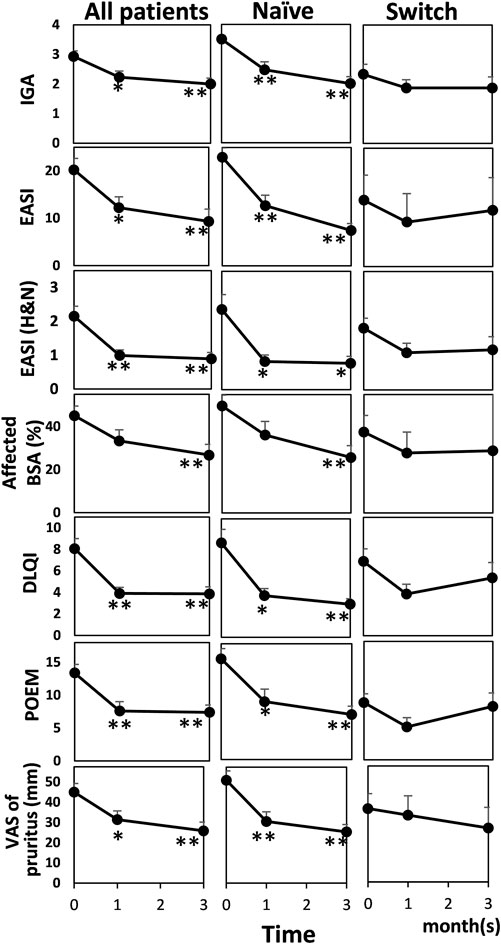
FIGURE 1. Effectiveness in objective severity assessments and patient-reported outcomes before and after baricitinib treatment for AD in all patients (n = 30), patients who did not receive any systemic therapy within 6 months before initiating baricitinib (Naïve) (n = 17), and patients who switched from other systemic therapy to baricitinib within 6 months before initiating baricitinib (Switch) (n = 13). Mean values are shown and bars represent standard errors. Shapiro-Wilk test was performed to test for normality, and then Kruskal-Wallis test or Dunnett’s test was conducted for comparison. *p < 0.05, **p < 0.01. IGA, investigator’s global assessment; EASI, eczema area and severity index; H&N, head and neck; BSA, body surface area; DLQI, dermatology life quality index; POEM, patient-oriented eczema measure; VAS, visual analogue scale.
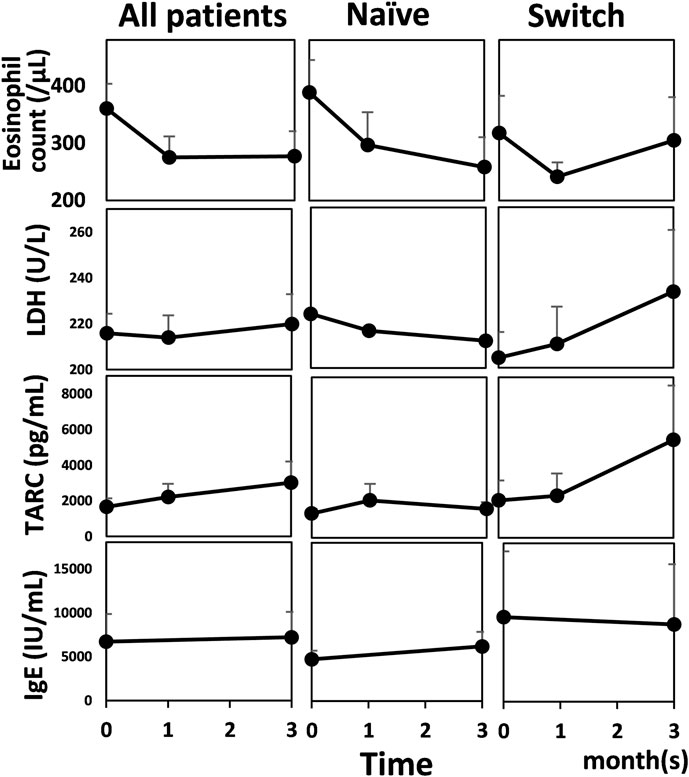
FIGURE 2. Biomarkers of atopic dermatitis before and after baricitinib treatment for AD in all patients (n = 30), patients who did not receive any systemic therapy within 6 months before initiating baricitinib (Naïve) (n = 17), and patients who switched from other systemic therapy to baricitinib within 6 months before initiating baricitinib (Switch) (n = 13). Mean values are shown and bars represent standard errors. LDH, lactate dehydrogenase; TARC, thymus and activation-regulated chemokine; IgE, immunoglobulin E.
In order to explore whether baseline clinical severity affected effectiveness, we compared baseline values in responders (patients who achieved EASI-50 at 3 months) with non-responders (patients with EASI reduction rate <50% at 3 months). No significant difference was observed (baseline EASI, 22.1 ± 8.7 vs. 24.8 ± 20.0, unpaired t-test, p = 0.6846; baseline affected BSA, 42 ± 20.7 vs. 51.4 ± 29.4, unpaired t-test, p = 0.4193). Neither was it when responders were defined as patients who achieved EASI-75 at 3 months (baseline EASI, 22.2 ± 4.7 vs. 23.3 ± 15.9, unpaired t-test, p = 0.8728; baseline affected BSA, 37.1 ± 17.5 vs. 48.7 ± 26.0, unpaired t-test, p = 0.3214).
Six patients (20%) discontinued baricitinib within 3 months (two in 1 month, four in 3 months) due to insufficient efficacy; four of them switched to upadacitinib and two switched to dupilumab. EASI at the withdrawal was 16.2 ± 8.0, which was significantly higher than that at 3 months in patients who continued baricitinib (8.67 ± 14.0, Mann Whitney test, p = 0.0171).
Regarding laboratory findings concerning the safety of baricitinib [7], no significant changes were observed (ordinary one-way analysis of variance or Kruskal-Wallis test) except for increased serum creatine phosphokinase (CPK) levels at 3 months compared with baseline values (Dunn’s multiple comparisons test, baseline vs. at 3 months, 106.5 ± 57.0 vs. 150.7 ± 79.2, p = 0.0297; Figure 3). As for adverse effects, acne and/or folliculitis was observed in seven patients (23.3%), elevated serum CPK levels in seven patients (23.3%), elevated liver enzymes in one patient (3.3%), and elevated total bilirubin in one patient (3.3%). No patient discontinued baricitinib due to these adverse effects. One case (3.3%) of herpes zoster was observed after 3 months of administering baricitinib, leading to discontinuation of baricitinib. One case (3.3%) of ocular herpes was observed after 3 months of administering baricitinib, leading to dose reduction. No malignancy, thrombosis/embolism, or cardiovascular event was observed.
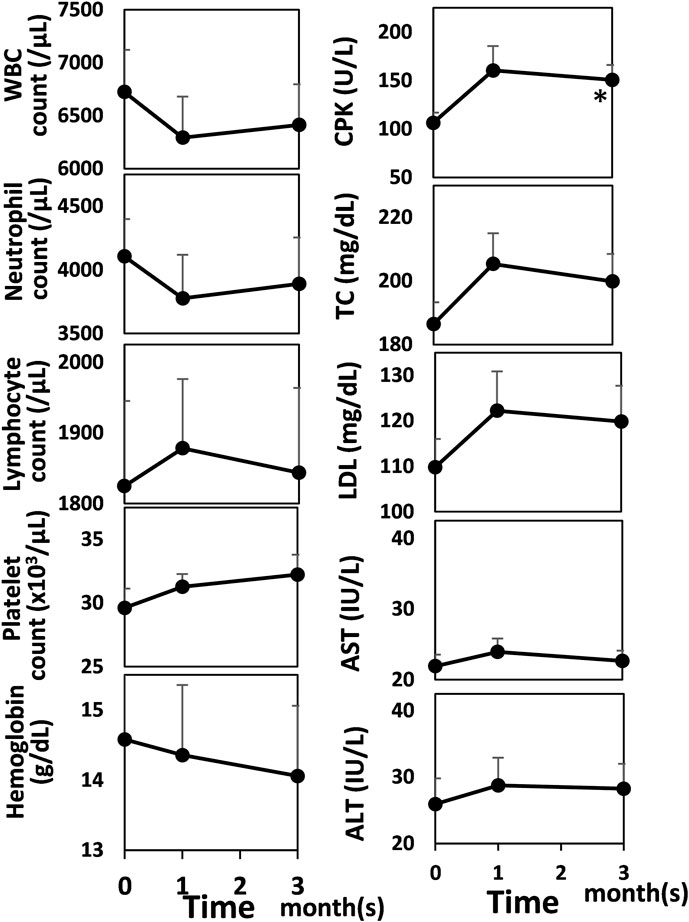
FIGURE 3. Laboratory data concerning the safety of baricitinib before and after baricitinib treatment for AD. Mean values are shown and bars represent standard errors. Shapiro-Wilk test was performed to test for normality, and then Dunnett’s test was conducted for comparison. *p < 0.05. WBC, white blood cell; CPK, creatine phosphokinase; TC, total cholesterol; LDL, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
The effectiveness and safety profiles in our study were similar to those obtained in clinical trials [2] and other real-world data [8–10]. In addition, our study demonstrated favorable laboratory data in AD patients receiving baricitinib in the real-world setting for the first time, as in clinical trials [7].
Our study indicated that AD biomarkers did not reflect clinical improvement during baricitinib treatment, whereas Uchiyama et al. [8] reported improvements in eosinophil count and serum thymus and activation-regulated chemokine (TARC) levels in a study of 14 patients. According to the report by Hagino et al. [9], after baricitinib treatment, eosinophil count and serum levels of TARC and lactate dehydrogenase (LDH) significantly decreased at week 4, compared with baseline levels. However, TARC and LDH levels significantly increased at week 12, compared with those at week 4. The IgE value was not altered at week 4 or 12, compared with baseline level. This inconsistency among these reports suggests that these biomarkers could not reflect clinical improvement during baricitinib treatment.
Thyssen et al. [11] identified and characterized patients who are likely to benefit most from baricitinib by analyzing data of clinical trials. They revealed that patients with moderate-to-severe AD and BSA affecting 10%–40% and severe itch were characterized as likely to benefit most from baricitinib 4-mg topical corticosteroid combination therapy, and were most likely to show favorable response rates in improving AD signs and symptoms, specifically itch. Furthermore, Silverberg et al. [12] identified responders/partial responders and non-responders at 16 weeks after baricitinib treatment, then, compared baseline characteristics including EASI and affected BSA. They revealed that the baseline EASI and affected BSA in responders/partial responders were lower than those in non-responders, indicating that moderate AD patients could benefit more from baricitinib than severe AD patients. In our study, for exploring whether baseline clinical severity affected effectiveness, we compared baseline EASI and affected BSA in responders with non-responders; however, no significant difference was observed. Further accumulation of real-world data is needed clarify this difference between clinical trials and real-word data.
In conclusion, baricitinib showed mild effectiveness and favorable safety including laboratory findings. Biomarkers did not reflect clinical improvement. Further accumulation of real-world data is needed to identify patients who can benefit most from baricitinib.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review board of Teikyo University. The studies were conducted in accordance with the local legislation and institutional requirements. An opt-out method on our website was used to collect informed consent.
Author contributions
AW, MK, SS, YO, MI, IM, HU, SE, CC, AH, SF, KH, AF, TT, TI, and YT performed the research and collected the data. MK and YT designed the research study. AW and MK analyzed the data. AW and MK wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
MK and YT received honoraria for lectures from Ely Lilly. YT received a grant for research from Ely Lilly that is not related to this article.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Part of our results was presented at EDVA Conference 2023 at Berline, Germany [13].
References
1. Kamata, M, and Tada, Y. Optimal use of jak inhibitors and biologics for atopic dermatitis on the basis of the current evidence. JID Innov (2023) 3:100195. doi:10.1016/j.xjidi.2023.100195
2. Simpson, EL, Lacour, JP, Spelman, L, Galimberti, R, Eichenfield, LF, Bissonnette, R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol (2020) 183:242–55. doi:10.1111/bjd.18898
3. Reich, K, Kabashima, K, Peris, K, Silverberg, JI, Eichenfield, LF, Bieber, T, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol (2020) 156:1333–43. doi:10.1001/jamadermatol.2020.3260
4. Katoh, N, Takita, Y, Isaka, Y, Nishikawa, A, Torisu-Itakura, H, and Saeki, H. Pooled safety analysis of baricitinib in adult participants with atopic dermatitis in the Japanese subpopulation from six randomized clinical trials. Dermatol Ther (Heidelb) (2022) 12:2765–79. doi:10.1007/s13555-022-00828-5
5. Uchida, H, Kamata, M, Mizukawa, I, Watanabe, A, Agematsu, A, Nagata, M, et al. Real-world effectiveness and safety of dupilumab for the treatment of atopic dermatitis in Japanese patients: a single-centre retrospective study. Br J Dermatol (2019) 181:1083–5. doi:10.1111/bjd.18163
6. Kamata, M, and Tada, Y. A literature review of real-world effectiveness and safety of dupilumab for atopic dermatitis. JID Innov (2021) 1:100042. doi:10.1016/j.xjidi.2021.100042
7. Bieber, T, Thyssen, JP, Reich, K, Simpson, EL, Katoh, N, Torrelo, A, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol (2021) 35:476–85. doi:10.1111/jdv.16948
8. Uchiyama, A, Fujiwara, C, Inoue, Y, and Motegi, SI. Real-world effectiveness and safety of baricitinib in Japanese patients with atopic dermatitis: a single-center retrospective study. J Dermatol (2022) 49:469–71. doi:10.1111/1346-8138.16350
9. Hagino, T, Saeki, H, Fujimoto, E, and Kanda, N. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol (2023) 50:869–79. doi:10.1111/1346-8138.16763
10. Vittrup, I, Elberling, J, Skov, L, Ibler, KS, Jemec, GBE, Mortz, CG, et al. Short-term real-world experience with baricitinib treatment in Danish adults with moderate-severe atopic dermatitis. J Eur Acad Dermatol Venereol (2023) 37:e543–e546. doi:10.1111/jdv.18804
11. Thyssen, JP, de Bruin-Weller, M, Costanzo, A, Grond, S, Schuster, C, Liu, C, et al. Baseline body surface area and itch severity define response to baricitinib in patients with moderate-to-severe atopic dermatitis at week 16. Adv Ther (2023) 40:3574–87. doi:10.1007/s12325-023-02528-8
12. Silverberg, JI, Simpson, EL, Thyssen, JP, Werfel, T, Cardillo, TE, Colvin, S, et al. Long-term efficacy (up to 68 weeks) of Baricitinib in combination with topical corticosteroids in adult patients with moderate-to-severe atopic dermatitis: analysis of treatment responders, partial responders and nonresponders originating from study BREEZE-AD7. J Eur Acad Dermatol Venereol (2023) 37:1036–45. doi:10.1111/jdv.18816
13. Watanabe, A, Kamata, M, Okada, Y, Suzuki, S, Ito, M, Mizukawa, I, et al. Real-world effectiveness and safety of baricitinib and its effect on biomarkers and laboratory data concerning its safety in Japanese adult patients with atopic dermatitis: A single-center retrospective study. Berlin, Germany: ePoster presentation, EADV Congress (2023). Available at: https://eadv.org/wp-content/uploads/scientificabstracts/EADV-congress-2023/Atopic-dermatitis-eczema.pdf.
Keywords: atopic dermatitis, baricitinib, Janus kinase, Janus kinase inhibitor, real world data
Citation: Watanabe A, Kamata M, Okada Y, Suzuki S, Ito M, Mizukawa I, Uchida H, Egawa S, Chijiwa C, Hiura A, Fukaya S, Hayashi K, Fukuyasu A, Tanaka T, Ishikawa T and Tada Y (2024) Real-world effectiveness and safety of baricitinib including its effect on biomarkers and laboratory data in Japanese adult patients with atopic dermatitis: a single-center retrospective study. J. Cutan. Immunol. Allergy 7:12455. doi: 10.3389/jcia.2024.12455
Received: 22 November 2023; Accepted: 12 February 2024;
Published: 23 February 2024.
Copyright © 2024 Watanabe, Kamata, Okada, Suzuki, Ito, Mizukawa, Uchida, Egawa, Chijiwa, Hiura, Fukaya, Hayashi, Fukuyasu, Tanaka, Ishikawa and Tada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Kamata, bWthbWF0YS10a3lAdW1pbi5hYy5qcA==
 Ayu Watanabe
Ayu Watanabe Masahiro Kamata
Masahiro Kamata