Abstract
Mycosis fungoides (MF) is a type of primary cutaneous T-cell lymphoma. The anti-cluster of differentiation (CD) 30 antibody agent, brentuximab vedotin (BV), has recently been developed for specific targets against CD30-expressed tumor cells with high efficacy against various lymphomas. Herein, we present a case of marginally CD30-expressed MF successfully treated with BV rechallenge.
Mycosis fungoides (MF) is a type of primary cutaneous T-cell lymphoma [1, 2]. The anti-cluster of differentiation (CD) 30 antibody agent, brentuximab vedotin (BV), has recently been developed for specific targets against CD30-expressed tumor cells with high efficacy against various lymphomas [3–7]. Herein, we present a case of marginally CD30-expressed MF successfully treated with BV rechallenge.
A 70-year-old man with previously identified early-stage MF was treated for 2 years at another institution with skin-targeted therapies such as narrowband ultraviolet B phototherapy and etretinate or interferon-gamma. Physical examination revealed multiple dark red papules and plaques throughout the entire body (Figure 1A). F-18 fluorodeoxyglucose positron emission tomography/computed tomography revealed accumulated lesions in the cervical, parotid, and axillary lymph nodes.
FIGURE 1
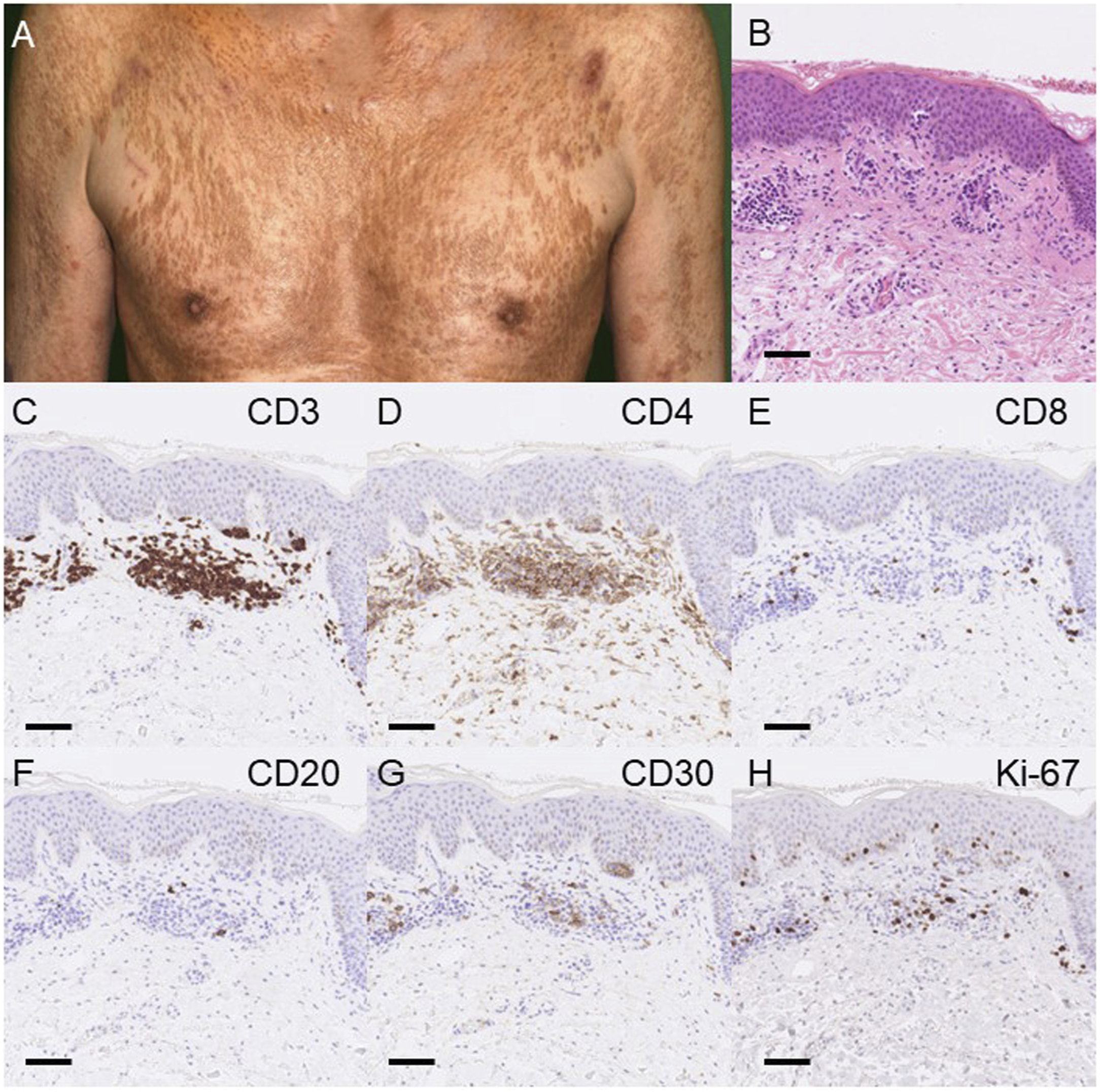
Clinical manifestations and histological analysis. (A) Clinical manifestation. Multiple dark red papules and plaques were observed throughout the entire body. (B) Histological analysis with hematoxylin and eosin staining. Skin biopsy showed an infiltration of small to medium-sized atypical lymphoid cells with hyperchromatic and irregular-shaped nuclei occasionally admixed with larger cells in the upper dermis and the epidermis (C–H). Immunostaining for CD3 (C), CD4 (D), CD8 (E), CD20 (F), CD30 (G), and Ki-67 (H). Immunohistochemical examination showed that atypical lymphoid cells were positive for CD3 and CD4, and 18.2% were also positive for CD30 in the atypical lymphocytes with a negative CD20. Twenty-five percent of the atypical lymphocytes in the skin were positive for Ki-67. Scale bar, 100 μm.
A skin biopsy revealed infiltration of small to medium-sized atypical lymphoid cells occasionally admixed with larger cells in the upper dermis and often infiltrating into the epidermis (Figure 1B). Atypical lymphoid cells were positive for CD3 and CD4, and some of them (18.2%) were also positive for CD30 (Figures 1C–H). In addition to plaques more than 10% of the body surface area of the skin, numerous atypical lymphocytes or small clusters of three to six cells were observed on lymph node biopsy indicating National Cancer Institute-Lymph Nodes (NCI-LN) 2 with negative clones. Although blood involvement quantification was not possible according to the guidelines, the presence of atypical lymphocytes in the peripheral blood exceeded 5%. Furthermore, no visceral involvement was found, leading to the diagnosis of mycosis fungoides T2BN1AM0BXA, Stage ⅡB based on the recently updated classification [2, 8].
In addition to CD30-positive cells, poor prognostic factors include advanced age at onset and male sex, indicating unfavorable clinical progress. Furthermore, because oral etanercept, bexarotene, and IFN were ineffective, BV was administered by physicians in the hematology department. Eight months after BV administration, skin eruptions improved without adverse reactions.
Mycosis fungoides was maintained with a complete response to skin-targeted treatment alone for 7 months. However, skin eruption flared up and the patient was started on bexarotene treatment, which was ineffective for skin eruption and peripheral lymphoid cells (2%) and swollen lymph nodes were observed when the disease advanced into T2AN2AM0BxA, Stage IIB, according to the recently updated classification [2, 8]. BV was rechallenged for mycosis fungoides, which achieved a complete response and is still ongoing for skin eruptions.
BV is effective in patients with CD30-positive MF and primary cutaneous anaplastic large cell lymphoma. Some studies indicated that BV treatment had better response rates and progression-free survival than other treatments in patients with CD30-positive MF, and these cases were independent of the degree of CD30 expression [9].
A previous study reported the importance of rechallenge treatment with BV in Hodgkin’s lymphoma, and a reduction in the measurable tumor volume [10] was exhibited. Therefore, BV may be a candidate therapeutic option independent of CD30 expression in MF. However, further investigations are necessary to clarify the actual effects of BV treatment in these cases.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Because this is a single case report, ethics approval was not required for this study. The patient gave us consent for their photographs and medical information to be published in print and online with the understanding that this information is publicly available.
Author contributions
HK, EO, and YS wrote manuscript and made figures. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Latzka J Assaf C Bagot M Cozzio A Dummer R Guenova E et al EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - update 2023. Eur J Cancer (2023) 195:113343. 10.1016/j.ejca.2023.113343
2.
Olsen EA Whittaker S Willemze R Pinter-Brown L Foss F Geskin L et al Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood (2022) 140(5):419–37. 10.1182/blood.2021012057
3.
Prince HM Kim YH Horwitz SM Dummer R Scarisbrick J Quaglino P et al Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet (2017) 390(10094):555–66. 10.1016/S0140-6736(17)31266-7
4.
Kim M Lee JO Koh J Kim TM Lee JY Jeon YK et al A phase II study of brentuximab vedotin in patients with relapsed or refractory Epstein-Barr virus-positive and CD30-positive lymphomas. Haematologica (2021) 106(8):2277–80. 10.3324/haematol.2021.278301
5.
Ribereau-Gayon E Donzel M Pham F Romain-Scelle N Perier-Muzet M Balme B et al Brentuximab-vedotin in combination with cyclophosphamide, doxorubicin, prednisolone for the treatment of aggressive CD30-positive cutaneous T-cell lymphomas. Leuk Lymphoma (2023) 64(8):1424–32. 10.1080/10428194.2023.2216820
6.
Lewis DJ Haun PL Samimi SS Vittorio CC Villasenor-Park J Barta SK et al Brentuximab vedotin for relapsed or refractory sézary syndrome. JAMA Dermatol (2021) 157(3):317–21. 10.1001/jamadermatol.2020.4901
7.
Horwitz S O'Connor OA Pro B Illidge T Fanale M Advani R et al Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (2019) 393(10168):229–40. 10.1016/S0140-6736(18)32984-2
8.
Hristov AC Tejasvi T Wilcox RA . Cutaneous B-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am J Hematol (2023) 98(1):1209–13. 10.1002/ajh.25970
9.
Kim YH Prince HM Whittaker S Horwitz SM Duvic M Bechter O et al Response to brentuximab vedotin versus physician's choice by CD30 expression and large cell transformation status in patients with mycosis fungoides: an ALCANZA sub-analysis. Eur J Cancer (2021) 148:411–21. 10.1016/j.ejca.2021.01.054
10.
Bartlett NL Chen R Fanale MA Brice P Gopal A Smith SE et al Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol (2014) 7:24. 10.1186/1756-8722-7-24
Summary
Keywords
mycosis fungoides, CD30, brentuximab vedotin, refractory, lymphoma
Citation
Kawahara H, Okada E and Sawada Y (2024) Rechallenge of brentuximab vedotin was effective for refractory mycosis fungoides: a case report. J. Cutan. Immunol. Allergy 7:12474. doi: 10.3389/jcia.2024.12474
Received
27 November 2023
Accepted
15 January 2024
Published
25 January 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Kawahara, Okada and Sawada.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hikaru Kawahara, hikaru-n@med.uoeh-u.ac.jp; Yu Sawada, long-ago@med.uoeh-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.