Dear Editors,Dupilumab is an anti-human IL-4/13 receptor monoclonal antibody [1] used for patients with atopic dermatitis (AD) who have shown an inadequate response to existing therapies. When dupilumab was introduced for AD treatment in the United States and Europe, the goal was to achieve a long drug survival period [2]. However, according to the Japanese guidelines for promoting optimal use [3], discontinuation of dupilumab could be considered approximately 6 months after remission of the skin rash. We previously reported that when dupilumab was discontinued in patients with AD who responded well, majority of the patients remained almost eczema-free (bio-free remission) for a long time period, whereas some patients experienced a relapse of skin rash within a few months [4]. However, reports in clinical practice on the favorability of dupilumab re-administration in patients whose AD skin rash recurred after its discontinuation are absent. Here, we report 10 patients with AD who were re-administered dupilumab.
This study was approved by the Hyogo Medical University Ethics Review Board. The study included 10 patients (eight males and two females) who underwent follow-ups for at least 16 weeks after dupilumab-retreatment from a total of 109 patients with AD who began dupilumab treatment in our hospital between April 2018 and July 2020. Topical steroids or calcineurin inhibitors were administered daily or intermittently, respectively. All patients received 600 mg of dupilumab as a loading or reloading dose, followed by 300 mg of dupilumab every 2 weeks.
The patient clinical courses are shown in Figure 1A. All 10 patients diagnosed with AD were administered dupilumab for 53.5 ± 14.2 (mean ± standard deviation) weeks and it was discontinued for 34.5 ± 49.4 weeks because of eczema remission; it was then re-administered because of skin rash relapse. The patients were followed up for 77.2 ± 26.8 weeks after re-administration and medication was not interrupted during that time.
FIGURE 1
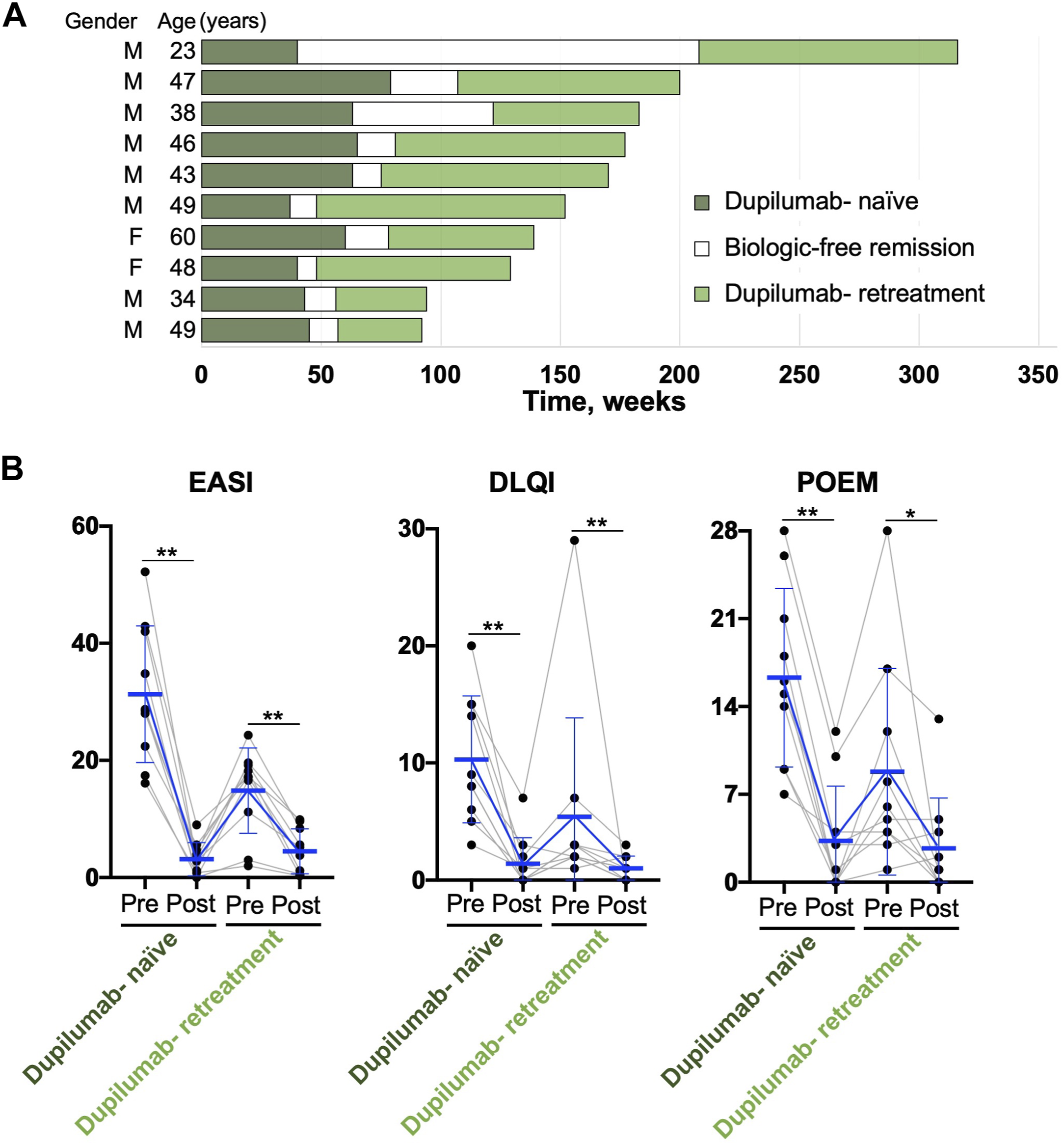
(A) Clinical information of each patient. M, Male; F, Female. (B) Changes in clinical data before (Pre) and after (Post) dupilumab treatment. The Pre and Post dates are dates of the start of administration and decision to end administration (or the last follow-up date), respectively. The last observation carried forward method was used to impute missing values. Each dot represents a value for each patient. The blue bold lines represent the estimated mean and the thin lines represent the standard deviation intervals. The data were analyzed using GraphPad Prism version 8 (GraphPad Software Inc., CA, United States). The Wilcoxon matched-pairs signed rank test was used to assess statistical significance. **p < 0.01; *p < 0.05. EASI, Eczema Area and Severity Index; DLQI, Dermatology Life Quality Index; POEM, Patient-Oriented Eczema Measure.
Patient clinical characteristics at the time of re-administration were as follows:
• Age: 43.7 ± 10.1 (mean ± standard deviation) years
• Eczema Area and Severity Index (EASI): 14.9 ± 7.3
• Dermatology Life Quality Index (DLQI): 5.4 ± 8.4
• Patient-Oriented Eczema Measure (POEM): 8.8 ± 8.2
All patients completed the 16-week retreatment period (
Figure 1A). At the latest follow-up, the following findings were obtained (
Figure 1B):
• EASI: 4.5 ± 3.8
• DLQI: 1.0 ± 1.1
• POEM: 3.0 ± 4.0
In a phase 3 open-label extension study, the dupilumab-naïve and retreatment subgroups showed improvement in EASI, consistent with the overall population [5]. However, in this phase 3 study, dupilumab was administered at various doses and dosing intervals. Therefore, this is the first report on dupilumab efficacy in the retreatment and dupilumab-naïve groups when AD was treated with dupilumab at the currently approved dosage in adults. No specific side effects were associated with dupilumab re-administration. Despite the small number of cases, this study suggests that dupilumab is effective when re-administered after discontinuation in real-world practice.
Statements
Data availability statement
Supporting data of this study are available from the corresponding author on request.
Ethics statement
The studies involving humans were approved by the Hyogo Medical University Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study has been certified by the Ethics Committee to omit written consent (Permit No. 3273). The reason for this is that this is a retrospective study that does not involve anything other than referring to medical records, and the opportunity to refuse participation (out-out) is provided by posting a document (information disclosure statement) on the website of the University of Hyogo Medical School regarding clinical research using the information in our possession. The legal basis in Japan is the national guideline “Ethical Guidelines for Medical Research Involving Human Subjects, Chapter 5: Informed Consent, etc., Section 12 1 (2) Procedures for Obtaining Informed Consent, etc.”
Author contributions
MM and YIm wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the Japan Society for the Promotion of Science Grantsin-Aid for Scientific Research (KAKENHI) Grant Number 21K08337 for YIm.
Acknowledgments
We would like to thank Editage for English language editing.
Conflict of interest
MM, YIm, MN, and NK received honoraria for lectures from Sanofi K. K.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Blauvelt A de Bruin-Weller M Gooderham M Cather JC Weisman J Pariser D et al Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303. 10.1016/S0140-6736(17)31191-1
2.
Spekhorst LS de Graaf M Zuithoff NPA van den Reek JMPA Kamsteeg M Boesjes CM et al Dupilumab drug survival and associated predictors in patients with moderate to severe atopic dermatitis: long-term results from the daily practice BioDay registry. JAMA Dermatol. 2022;158(9):1048–56. 10.1001/jamadermatol.2022.3014
3.
Matsutani M Imai Y Inoue Y Hosotani Y Kusakabe M Natsuaki M et al Real-world use of dupilumab for 53 patients with atopic dermatitis in Japan. J Cutan Immunol Allergy. 2020;3(2):35–6. 10.1002/cia2.12099
4.
Miyamoto S Imai Y Natsuaki M Yamanishi K Kanazawa N . Long-term remission of atopic dermatitis after discontinuation of dupilumab. Acta Dermato-Venereologica2022; 102: adv00731, 10.2340/actadv.v102.295
5.
Deleuran M Thaçi D Beck LA de Bruin-Weller M Blauvelt A Forman S et al Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–88. 10.1016/j.jaad.2019.07.074
Summary
Keywords
dupilumab, atopic dermatitis, eczema, skin rash, biologic-free remission
Citation
Matsutani M, Imai Y, Miyamoto S, Inoue Y, Natsuaki M and Kanazawa N (2024) Real-world efficacy of dupilumab re-administration after discontinuation in patients with atopic dermatitis. J. Cutan. Immunol. Allergy 7:12480. doi: 10.3389/jcia.2024.12480
Received
27 November 2023
Accepted
08 January 2024
Published
22 January 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Matsutani, Imai, Miyamoto, Inoue, Natsuaki and Kanazawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasutomo Imai, imai-yas@hyo-med.ac.jp
ORCID: Yasutomo Imai, orcid.org/0000-0003-3169-5717; Nobuo Kanazawa, orcid.org/0000-0003-3000-9711
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.