- Department of Dermatology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan
Dear Editors,
Prurigo is a reactive skin disorder marked by pruriginous lesions such as papules, nodules, and erythema. In Japan it is classified into three types according to clinical skin symptoms: prurigo nodularis (PN), prurigo chronica multiformis (PCM), prurigo (not otherwise specified). In recent years, several studies have suggested that Th2 cytokines are involved in the pathological mechanisms of prurigo [1, 2]. The transcript levels of Th2 cytokines including interleukin (IL)-4 and IL-31 were reported to be significantly higher in papulonodular lesions of subacute/chronic prurigo compared with normal skin and psoriatic lesions [1]. Another study demonstrated that keratinocytes in PN present more marked nuclear translocation of pSTAT6 and pSTAT3 than of pSTAT1 [2].
Dupilumab is a humanized monoclonal antibody that targets the alpha chain of the IL-4 receptor to block IL-4/13 signaling, and is an effective drug for type 2 inflammation-associated diseases such as atopic dermatitis, bronchial asthma, and chronic rhinosinusitis with nasal polyps. Efficacy of dupilumab for treatment of PN has been reported recently [3]. The retrospective multiple-center study of 27 PN patients in Italy demonstrated that skin symptoms determined by investigator global assessment scale and itch numerous rating scale (NRS) were improved following the 16 weeks administration of dupilumab [3]. These findings support the idea that type 2 inflammation driven by IL-4 and IL-13 play important roles in the pathological mechanisms of PN. However, it remains unclear if the pathogenesis of PCM is also regulated by type 2 inflammation. To this end, we evaluated the therapeutic effects of dupilumab in PCM cases.
This pilot study enrolled two Japanese patients (49-year-old male and 59-year-old male) who were hospitalized due to PCM at the Department of Dermatology, Tokyo Medical and Dental University Hospital. The diagnosis of PCM was based on the clinical criteria presented in the Japanese Dermatological Association guidelines. These patients were treated with 300 mg dupilumab every 2 weeks for 6 weeks, following the initial administration of 600 mg dupilumab (Figure 1A) after the informed consent was obtained. We evaluated the visual analogue scale (VAS), skin scores, and blood biomarkers before and after the treatment (week -4-0, week 8 and week 16). The skin scores were calculated using the skin-score measurement scale as described in the legend of Figure 1. This study was approved by the ethics committee of Tokyo Medical and Dental University (Approval number: NR2019-003), registered on Japan Registry of Clinical Trials (Registration number: jRCTs031200073), and conformed to the Helsinki Declaration of 1975, as amended in 1983.
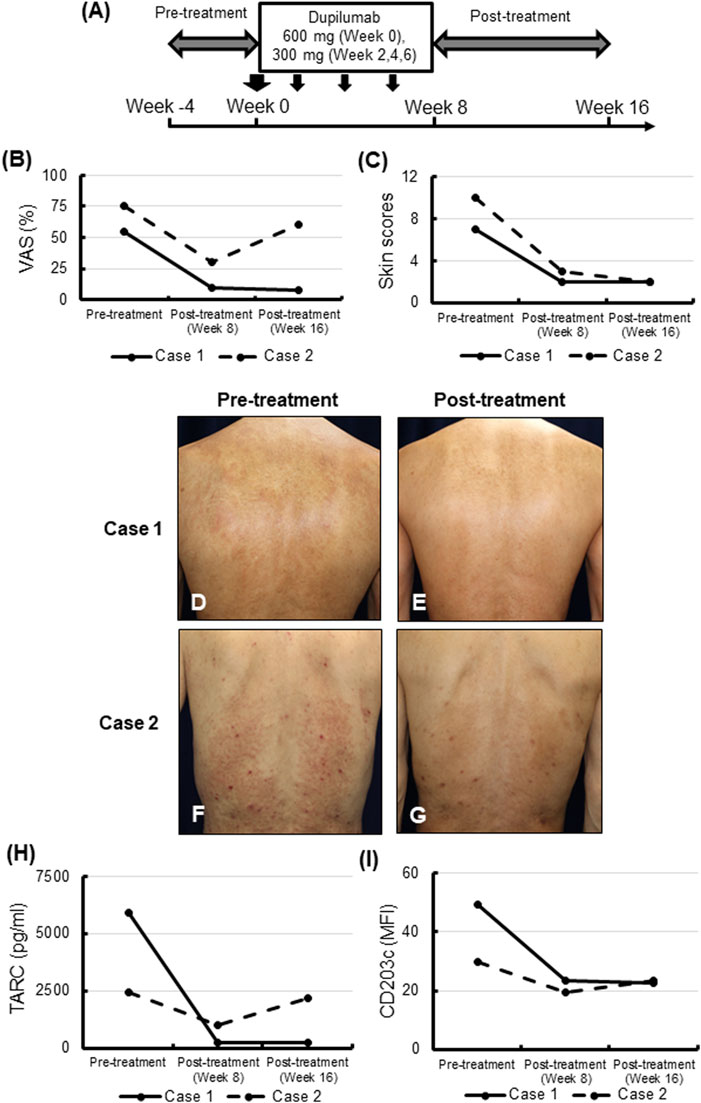
FIGURE 1. Visual analogue scale (VAS) scores, skin symptom, and blood biomarkers before and after treatment with dupilumab. (A) Study design. (B,C) VAS scores (B) and skin scores (C) of each case before (week 0) and after the 6-week treatment with dupilumab (week 8 and week 16). The skin scores were calculated using the skin-score measurement scale as described below. The number of lesions per body, thickness, body area (%), and redness of each skin symptom (papule, nodule, or erythema) were assessed on a 0–3 scale. The papule scales and the nodule scales were calculated by [lesions per body] × [thickness]. The erythema scales were calculated by [body area (%)] × [redness]. The total skin scores were expressed as the sum of each score. (D–G) Skin manifestations on the backs in case 1 (D,E) and case 2 (F,G) before (D,F) and after (E,G) the 6-week administration of dupilumab. Case 1, erythematous macules were spread over the back (D). Erythematous macules on the back were highly improved after the treatment (E). Case 2, erythematous macules and nodules associated with scratch marks were spread over the back (F). Erythematous macules and nodules on the back were highly improved after the treatment (G). (H,I) Serum TARC/CCL17 (H), and the mean fluorescence intensity (MFI) of CD203c expression on blood basophils (I) in the two cases before the 6-week administration of dupilumab, and after the treatment (week 8 and week 16).
The VAS scores representing pruritis were over 50% before the administration of dupilumab in both cases (Figure 1B). Following the administration of dupilumab four times, VAS scores at week 8 were 63.6% and 60% reduced in Case 1 and Case 2, respectively, compared with their baselines. Ten weeks after the last administration of dupilumab (week 16), pruritis flared up in Case 2, while it was stable in Case 1 (Figure 1B).
Skin symptoms were also improved after administration of dupilumab four times (Figures 1C–G). The skin scores at week 8 were 71.4% and 70% decreased in Case 1 and Case 2, respectively, and they were stable in both cases at week 16 (Figure 1C). In Case 2 at week 16 pruritis relapsed, but skin symptoms did not flare up.
While blood eosinophil counts decrease after treatment (at week 8). Moreover, they returned to the baseline level 10 weeks after the last administration of dupilumab (week 16) in both cases (data not shown). Serum levels of IgE declined after the administration of dupilumab in both cases (data not shown). Serum levels of TARC/CCL17 were higher than the normal range (<450 pg/mL) in both cases and were similarly decreased just after treatment (at week 8). At week 16, TARC/CCL17 returned to baseline in Case 2 in conjunction with relapse of pruritis, while levels remained stable in Case 1 (Figure 1H).
Our previous study demonstrated that basophil activation was observed in some patients with chronic prurigo [4]. The expression of CD203c on blood basophils was downregulated at week 8 in both cases; however, returned to baseline at week 16 in Case 2 (Figure 1I).
Here, this prospective clinical study showed the effectiveness of dupilumab, which targets major Th2 cytokines, IL-4 and IL-13, in cases with PCM for the first time. Pruritis and skin lesions were improved in both cases following a 6-week administration of dupilumab. In our previous study of 168 patients with chronic prurigo, the serum levels of TARC/CCL17 were higher in PCM patients than in PN patients [5], which suggests that PCM exhibits a more marked type 2 inflammatory pattern than does PN. In a similar manner, in both cases in the present study serum levels of TARC/CCL17 at baseline were higher than normal, and declined along with amelioration of itch and skin symptoms. This data supports the theory that IL-4 and IL-13, key players in type 2 inflammation, are involved in the pathological mechanisms of PCM. Moreover, the symptoms of PCM improved following a shorter dupilumab treatment period than was seen for PN in the previous trial [3].
Case 2 had a relapse of pruritis, but not of skin lesions, along with an increase of TARC/CCL17 serum level and upregulation of CD203c expression on blood basophils, within 10 weeks after the termination of dupilumab treatment. This result indicated that itch intensity in PCM might be correlated with basophil activation as well as type 2 inflammation.
In summary, two cases with PCM were successfully treated with dupilumab, indicating that type 2 inflammation is important for the pathogenesis of PCM. Further studies on a larger number of cases are required to confirm these results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Tokyo Medical and Dental University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TU and HY designed the study. TU contributed to data collection. TU and NO wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by KAKENHI from the Japan Society for the Promotion of Science (JSPS, 19K08767 for HY and TU) and the grant from the consortium of research on refractory diseases at TMDU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Chiyako Miyagishi for her technical assistance.
References
1. Park, K, Mori, T, Nakamura, M, and Tokura, Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol (2011) 21(1):135–6. doi:10.1684/ejd.2010.1196
2. Fukushi, S, Yamasaki, K, and Aiba, S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol (2011) 165(5):990–6. doi:10.1111/j.1365-2133.2011.10498.x
3. Chiricozzi, A, Maurelli, M, Gori, N, Argenziano, G, De Simone, C, Calabrese, G, et al. Dupilumab improves clinical manifestations, symptoms, and quality of life in adult patients with chronic nodular prurigo. J Am Acad Dermatol (2020) 83(1):39–45. doi:10.1016/j.jaad.2020.03.049
4. Ito, Y, Satoh, T, Takayama, K, Miyagishi, C, Walls, AF, and Yokozeki, H. Basophil recruitment and activation in inflammatory skin diseases. Allergy (2011) 66(8):1107–13. doi:10.1111/j.1398-9995.2011.02570.x
Keywords: dupilumab, prurigo chronica multiformis, prurigo nodularis, pruritis, type 2 inflammation
Citation: Ugajin T, Yokozeki H, Namiki T and Okiyama N (2024) Dupilumab for prurigo chronica multiformis: a pilot case study. J. Cutan. Immunol. Allergy 7:12518. doi: 10.3389/jcia.2024.12518
Received: 05 December 2023; Accepted: 08 January 2024;
Published: 19 January 2024.
Copyright © 2024 Ugajin, Yokozeki, Namiki and Okiyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsukasa Ugajin, dWdhamluLmRlcm1AdG1kLmFjLmpw
 Tsukasa Ugajin
Tsukasa Ugajin Hiroo Yokozeki
Hiroo Yokozeki Naoko Okiyama
Naoko Okiyama