Dear Editors,
A 55-year old man with a 10-year history of psoriasis vulgaris had shown little response to oral apremilast and was introduced to our department. On the first visit, he showed multiple scaly erythemas on the trunk and extremities (PASI:15, DLQI:10, BSA:18.5%) (Figure 1A). As the lower risk for severe infection was expected, the patient selected oral deucravacitinib for the next treatment. After 2 months of deucravacitinib application, skin lesions became widely in remission (PASI:6, DLQI: 4) (Figure 1B). However, soon after that, he suddenly developed painful photophobia and hyperemia of the left eye, and deucravacitinib was discontinued. Examination of the anterior segment of the left eye showed ciliary hyperemia and anterior chamber cells, while fundus findings included vitreous opacification and retinal hemorrhage. Branch retinal vein occlusion and uveitis were given as the ophthalmologic diagnosis, although their origin was unclear. By the application of tropicanide eye drop, 0.01% betamethasone butyrate ester eye drop, 0.3% gatifloxacin hydrate eye drop, and Tenon’s intracapsular triamcinolone acetonide injection, the hyperemia and swelling of his left eye were rapidly subsided. For skin lesions, topical betamethasone dipropionate ointment was applied as an alternative treatment. However, after 3 months of the discontinuation of deucravacitinib, his rash became in relapse and was again keratotic and wide-spread (Figure 1C). As uveitis was unilateral and had not been reported domestic and overseas as a side effect or paradoxical reaction of deucravacitinib, psoriatic uveitis was strongly suspected and deucravacitinib was restarted according to the patient’s consent. This time, his eye lesions did not deteriorate and skin condition again got improved. No uveitis findings were revealed in his left eye, even 3 months after the deucravacitinib restart.
FIGURE 1
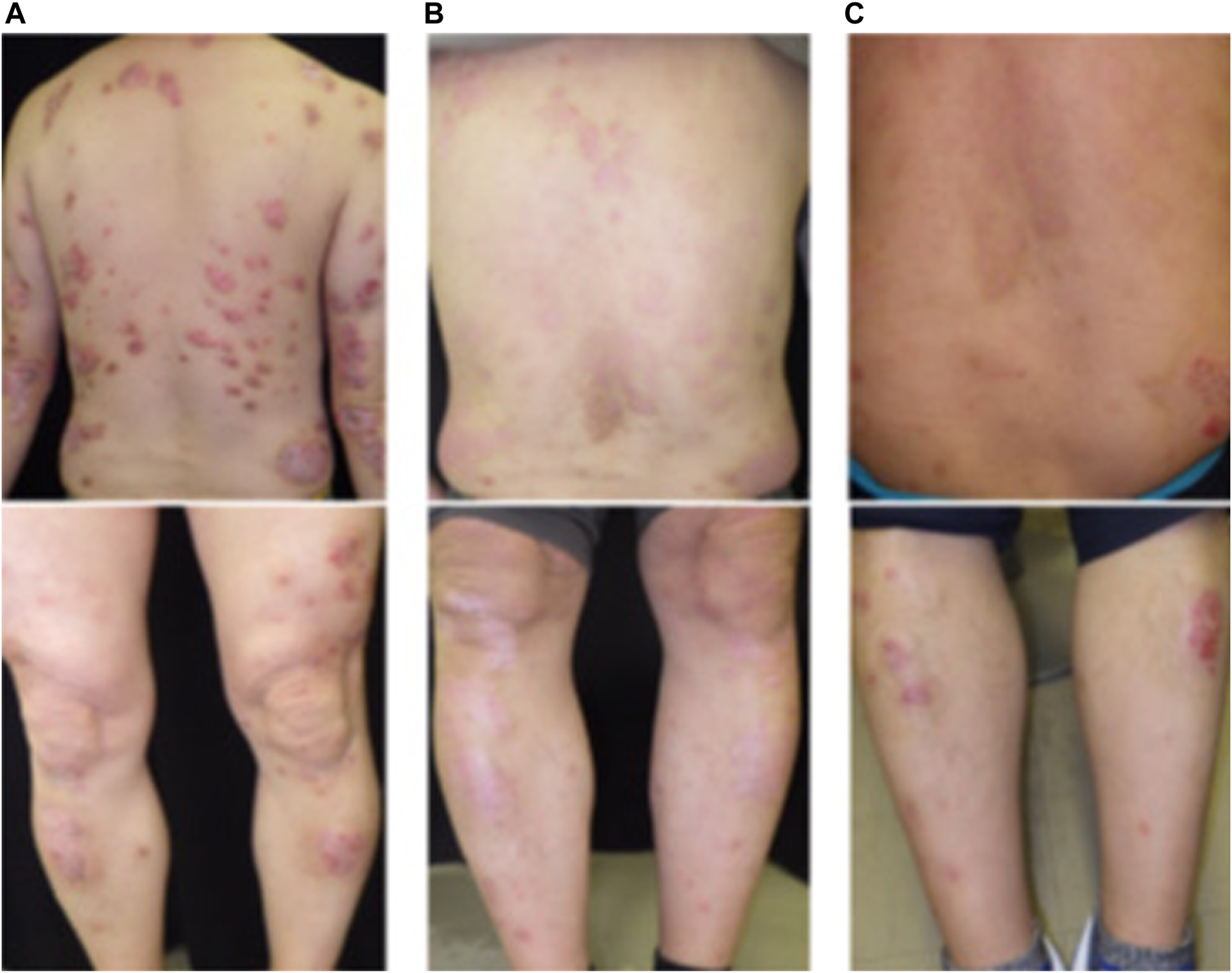
Skin manifestation in our case during the course of deucravacitinib treatment: (A) on the first visit, (B) 3 weeks after the deucravacitinib application, (C) 3 months after the deucravacitinib discontinuation.
Characteristic acute unilateral anterior uveitis observed in our case is not specific but compatible with psoriatic uveitis. Psoriatic uveitis is a rare complication of psoriasis and is reportedly recognized in nearly 3% of psoriasis patients [1, 2]. As the pathogenesis of psoriatic uveitis has not been well-clarified, therapeutic effect of deucravacitinib, a specific tyrosine kinase 2 inhibitor, on psoriatic uveitis might be less than that on psoriasis vulgaris [3]. Although patients with psoriatic arthritis and severe psoriasis are reportedly more prone to psoriatic uveitis, our case is neither so severe nor accompanied with arthritis. These facts suggest that, in our case, deucravacitinib might have induced uveitis as the paradoxical reaction even without reproducibility of the symptoms. Indeed, cases with biologics-induced uveitis and those with Janus kinase inhibitors-induced inflammatory skin diseases have been reported as the paradoxical reaction [4, 5]. Notably, when the original disease recurred after discontinuing the medication, readministration was successful in some cases, but in others it again induced the paradoxical reaction. In our case, no recurrence of uveitis findings has been observed after deucravacitinib was readministered, but careful follow-up is required. In order to verify the role of deucravacitinib on the development of uveitis in our case, further study of case series should be required.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This retrospective study was approved by the Ethics Committee of Hyogo Medical University. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Kobayashi S Kishimoto M . Comorbidities of psoriasis vulgaris. Pharma Med (2021) 38:10.
2.
Charlton T Green A Shaddick G Snowball J Nightingale A Tillett W et al Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis (2018) 77:277–80. 10.1136/annrheumdis-2017-212328
3.
Wrobleski ST Moslin R Lin S Zhang Y Spergel S Kempson J et al Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem (2019) 62:8973–95. 10.1021/acs.jmedchem.9b00444
4.
Zheng Q Zhu Y Cheng H Zhu K . Uveitis occurring in a patient with psoriasis during adalimumab therapy: a case report. Indian J Dermatol (2022) 67:207. 10.4103/ijd.ijd_366_21
5.
Shibata T Muto J Hirano Y Takama H Yanagishita T Ohshima Y et al Palmoplantar pustulosis-like eruption following tofacitinib therapy for juvenile idiopathic arthritis. JAAD Case Rep (2019) 5:518–21. 10.1016/j.jdcr.2019.03.024
Summary
Keywords
deucravacitinib, uveitis, psoriatic uveitis, paradoxical reaction, psoriasis vulgaris
Citation
Watanabe N, Murata T, Sugisawa T, Gomi F and Kanazawa N (2024) Uveitis in a psoriasis vulgaris patient receiving deucravacitinib: a case report. J. Cutan. Immunol. Allergy 7:12545. doi: 10.3389/jcia.2024.12545
Received
10 December 2023
Accepted
23 February 2024
Published
05 March 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Watanabe, Murata, Sugisawa, Gomi and Kanazawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nobuo Kanazawa, nkanazaw@hyo-med.ac.jp
ORCID: Natsu Watanabe, orcid.org/0009-0003-3065-2659; Teruasa Murata, orcid.org/0000-0001-8350-1289; Aya Gomi, orcid.org/0000-0003-0807-8817; Nobuo Kanazawa, orcid.org/0000-0003-3000-9711
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.