Dear Editors,
Tyrosine kinase 2 (TYK2) is a member of Janus kinase (JAK) family. Although, clinical efficacy of TYK2 inhibitor, deucravacitinib, for psoriatic arthritis (PsA) has been reported [1], the radiological assessment was not conducted in the study. We herein report the first case of radiological improvement in PsA after deucravacitinib treatment.
A 46-year-old Japanese woman with a 25-year history of plaque psoriasis had been treated with 8 mg weekly of methotrexate for the last 2 years for concomitant PsA on the right wrist, fingers, and knee, which developed 3 years before. While her joint symptoms improved, skin lesions were not sufficiently ameliorated. At presentation, she had extensive scaly erythema on the trunk and extremities, with psoriasis area and severity index (PASI) and body surface area (BSA) 14.8% and 28%, respectively (Figure 1A). She was enrolled in POETYK PSO-4 trial (NCT03924427), a prospective, open-label, single-arm, phase 3 trial evaluating efficacy and safety of deucravacitinib for moderate to severe plaque psoriasis and its extension trail POETYK PSO-LTE (NCT04036435). Two years later, her PASI and BSA improved to 1 and 0.5, respectively, without adverse events (Figure 1B). Although joint assessment was not included in the study protocols, clinical and radiographical assessment for joint symptoms was also conducted. Her arthritis remained in clinical remission during the 2 years, and X-ray image showed disappearance of bone erosion in the right third distal interphalangeal joint (DIP) as compared to the findings before the initiation of deucravacitinib treatment (Figures 1C–E).
FIGURE 1
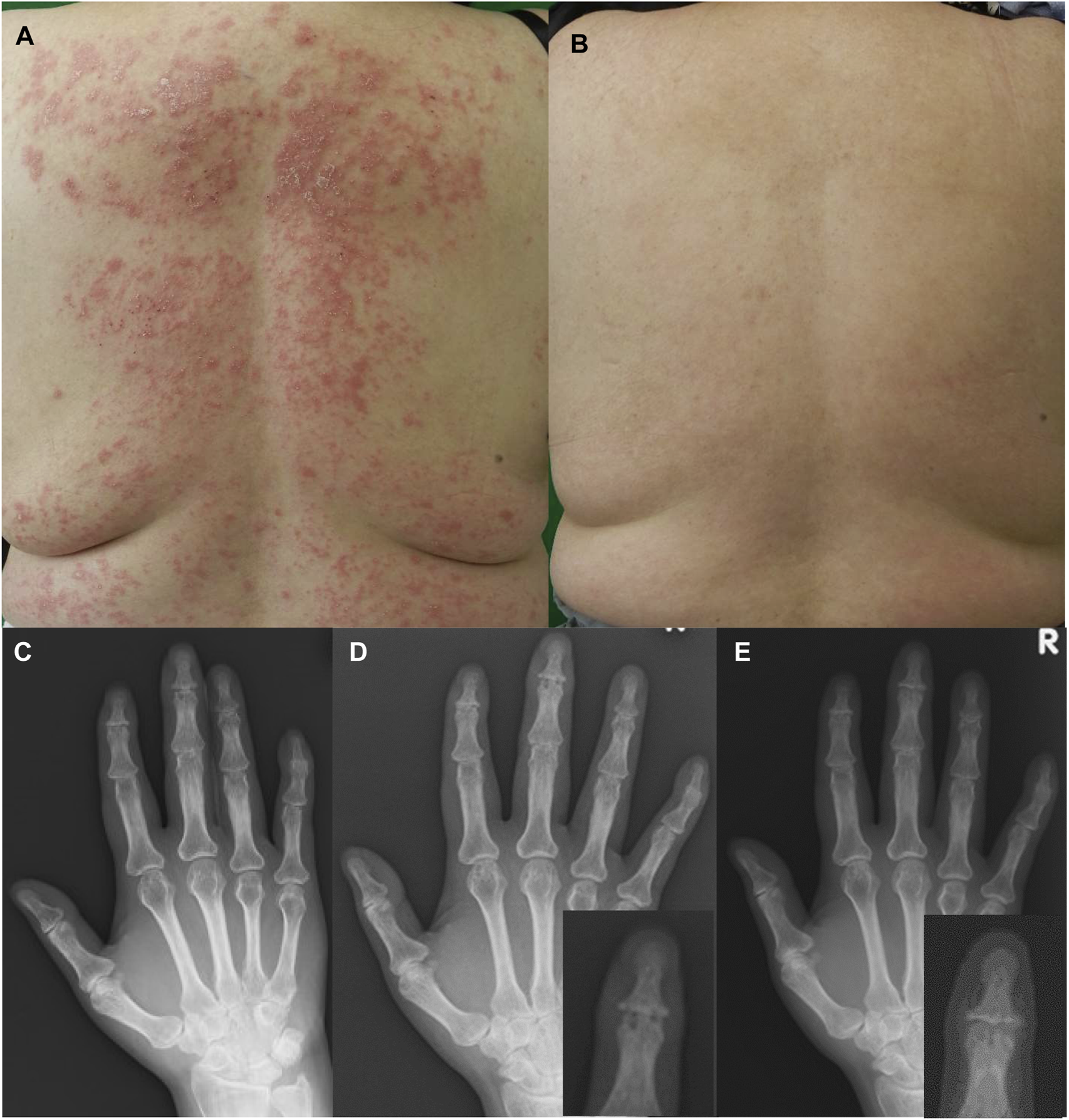
Clinical features of plaque psoriasis on the back (A) before and (B) 48-week after treatment with deucravacitinib. X-ray showed a bone erosion in the right third distal interphalangeal joint (C) 1-year before deucravacitinib treatment and (D) at the initiation of deucravacitinib treatment. (E) Magnified images of the third distal interphalangeal joint are shown in right lower panels (D, E). Bone erosion in the right third distal interphalangeal joint disappeared 2 years after the treatment of deucrabacitinib.
Deucravacitinib is currently approved for plaque psoriasis but not for PsA. The involvement of TYK2 in the pathophysiology of psoriatic arthritis (PsA) is still to be elucidated. TYK2 medicates intracellular signaling of interferon-α/β, interleukin (IL)-12, IL-23, IL-10 family cytokines (IL-10, IL-26, and IL-22), IL-6, and IL-13, by pairing with JAK1 or JAK2 [2]. On the other hand, IL-23 and its downstream IL-17 are pathogenic cytokines not only in plaque psoriasis but also in PsA [3]. Structural bone damage is a feature of PsA as a result of joint inflammation affecting cartilage and bone. Recently, the efficacy of IL-6 inhibitors for joint/bone repair in inflammatory arthritis draws much attention. In rheumatoid arthritis, an IL-6 inhibitor tocilizumab has been shown to repair bone erosion [4]. Moreover, Kutsuna et al. reported effectiveness of tocilizumab in a patient with progressive and destructive PsA [5]. They confirmed radiological repair of the bone cyst at the lateral humeral condyle in the elbow and repair of the DIP joints [5]. Since deucravacitinib also suppresses IL-6 signaling, inhibition of IL-6 in addition to IL-23 may have contributed to bone erosion repair in our case. Further research is necessary to elucidate the efficacy of deucravacitinib for PsA.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The patients provided informed consent for the publication of the images submitted with this article.
Author contributions
YN and HF collected the data, all participated in analyzing the results of the data and reviewing the manuscript, and NI and HF wrote the manuscript. All members approved the final draft.
Acknowledgments
This is a case report of a patient who was enrolled in a phase 3 open-label clinical trial of deucravacitinib, a drug developed by Bristol Myers Squibb. Bristol Myers Squibb has approved publication of this case report. We did not receive any funds to develop and publish this article.
Conflict of interest
NI has received honoraria for speaker from AbbVie, Amgen, Asahi-Kasei, Eisai, Janssen, Kyowa-Kirin, Maruho, Novartis, Taiho, and UCB. YN has received honoraria for speaker from Asahi-Kasei, Eisai, Eli Lilly, Mitasubishi-Tanabe, and Nippon Shinyaku. HN has received research grant or honoraria for speaker from AbbVie, Asahi Kasei, Astellas, Astra Zeneka, Boehringer-Ingelheim, Bristol Myers Squibb, Chugai, Eisai, Eli Lilly, Glaxo Smith Kline, Janssen, Mitsubishi Tanabe, Nippon Shinyaku, Novartis, Ono, Pfizer, Taisho, Takeda, and UCB. HF has received research grant or honoraria for speaker and/or consultancy from AbbVie, Amgen, Boehringer-Ingelheim, Bristol Myers Squibb, Eisai, Eli Lilly, Kyowa Kirin, LEO, Mitsubishi Tanabe, Novartis, Janssen, Maruho, Sanofi, Sun Pharma, Torii, Taiho, and UCB.
References
1.
Mease PJ Deodhar AA van der Heide D Behrens F Kivitz AJ Neal J et al Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis (2022) 81:815–22. 10.1136/annrheumdis-2021-221664
2.
Strobl B Stoiber D Sexl V Mueller M . Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front Biosci (2011) 16:3214–32. 10.2741/3908
3.
Ritchlin CT Colbert RA Gladman DD . Psoriatic arthritis. N Engl J Med (2017) 376:957–70. 10.1056/NEJMra1505557
4.
Finzel S Kraus S Figueiredo CP Regensburger A Kocijan R Rech J et al Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann Rheum Dis (2019) 78:1186–91. 10.1136/annrheumdis-2018-214894
5.
Kutsuna T Hino K Hasegawa H Watamori K Kidani T Imai H et al Psoriatic arthritis successfully treated with second-line anti-interleukin-6 treatment: a case report and review of the literature. J Med Case Rep (2022) 16:402. 10.1186/s13256-022-03624-z
Summary
Keywords
psoriatic arthritis, plaque psoriasis, TKY2 inhibitor, deucravacitinib, bone erosion
Citation
Ikumi N, Nagasawa Y, Nakamura H and Fujita H (2024) Radiological improvement of peripheral arthritis in a patient with psoriatic arthritis treated with deucravacitinib. J. Cutan. Immunol. Allergy 7:12486. doi: 10.3389/jcia.2024.12486
Received
28 November 2023
Accepted
16 January 2024
Published
29 January 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Ikumi, Nagasawa, Nakamura and Fujita.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hideki Fujita, fujita.hideki@nihon-u.ac.jp
ORCID: Natsumi Ikumi, orcid.org/0000-0002-3193-5779; Hideki Fujita, orcid.org/0000-0001-8657-0618
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.